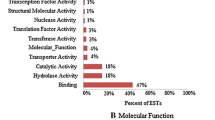
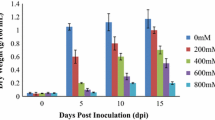
Abstract
Salinity, one of the most deleterious stresses, affects growth and overall yield of crop plants. To identify new “candidate genes” having potential role in salinity tolerance, we have carried out ‘functional screening’ of a cDNA library (made from a salt tolerant rice—Pokkali). Based on this screening, we identified a cDNA clone that was allowing yeast cells to grow in the presence of 1.2 M NaCl. Sequencing and BLAST search identified it as mannose-1-phosphate guanyl transferase (OsMPG1) gene from rice. Analysis of rice genome sequence database indicated the presence of 3 additional genes for MPG. Out of four, three MPG genes viz. OsMPG1, 3 and 4 were able to functionally complement yeast MPG mutant -YDL055C. We have carried out detailed transcript profiling of all members of MPG family by qRT-PCR using two contrasting rice genotypes (IR64 and Pokkali) under different abiotic stresses (salinity, drought, oxidative stress, heat stress, cold or UV light). These MPG genes showed differential expression under various abiotic stresses with two genes (OsMPG1 and 3) showing high induction in response to multiple stresses. Analysis of rice microarray data indicated higher expression levels for OsMPG1 in specific tissues such as roots, leaves, shoot apical meristem and different stages of panicle and seed development, thereby indicating its developmental regulation. Functional validation of OsMPG1 carried out by overexpression in the transgenic tobacco revealed its involvement in enhancing salinity stress tolerance.








Similar content being viewed by others
References
Agaphonov MO, Packeiser AN, Chechenova MB, Choi ES, Ter-Avanesyan MD (2001) Mutation of the homologue of GDP-mannose pyrophosphorylase alters cell wall structure, protein glycosylation and secretion in Hansenula polymorpha. Yeast 18:391–402
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiporter in Arabidopsis. Science 285:1256–1258
Arora R, Agarwal P, Ray S, Singh AK, Singh VP, Tyagi AK, Kapoor S (2007) MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics 8:242
Barth C, Gouzd ZA, Steele HP, Imperio RM (2010) A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J Exp Bot 61:379–394
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Branduardi P, Fossati T, Sauer M, Pagani R, Mattanovich D et al (2007) Biosynthesis of vitamin C by yeast leads to increased stress resistance. PLoS ONE 2(10):e1092
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and H+-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301–308
Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60:765–778
Chinnusamy V, Zhu J, Zhu JK (2006) Salt stress signaling and mechanisms of plant salt tolerance. Genet Eng 27:141–177
Conklin PL (2001) Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant, Cell Environ 24:383–394
Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL (1999) Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proc Natl Acad Sci USA 96:4198–4203
Cuartero J, Bolarin MC, Asins MJ, Moreno V (2006) Increasing salt tolerant in the tomato. J Exp Bot 57:1045–1058
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Ezawa S, Tada Y (2009) Identification of salt tolerance genes from the mangrove plant Bruguiera gymnorrhiza using Agrobacterium functional screening. Plant Sci 176:272–278
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Forment J, Naranjo MA, Roldan M, Serrano R, Vicente O (2002) Expression of Arabidopsis SR-like splicing proteins confers salt tolerance to yeast and transgenic plants. Plant J 30:511–519
Gentzsch M, Tanner W (1996) The PMT gene family: protein O-glycosylation in Saccharomyces cerevisiae is vital. EMBO J 15:5752–5759
Gibeaut DM (2000) Nucleotide sugars and glycosyltransferases for synthesis of cell wall matrix polysaccharides. Plant Physiol Biochem 38:69–80
Hashimoto H, Sakakibara A, Yamasaki M, Yoda K (1997) Saccharomyces cerevisiae VIG9 encodes GDP-mannose pyrophosphorylase, which is essential for protein glycosylation. J Biol Chem 272:16308–16314
Herscovics A, Orlean P (1993) Glycoprotein biosynthesis in yeast. FASEB J 7:540–550
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Itoh H, Sasaki A, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Hasegawa Y, Minami E, Ashikari M, Matsuoka M (2005) Dissection of the phosphorylation of rice DELLA protein, SLENDER RICE1. Plant Cell Physiol 46:1392–1399
