Abstract
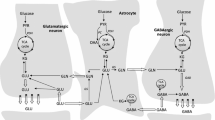
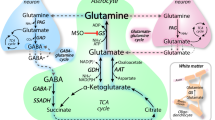
In the brain, glutamine synthetase (GS), which is located predominantly in astrocytes, is largely responsible for the removal of both blood-derived and metabolically generated ammonia. Thus, studies with [13N]ammonia have shown that about 25 % of blood-derived ammonia is removed in a single pass through the rat brain and that this ammonia is incorporated primarily into glutamine (amide) in astrocytes. Major pathways for cerebral ammonia generation include the glutaminase reaction and the glutamate dehydrogenase (GDH) reaction. The equilibrium position of the GDH-catalyzed reaction in vitro favors reductive amination of α-ketoglutarate at pH 7.4. Nevertheless, only a small amount of label derived from [13N]ammonia in rat brain is incorporated into glutamate and the α-amine of glutamine in vivo. Most likely the cerebral GDH reaction is drawn normally in the direction of glutamate oxidation (ammonia production) by rapid removal of ammonia as glutamine. Linkage of glutamate/α-ketoglutarate-utilizing aminotransferases with the GDH reaction channels excess amino acid nitrogen toward ammonia for glutamine synthesis. At high ammonia levels and/or when GS is inhibited the GDH reaction coupled with glutamate/α-ketoglutarate-linked aminotransferases may, however, promote the flow of ammonia nitrogen toward synthesis of amino acids. Preliminary evidence suggests an important role for the purine nucleotide cycle (PNC) as an additional source of ammonia in neurons (Net reaction: l-Aspartate + GTP + H2O → Fumarate + GDP + Pi + NH3) and in the beat cycle of ependyma cilia. The link of the PNC to aminotransferases and GDH/GS and its role in cerebral nitrogen metabolism under both normal and pathological (e.g. hyperammonemic encephalopathy) conditions should be a productive area for future research.



Similar content being viewed by others
Notes
Ammonia free base (NH3) has a pK a of ~9.2. Thus, under normal intracellular physiological conditions (pH 7.2–7.4) ammonia exists predominantly (~99 %) as the conjugate acid, ammonium (NH4 +). For convenience, unless otherwise stated, the term ammonia is used throughout the text to indicate the sum of NH3 plus NH4 +.
This finding appears to have been the basis for the model developed by the Shulman laboratory for the NMR measurement of TCA flux using [13C]glucose cf. [11, 31, 32]. Due to the very rapid exchange of nitrogen in the AAT reaction (considerably faster than carbon flux through the TCA cycle) label in glutamate is a good proxy for that in the much smaller pool of α-ketoglutarate.
Abbreviations
- AAT:
-
Aspartate aminotransferase
- AMP:
-
Adenosine monophosphate
- BBB:
-
Blood–brain barrier
- BCA:
-
Branched-chain amino acid
- BUI:
-
Brain uptake index
- cAAT:
-
Cytosolic aspartate aminotransferase
- CSF:
-
Cerebrospinal fluid
- GDH:
-
Glutamate dehydrogenase
- GS:
-
Glutamine synthetase
- HE:
-
Hepatic encephalopathy
- HPLC:
-
High performance liquid chromatography
- IMP:
-
Inosine monophosphate
- mAAAT:
-
Mitochondrial aspartate aminotransferase
- MAS:
-
Malate-aspartate shuttle
- MSKCC:
-
Memorial Sloan Kettering Cancer center
- NMR:
-
Nuclear magnetic resonance
- MSO:
-
l-Methionine-S,R-sulfoximine
- PCS:
-
Portacaval shunt
- PET:
-
Positron emission tomography
- TCA:
-
Tricarboxylic acid
References
Berl S, Takagaki G, Clarke DD, Waelsch H (1962) Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem 237:2562–2569
Gebhardt R, Ebert A, Bauer G (1988) Heterogeneous expression of glutamine synthetase mRNA in rat liver parenchyma revealed by in situ hybridization and Northern blot analysis of RNA from periportal and perivenous hepatocytes. FEBS Lett 241:89–93
Brosnan ME, Brosnan JT (2009) Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 90:857S–861S
Berl S, Takagaki G, Clarke DD, Waelsch H (1962) Carbon dioxide fixation in the brain. J Biol Chem 237:2570–2573
