Abstract
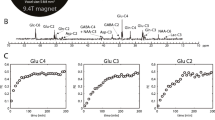
Metabolic alterations in the brain underly many of the mechanisms leading to acute and chronic Hepatic Encephalopathy (HE). Controversy exists about the role of glutamine accumulation as a causal factor in HE. Glutamine formation contributes to detoxify ammonia, whereby anaplerotic mechanisms in the astrocytes have to be sufficient to replenish Krebs cycle intermediates. The application of ex vivo high-resolution nuclear magnetic resonance (NMR) spectroscopy permits direct measurements of metabolites and different metabolic pathways. Ex vivo 13C-NMR studies in experimental animal models of acute and chronic HE have provided new insights. In an experimental rat model of ALF, 13C isotopomer analysis of glucose metabolism showed that alterations of glucose flux through astrocytic pyruvate carboxylase might be linked to the pathogenesis of ALF as a limited anaplerotic flux in the brain, but not in the muscle, correlates with the development of brain edema. Moreover, 13C-NMR data from a rat model of mild HE demonstrated relative differences in the pathway of glucose through pyruvate carboxylase in thalamus compared to frontal cortex, which might explain the vulnerability of this brain region compared to thalamus. These findings further support that glutamine accumulation might be not the primary cause of neurological symptoms in HE, and show that anaplerotic mechanisms could be essential for ammonia detoxification in HE.






Similar content being viewed by others
References
Adams RD, Foley JM (1953) The neurological disorders associated with liver disease. Annu Rev Nerv Ment Dis Proc 32:198
Ahl B, Weissenborn K, van den Hoff J, Fischer-Wasels, D, Kostler H, Hecker H, Burchert W (2004) Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology 40:73–79
Albrecht J (2003) Glucose-derived osmolytes and energy impairment in brain edema accompanying liver failure: the role of glutamine reevaluated. Gastroenterology 125:76–978
Albrecht J, Dolinska M (2001) Glutamine as a pathogenic factor in hepatic encephalopathy. J Neurosci Res 65:1–5
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21:1133–1145
Aureli T, Di Cocco ME, Calvani M, Conti F (1997) The entry of [1-13C]glucose into biochemical pathways reveals a complex compartmentation and metabolite trafficking between glia and neurons: a study by 13C–NMR spectroscopy. Brain Res 765:218–227
Balanzs R, Machiyama Y, Hammond BJ, Julian T, Richter D (1970) The operation of the gamma-aminobutyrate bypath of the tricarboxylic acid cycle in brain tissue in vitro. Biochem J 116:445–461
Berl S, Clarke DD (1969) Compartmentation of amino acid metabolism. In: Lajtha A (ed) Handbook of neurochemistry (Vol. 2, pp 447–472). Plenum Press, New York
Berl S, Nicklas WJ, Clarke DD (1970) Compartmentation of citric acid cycle metabolism in brain: labelling of glutamate, glutamine, aspartate and GABA by several radioactive tracer metabolites. J Neurochem 17:1009–1015
Blei A-T, Olafsson S, Therrien G, Butterworth R-F (1994) Ammonia-induced brain edema and intracranial hypertension in rats after portacaval anastomosis. Hepatology 19:1437–1444
Bluml S, Moreno-Torres A, Shic F, Nguy CH, Ross BD (2002) Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed 15:1–5
Bosman DK, Deutz NE, Maas MA, van Eijk HM, Smit JJ, de Haan JG, Chamuleau RA (1992) Amino acid release from cerebral cortex in experimental acute liver failure, studied by in vivo cerebral cortex microdialysis. J Neurochem 59:591–599
Butterworth RF (2000) Hepatic encephalopathy: a neuropsychiatric disorder involving multiple neurotransmitter systems. Curr Opin Neurol 13:721–727
Butterworth RF (2003) Pathogenesis of hepatic encephalopathy: new insights from neuroimaging and molecular studies. J Hepatol 39:278–285
Butterworth RF, Girard G, Giguere JF (1988) Regional differences in the capacity for ammonia removal by brain following portocaval anastomosis. J Neurochem 51:486–490
Cesar M, Hamprecht B (1995) Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem 64:2312–2318
Chatauret N, Zwingmann C, Rose C, Leibfritz D, Butterworth R-F (2003) Effects of hypothermia on brain glucose metabolism in acute liver failure: a H/C-nuclear magnetic resonance study. Gastroenterology 125:815–824
Cooper AJ, Lai JC (1987) Cerebral ammonia metabolism in normal and hyperammonemic rats. Neurochem Pathol 6:67–95
Cordoba J, Blei A-T (1995) Cerebral edema and intracranial pressure monitoring. Liver Transplant Surg 1:187–194
Cordoba J, Crespin J, Gottstein J, Blei AT (1999) Mild hypothermia modifies ammonia-induced brain edema in rats after portacaval anastomosis. Gastroenterology 116:686–693
Cruz F, Cerdan S (1999) Quantitative 13C NMR studies of metabolic compartmentation in the adult mammalian brain. NMR Biomed 12:451–462
Deutz NEP, De Graaf AA, De Haan JG, Bovée WMMJ, Chamuleau RAFM (1988) in vivo brain 1H-NMR spectroscopy (1H-NMRS) during acute hepatic encephalopathy (HE). In: Soeters PB, Wilson JHP, Meijer AJ, Holm E (eds) Advances in ammonia metabolism and hepatic encephalopathy. Chap. 57. Amsterdam. Excerpta Media, pp 439–446
Faff-Michalak L, Albrecht J (1991) Aspartate aminotransferase, malate dehydrogenase, and pyruvate carboxylase activities in rat cerebral synaptic and nonsynaptic mitochondria: effects of in vitro treatment with ammonia, hyperammonemia and hepatic encephalopathy. Metab Brain Dis 6:187–197