Iverson TM, Alber BE, Kisker C, Ferry JG, Rees DC (2000) A closer look at the active site of gamma-class carbonic anhydrases: high-resolution crystallographic studies of the carbonic anhydrase from Methanosarcina thermophila. Biochemistry 39:9222–9231
Joshi A, Dang HQ, Vaid N, Tuteja N (2009) Isolation of high salinity stress tolerant genes from Pisum sativum by random overexpression in Escherichia coli and their functional validation. Plant Signal Behav 4:400–412
Kanhonou R, Serrano R, Palau RR (2001) A catalytic subunit of the sugar beet protein kinase CK2 is induced by salt stress and increases NaCl tolerance in Saccharomyces cerevisiae. Plant Mol Biol 47:571–579
Karan R, Singla-Pareek SL, Pareek A (2009) Histidine kinase and response regulator genes as they relate to salinity tolerance in rice. Funct Integr Genomics 9:411–417
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Koropatkin NM, Holden HM (2004) Molecular structure of alpha-d-glucose-1-phosphate cytidylyltransferase from Salmonella typhi. J Biol Chem 279:44023–44029
Kumar G, Purty RS, Sharma MP, Singla-Pareek SL, Pareek A (2009) Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. J Plant Physiol 166:507–520
Kumari S, Sabharwal VP, Khushwaha HR, Sopory SK, Singla-Pareek SL, Pareek A (2009a) Transcriptome map for seedling stage specific salinity response indicates a specific set of genes as candidate for saline tolerance in Oryza sativa L. Funct Integr Genomics 9:109–123
Kumari S, Singh P, Singla-Pareek SL, Pareek A (2009b) Heterologous expression of a salinity and developmentally regulated rice cyclophilin gene (OsCyp2) in E. coli and S. cerevisiae confers tolerance towards multiple abiotic stresses. Mol Biotechnol 42:195–204
Kushwaha HR, Singh AK, Sopory SK, Singla-Pareek SL, Pareek A (2009) Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics 10:200
Kushwaha HR, Kumar G, Verma PK, Singla-Pareek SL, Pareek A (2011) Analysis of a salinity induced BjSOS3 protein from Brassica indicate it to be structurally and functionally related to its ortholog from Arabidopsis. Plant Physiol Biochem 49:996–1004
Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA et al (2007) Clustal W and ClustalX version 2.0. Bioinformatics 23:2947–2948
Leidich SD, Kostova Z, Latek RR, Costello LC, Drapp DA, Gray W et al (1995) Temperature sensitive yeast GPI anchoring mutants gpi2 and gpi3 are defective in the synthesis of N-acetylglucosaminyl phosphatidylinositol. Cloning of the GPI2 gene. J Biol Chem 270:13029–13035
Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR (2001) Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA 98:2262–2267
Mochalkin I, Lightle S, Zhu Y, Ohren JF, Spessard C, Chirgadze NY, Banotai C, Melnick M, McDowell L (2007) Characterization of substrate binding and catalysis in the potential antibacterial target N-acetylglucosamine-1-phosphate uridyltransferase (GlmU). Protein Sci 16:2657–2666
Mundree SG, Whittaker A, Thomson JA, Farrant JM (2000) An aldose reductase homolog from the resurrection plant Xerophyta viscosa. Planta 211:693–700
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell Environ 25:239–250
Mustafiz A, Sahoo KK, Singla-Pareek SL, Sopory SK (2010) Metabolic engineering of glyoxalase pathway for enhancing stress tolerance in plants. Methods Mol Biol 639:95–118
Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct Integr Genomics 11:293–305
Orlean P (1997) Biogenesis of yeast wall and surface components. In: Pringle JR, Broach JR, Jones EW (eds) The molecular biology of the yeast Saccharomyces, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 229–362
Owens S (2001) Salt of the earth. Genetic engineering may help to reclaim agricultural land lost due to salinization. EMBO Rep 2(10):877–879
Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL (2006) Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol 142:380–397
Pareek A, Sopory SK, Bohnert HJ, Govindjee (2010) Abiotic stress adaptation in plants: Physiological, Molecular and Genomic Foundation, 1st edn. Springer, The Netherlands
Piao HL, Lim JH, Kim SJ, Cheong GW, Hwang I (2001) Constitutive over-expression of AtGSK1 induces NaCl stress responses in the absence of NaCl stress and results in enhanced NaCl tolerance in Arabidopsis. Plant J 27:305–314