Lapidot A, Gopher A (1994) Cerebral metabolic compartmentation. Estimation of glucose flux via pyruvate carboxylase/pyruvate dehydrogenase by 13C NMR isotopomer analysis of D-[U-13C]glucose metabolites. J Biol Chem 269:27198–27208
Lapidot A, Gopher A (1997) Quantitation of metabolic compartmentation in hyperammonemic brain by natural abundance 13C-NMR detection of 13C-15N coupling patterns and isotopic shifts. Eur J Biochem 243:597–604
Zwingmann C, Brand A, Richter-Landsberg C, Leibfritz D (1998) Multinuclear NMR spectroscopy studies on NH4Cl-induced metabolic alterations and detoxification processes in primary astrocytes and glioma cells. Dev Neurosci 20:417–426
Kanamatsu T, Tsukada Y (1999) Effects of ammonia on the anaplerotic pathway and amino acid metabolism in the brain: an ex vivo 13C NMR spectroscopic study of rats after administering [2-13C] glucose with or without ammonium acetate. Brain Res 84:11–19
Zwingmann C (2007) The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis 22:235–249
Dadsetan S, Bak LK, Sørensen M, Keiding S, Vilstrup H, Ott P, Leke R, Schousboe A, Waagepetersen HS (2011) Inhibition of glutamine synthesis induces glutamate dehydrogenase-dependent ammonia fixation into alanine in co-cultures of astrocytes and neurons. Neurochem Int 59:482–488
Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG (2001) In vivo 13C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during [2-13C]glucose infusion. J Neurochem 76:975–989
Duarte JMN, Lanz B, Gruetter R (2011) Compartmentalised cerebral metabolism of [1,6-13C]glucose determined by in vivo 13C NMR spectroscopy at 14.1 T. Front Neuroenergetics 3:3
Hassel B (2000) Carboxylation and anaplerosis in neurons and glia. Mol Neurobiol 22:21–40
Cooper AJL (2011) 13N as a tracer for studying glutamate metabolism. Neurochem Int 59:456–464
Cooper AJL, Gelbard AS, Freed BR (1985) Nitrogen-13 as a biochemical tracer. Adv Enzymol Relat Areas Mol Biol 57:251–356
Cooper AJL, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE (1979) The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 254:4982–4992
Lockwood AH, Finn RD, Campbell JA, Richman TB (1980) Factors that affect the uptake of ammonia by the brain: the blood-brain pH gradient. Brain Res 181:259–266
Raichle ME, Larson KB (1981) The significance of the NH3-NH4 + equilibrium on the passage of 13N-ammonia from blood to brain. A new regional residue detection model. Circ Res 48:913–937
Carter CC, Lifton JF, Welch MJ (1973) Organ uptake and blood pH and concentration effects of ammonia in dogs determined with ammonia labeled with 10 minute half-lived nitrogen 13. Neurology 23:204–213
Cooper AJL, Freed BR (2005) Metabolism of [13N]ammonia in rat lung. Neurochem Int 47:103–118
Cooper AJL, Lai JCK, Gelbard AS (1988) Ammonia metabolism in normal and hyperammonemic rat brain. In: Norenberg MD, Hertz L, Schousboe A (eds) The Biochemical pathology of astrocytes. Alan R Liss, Inc, New York, pp 419–434
Glutamine WuC, Synthetase I (1963) A comparative study of its distribution in animals and its inhibition by DL-allo-δ-hydroxylysine. Comp Biochem Physiol 34:335–3351
Pamiljans V, Krishnaswamy PR, Dumville G, Meister A (1962) Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry 1:153–158
Martinez-Hernandez A, Bell KP, Norenberg MD (1977) Glutamine synthetase: glial localization in brain. Science 195:1356–1358
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Hertz L, Schousboe A (1988) Metabolism of glutamate and glutamine in neurons and astrocytes in primary cultures. In: Kvamme E (ed) Glutamine and glutamate in mammals, vol II. CRC Press Inc, Boca Raton, pp 39–55
Kanamori K, Parivar F, Ross BD (1993) A 15N NMR study of in vivo cerebral glutamine synthesis in hyperammonemic rats. NMR Biomed 6:21–26
Kanamori K, Ross BD (1993) 15N n.m.r. measurement of the in vivo rate of glutamine synthesis and utilization at steady state in the brain of the hyperammonaemic rat. Biochem J 293:461–468