Rausell A, Kanhonou R, Yenush L, Serrano R, Ros R (2003) The translation initiation factor eIF1A is an important determinant in the tolerance to NaCl stress in yeast and plants. Plant J 34:257–267
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakamoto A, Murata A, Murata N (1998) Metabolic engineering of rice leading to biosynthesis of glycine betaine and tolerance to salt and cold. Plant Mol Biol 38:1011–1019
Seki M, Kamei A, Yamaguchi-Shinozaki K, Shinozaki K (2003) Molecular responses to drought, salinity and frost: common and different paths for plant protection. Curr Opin Biotechnol 14:194–199
Serrano R, Gaxiola R (1994) Microbial models and salt stress tolerance in plants. CRC Crit Rev Plant Sci 13:121–138
Shi H, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Singh AK, Ansari MW, Pareek A, Singla-Pareek SL (2008) Raising salinity tolerant rice: recent progress and future perspectives. Physiol Mol Biol Plants 14:137–154
Singh AK, Kumar R, Pareek A, Sopory SK, Singla-Pareek SL (2012) Overexpression of rice CBS domain containing protein improves salinity, oxidative, and heavy metal tolerance in transgenic tobacco. Mol Biotechnol. doi:10.1007/s12033-011-9487-2
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci USA 100:14672–14677
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17:171–180
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Uddin MI, Qi Y, Yamada S, Shibuya I, Deng XP, Kwak SS, Kaminak H, Tanak K (2008) Overexpression of a new rice vacuolar antiporter regulating protein OsARP improves salt tolerance in tobacco. Plant Cell Physiol 49:880–890
Vandesompele J, Kubista M, Pfaffl MW (2009) Reference gene validation software for improved normalization. In: Logan J, Edwards K, Saunders N (eds) Real-time PCR: current technology and applications, vol 47. Caister Academic Press, Norfolk, pp 64–90
Velasquez SM, Ricardi MM, Dorosz JG, Fernandez PV, Nadra AD et al (2011) O-glycosylated cell wall proteins are essential in root hair growth. Science 332:1401–1403
Verma D, Singla-Pareek SL, Rajagopal D, Reddy MK, Sopory SK (2007) Functional validation of a novel isoform of Na+/H+ antiporter from Pennisetum glaucum for enhancing salinity tolerance in rice. J Biosci 32:621–628
Wang XG, Olsen LR, Roderick SL (2002) Structure of the lac operon galactoside acetyltransferase. Structure 10:581–588
Wang WB, Vinocur A, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Warit S, Zhang N, Short A, Walmsley RM, Oliver SG, Stateva LI (2000) Glycosylation deficiency phenotypes resulting from depletion of GDP-mannose pyrophosphorylase in two yeast species. Mol Microbiol 36:1156–1166
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 39:365–369
Yamada A, Tsutsumi K, Tanimoto S, Ozeki Y (2003) Plant RelA/SpoT homolog confers salt tolerance in Escherichia coli and Saccharomyces cerevisiae. Plant Cell Physiol 44:3–9
Yoda K, Kawada T, Kaibara C, Fujie A, Abe M, Hashimoto H, Shimizu J, Tomishige N, Noda Y, Yamasaki M (2000) Defect in cell wall integrity of the yeast Saccharomyces cerevisiae caused by a mutation of the GDP-mannose pyrophosphorylase gene VIG9. Biosci Biotechnol Biochem 64:1937–1941
Yoshida S, Forno DA, Cock JH, Gomez KA (1972) Laboratory manual for physiological studies of rice, 3rd edn. International Rice Research Institute, Manila, pp 1–83
Acknowledgments
Authors are thankful for the funds received from International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi and Department of Biotechnology (DBT) and Department of Science & Technology (DST), Government of India. AM thanks ICGEB for providing post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ritesh Kumar and Ananda Mustafiz contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2012_9928_MOESM2_ESM.ppt
Representative picture to show screening of rice cDNA library in yeast system under high salinity. The rectangle marks one of the yeast clones showing normal robust growth and maintaining its rounded morphology on the medium containing 900 mM NaCl, while all other yeast clones showed scattered and irregular growth on this media. (PPT 222 kb)
Rights and permissions
About this article
Cite this article
Kumar, R., Mustafiz, A., Sahoo, K.K. et al. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol Biol 79, 555–568 (2012). https://doi.org/10.1007/s11103-012-9928-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-012-9928-8