Kanamori K, Ross BD (1995) Steady-state in vivo glutamate dehydrogenase activity in rat brain measured by 15N NMR. J Biol Chem 270:24805–24809
Kanamori K, Ross BD, Chung JC, Kuo EL (1996) Severity of hyperammonemic encephalopathy correlates with brain ammonia level and saturation of glutamine synthetase in vivo. J Neurochem 67:1584–1594
Shen J, Sibson NR, Cline G, Behar KL, Rothman DL, Shulman RG (1998) 15N-NMR spectroscopy studies of ammonia transport and glutamine synthesis in the hyperammonemic rat brain. Dev Neurosci 20:434–443
Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, Shulman RG (1997) In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci USA 94:2699–2704
Cudalbu C, Lanz B, Duarte JM, Morgenthaler FD, Pilloud Y, Mlynárik V, Gruetter R (2012) Cerebral glutamine metabolism under hyperammonemia determined in vivo by localized 1H and 15N NMR spectroscopy. J Cereb Blood Flow Metab 32:696–708
Cooper AJL, Mora SN, Cruz NF, Gelbard AS (1985) Cerebral ammonia metabolism in hyperammonemic rats. J Neurochem 44:1716–1723
Desjardins P, Rao KV, Michalak A, Rose C, Butterworth RF (1999) Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis 14:273–280
Chatauret N, Desjardins P, Zwingmann C, Rose C, Rao KV, Butterworth RF (2006) Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J Hepatol 44:1083–1088
Desjardins P, Du T, Jiang W, Peng L, Butterworth RF (2012) Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure: role of glutamine redefined. Neurochem Int. 2012 Feb 21. [Epub ahead of print]
Bosman DK, Deutz NE, De Graaf AA, vd Hulst RW, Van Eijk HM, Bovée WM, Maas MA, Jörning GG, Chamuleau RA (1990) Changes in brain metabolism during hyperammonemia and acute liver failure: results of a comparative 1H-NMR spectroscopy and biochemical investigation. Hepatology 12:281–290
Levintow L, Meister A (1954) Reversibility of the enzymatic synthesis of glutamine. J Biol Chem 209:265–280
Wedler FC (1974) Mechanisms of substrate binding with glutamine synthetase. Equilibrium isotope exchanges with the ovine brain, pea seed, and Escherichia coli enzymes. J Biol Chem 249:5080–5087
Hindfelt B, Plum F, Duffy TE (1977) Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest 59:386–396
Meister A (1985) Glutamine synthetase from mammalian tissues. Methods Enzymol 113:185–199
Minet R, Villie F, Marcollet M, Meynial-Denis D, Cynober L (1997) Measurement of glutamine synthetase activity in rat muscle by a colorimetric assay. Clin Chim Acta 268:121–132
Santoro JC, Harris G, Sitlani A (2001) Colorimetric detection of glutamine synthetase-catalyzed transferase activity in glucocorticoid-treated skeletal muscle cells. Anal Biochem 289:18–25
Cooper AJL, Vergara F, Duffy TE (1983) Cerebral glutamine synthetase. In: Hertz L, Kvamme E, McGeer E, Schousboe A (eds) Glutamine, glutamate and GABA in the central nervous system. Alan R Liss Inc, New York, pp 77–93
Vaquero J, Butterworth RF (2006) The brain glutamate system in liver failure. J Neurochem 98:661–669
Lockwood AH, Yap EW, Wong WH (1991) Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. J Cereb Blood Flow Metab 11:337–341
Ahl B, Weissenborn K, van den Hoff J, Fischer-Wasels D, Köstler H, Hecker H, Burchert W (2004) Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology 40:73–79
Keiding S, Sørensen M, Bender D, Munk OL, Ott P, Vilstrup H (2006) Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology 43:42–50
Sørensen M, Keiding S (2007) New findings on cerebral ammonia uptake in HE using functional 13N-ammonia PET. Metab Brain Dis 22:277–284
Sørensen M, Munk OL, Keiding S (2009) Backflux of ammonia from brain to blood in human subjects with and without hepatic encephalopathy. Metab Brain Dis 24:237–242
Keiding S, Sørensen M, Munk OL, Bender D (2010) Human 13N-ammonia PET studies: the importance of measuring 13N-ammonia metabolites in blood. Metab Brain Dis 25:49–56
Cooper AJL, Plum F (1987) Biochemistry and physiology of brain ammonia. Physiol Rev 67:440–519
Lavoie J, Giguère JF, Layrargues GP, Butterworth RF (1987) Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem 49:692–697
Córdoba J, Sanpedro F, Alonso J, Rovira A (2002) 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis 17:415–429
Rovira A, Alonso J, Córdoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29:1612–1621
Mardini H, Smith FE, Record CO, Blamire AM (2011) Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol 54:1154–1160
Manning JM, Moore S, Rowe WB, Meister A (1969) Identification of l-methionine S-sulfoximine as the diastereoisomer of l-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry 8:2681–2685
Gibson GE, Zimber A, Krook L, Richardson EP, Visek WJ (1974) Brain histology and behavior of mice injected with urease. J Neuropathol Exp Neurol 33:201–211
Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A (2007) Glutamine in the central nervous system: function and dysfunction. Front Biosci 12:332–343
Hertz L (2011) Astrocytic energy metabolism and glutamate formation–relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging 29:1319–1329
Hogstad S, Svenneby G, Torgner IA, Kvamme E, Hertz L, Schousboe A (1988) Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem Res 13:383–388
Chee PY, Dahl JL, Fahien LA (1979) The purification and properties of rat brain glutamate dehydrogenase. J Neurochem 33:53–60
Yu ACH, Schousboe A, Hertz L (1982) Metabolic fate of 14C-labeled glutamate in astrocytes in primary cultures. J Neurochem 39:954–960
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
Hertz L, Hertz E (2003) Cataplerotic TCA cycle flux determined as glutamate-sustained oxygen consumption in primary cultures of astrocytes. Neurochem Int 43:355–361
Lowenstein JM (1972) Ammonia production in muscle and other tissues: the purine nucleotide cycle. Physiol Rev 52:382–414
Schultz V, Lowenstein JM (1976) Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem 251:485–492
Schultz V, Lowenstein JM (1978) The purine nucleotide cycle. Studies of ammonia production and interconversions of adenine and hypoxanthine nucleotides and nucleosides by rat brain in situ. J Biol Chem 253:1938–1943
Chapman AG, Miller AL, Atkinson DE (1976) Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res 36:1144–1150
Knecht K, Wiesmüller KH, Gnau V, Jung G, Meyermann R, Todd KG, Hamprecht B (2001) AMP deaminase in rat brain: localization in neurons and ependymal cells. J Neurosci Res 66:941–950
Braunstein AE (1957) Principal ways of assimilation & dissimilation of nitrogen in animals. Adv Enzymol Relat Sub Biochem 19:335–389 (French)
Cooper AJL, Meister A (1989) An appreciation of Professor Alexander E. Braunstein. The discovery and scope of enzymatic transamination. Biochimie 71:387–404
Hutson SM, Islam MM, Zaganas I (2011) Interaction between glutamate dehydrogenase (GDH) and l-leucine catabolic enzymes: intersecting metabolic pathways. Neurochem Int 59:518–524
Spanaki C, Plaitakis A (2012) The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res 21:117–127
Leke R, Bak LK, Anker M, Melø TM, Sørensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Sonnewald U, Schousboe A, Waagepetersen HS (2011) Detoxification of ammonia in mouse cortical GABAergic cell cultures increases neuronal oxidative metabolism and reveals an emerging role for release of glucose-derived alanine. Neurotox Res 19:496–510
Benuck M, Stern F, Lajtha A (1971) Transamination of amino acids in homogenates of rat brzain. J Neurochem 18:1555–1567
Oldendorf WH, Szabo J (1976) Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol 230:94–98
Young RL, Lowry OH (1966) Quantitative methods for measuring the histochemical distribution of alanine, glutamate and glutamine in brain. J Neurochem 13:785–793
Yudkoff M, Nissim I, Hertz L (1990) Precursors of glutamic acid nitrogen in primary neuronal cultures: studies with 15N. Neurochem Res 15:1191–1196
Tofteng F, Hauerberg J, Hansen BA, Pedersen CB, Jørgensen L, Larsen FS (2006) Persistent arterial hyperammonemia increases the concentration of glutamine and alanine in the brain and correlates with intracranial pressure in patients with fulminant hepatic failure. J Cereb Blood Flow Metab 26:21–27
Bjerring PN, Hauerberg J, Frederiksen HJ, Jorgensen L, Hansen BA, Tofteng F, Larsen FS (2008) Cerebral glutamine concentration and lactate-pyruvate ratio in patients with acute liver failure. Neurocrit Care 9:3–7
Parli JA, Godfrey DA, Ross CD (1987) Separate enzymatic microassays for aspartate aminotransferase isoenzymes. Biochim Biophys Acta 925:175–184
Fitzpatrick SM, Cooper AJL, Duffy TE (1983) Use of β-methylene-dl-aspartate to assess the role of aspartate aminotransferase in cerebral oxidative metabolism. J Neurochem 41:1370–1383
Hertz L, Murthy CR, Lai JC, Fitzpatrick SM, Cooper AJL (1987) Some metabolic effects of ammonia on astrocytes and neurons in primary cultures. Neurochem Pathol 6:97–129
Fitzpatrick SM, Cooper AJL, Hertz L (1988) Effects of ammonia and β-methylene-dl-aspartate on the oxidation of glucose and pyruvate by neurons and astrocytes in primary culture. J Neurochem 51:1197–1203
Hertz L, Dienel GA (2002) Energy metabolism in the brain. Int Rev Neurobiol 51:1–102
Hertz L, Kala G (2007) Energy metabolism in brain cells: effects of elevated ammonia concentrations. Metab Brain Dis 22:199–218
Williamson DH, Lund P, Krebs HA (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103:514–527
Treberg JR, Brosnan ME, Watford M, Brosnan JT (2010) On the reversibility of glutamate dehydrogenase and the source of hyperammonemia in the hyperinsulinism/hyperammonemia syndrome. Adv Enzyme Reg 50:34–43
Howse DC, Duffy TE (1975) Control of the redox state of the pyridine nucleotides in the rat cerebral cortex. Effect of electroshock-induced seizures. J Neurochem 24:935–940
Cooper AJL, Nieves E, Coleman AE, Filc-DeRicco S, Gelbard AS (1987) Short-term metabolic fate of [13N]ammonia in rat liver in vivo. J Biol Chem 262:1073–1080
Müller AF, Leuthardt F (1950) Die Umwandlung der Glutaminsäure in Asparaginsäure in den Mitochondrien der Leber. (mit Bemerkung über das Vorkommen einer Transaminase in Clostridium Welchii). Helv Chim Acta 33:268–273
Lowenstein JM (1967) The tricarboxylic acid cycle. In: Greenberg DM (ed) Metabolic pathways, vol 1. Academic Press, New York, pp 146–270
Cooper AJL (1981) l-Glutamate (2-oxoglutarate) aminotransferases. In: Kvamme E (ed) Glutamine and glutamate in mammals, vol I. CRC Press Inc, Boca Raton, pp 123–152
McKenna MC, Stevenson JH, Huang X, Hopkins IB (2000) Differential distribution of the enzymes glutamate dehydrogenase and aspartate aminotransferase in cortical synaptic mitochondria contributes to metabolic compartmentation in cortical synaptic terminals. Neurochem Int 37:229–241
Pardo B, Rodrigues TB, Contreras L, Garzón M, Llorente-Folch I, Kobayashi K, Saheki T, Cerdan S, Satrústegui J (2011) Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J Cereb Blood Flow Metab 31:90–101
Hertz L (2011) Brain glutamine synthesis requires neuronal aspartate: a commentary. J Cereb Blood Flow Metab 31:384–387
Lowenstein JM, Goodman MN (1978) The purine nucleotide cycle in skeletal muscle. Fed Proc 37:2308–2312
Bergmeyer HU (1974) Methods of enzymatic analysis. Academic Press Inc. New York, pp. 2284–2285
Acknowledgments
I thank Dr. Boris Krasnikov (New York Medical College) for his help in preparing this manuscript. I also thank Dr. Kevin Behar (Yale University School of Medicine) for valuable discussion. Part of the work described in this review was supported by NIH grant DK 16739.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: in honour of Leif Hertz.
Rights and permissions
About this article
Cite this article
Cooper, A.J.L. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem Res 37, 2439–2455 (2012). https://doi.org/10.1007/s11064-012-0803-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0803-4