Gamberino WC, Berkich DA, Lynch CJ, Xu B, LaNoue KF (1997) Role of pyruvate carboxylase in facilitation of synthesis of glutamate and glutamine in cultured astrocytes. J Neurochem 69:2312–2325
Gjedde A, Marrett S (2001) Glycolysis in neurons, not astrocytes, delays oxidative metabolism of human visual cortex during sustained checkerboard stimulation in vivo. J Cereb Blood Flow Metab 21:1384–1392
Grill V, Bjorkman O, Gutniak M, Lindqvist M (1992) Brain uptake and release of amino acids in nondiabetic and insulin-dependent diabetic subjects: important role of glutamine release for nitrogen balance. Metabolism 41:28–32
Gruetter R (2002) In vivo 13C NMR studies of compartmentalized cerebral carbohydrate metabolism. Neurochem Int 41:143–154
Gruetter R, Seaquist ER, Kim S, Ugurbil K (1998) Localized in vivo 13C-NMR of glutamate metabolism in the human brain: initial results at 4 tesla. Dev Neurosci 20:380–388
Gruetter R, Seaquist ER, Ugurbil K (2001) A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab 281:E100–E112
Hassel B, Paulsen RE, Johnsen A, Fonnum F (1992) Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res 576:120–124
Hertz L, Dienel GA (2002) Energy metabolism in the brain. Int Rev Neurobiol 51:1–102
Hertz L, Dringen R, Schousboe A, Robinson SR (1999) Astrocytes: glutamate producers for neurons. J Neurosci Res 57:417–428
Hertz L, Yu AC, Kala G, Schousboe A (2000) Neuronal-astrocytic and cytosolic-mitochondrial metabolite trafficking during brain activation, hyperammonemia and energy deprivation. Neurochem Int 37:83–102
Kanamatsu T, Tsukada Y (1999) Effects of ammonia on the anaplerotic pathway and amino acid metabolism in the brain: an ex vivo 13C NMR spectroscopic study of rats after administering [2-13C] glucose with or without ammonium acetate. Brain Res 841:11–19
Kanamori K, Ross BD, Chung JC, Kuo EL (1996) Severity of hyperammonemic encephalopathy correlates with brain ammonia level and saturation of glutamine synthetase in vivo. J Neurochem 67:1584–1594
Kaufman EE, Driscoll BF (1993) Evidence for cooperativity between neurons and astroglia in the regulation of CO2 fixation in vitro. Dev Neurosci 15:299–305
Lapidot A, Gopher A (1994) Cerebral metabolic compartmentation. Estimation of glucose flux via pyruvate carboxylase/pyruvate dehydrogenase by 13C NMR isotopomer analysis of D–[U–13C]glucose metabolites. J Biol Chem 269:27198–27208
Larsen F-S, Gottstein J, Blei A-T (2001). Cerebral hyperemia and nitric oxide synthase in rats with ammonia-induced brain edema. J Hepatol 34:548–554
Lavoie J, Giguere JF, Layrargues GP, Butterworth RF (1987) Amino acid changes in autopsied brain tissue from cirrhotic patients with hepatic encephalopathy. J Neurochem 49:692–697
Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL (2002). Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22:1523–1531
Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM (2001). Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem 76:1712–1723
Lockwood AH, Yap EW, Rhoades HM, Wong WH (1991) Altered cerebral blood flow and glucose metabolism in patients with liver disease and minimal encephalopathy. J Cereb Blood Flow Metab 11:331–336
Magistretti PJ, Sorg O, Yu N, Martin JL, Pellerin L (1993) Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev Neurosci 15:306–312
Martin M, Portais JC, Voisin P, Rousse N, Canioni P, Merle M (1995) Comparative analysis of 13C-enriched metabolites released in the medium of cerebellar and cortical astrocytes incubated with [1-13C]glucose. Eur J Biochem 231:697–703
Martin M, Canioni P, Merle M (1997). Analysis of carbon metabolism in cultured cerebellar and cortical astrocytes. Cell Mol Biol (Noisy-le-grand) 43:631–643
Michalak A, Rose C, Butterworth J, Butterworth, R.-F. (1996). Neuroactive amino acids and glutamate (NMDA) receptors in frontal cortex of rats with experimental acute liver failure. Hepatology 24:908–913
Minchin MC, Beart PM (1975). Compartmentation of amino acid metabolism in the rat dorsal root ganglion; a metabolic and autoradiographic study. Brain Res 83:437–449
Naruse H, Cheng SC, Waelsch H (1966a) CO2 fixation in the nervous system. V. CO2 fixation and citrate metabolism in rabbit nerve. Exp Brain Res 1:291–298 contd
Naruse H, Cheng SC, Waelsch H (1966b) CO2 fixation in the nervous tissue. IV. CO2 fixation and citrate metabolism in lobster nerve. Exp Brain Res 1:284–290 contd
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R (2004) Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci 24:11273–22379
Patel MS (1974) The relative significance of CO2-fixing enzymes in the metabolism of rat brain. J Neurochem 22:717–724
Pellerin L, Magistretti PJ (2003) How to balance the brain energy budget while spending glucose differently. J Physiol 15:551–564
Shank RP, Aprison MH (1981) The present status and significance of the glutamine cycle in neuronal tissues. Life Sci 28:837–842
Shank RP, Campbell GL (1983) Glutamate in the CNS. In: Lajtha A (ed) Handbook of neurochemistry (vol. 3). Plenum, New York, pp 381–404
Shank RP, Bennett GS, Freytag SO, Campbell GL (1985) Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 329:364–367
Sibson NR, Mason GF, Shen J, Cline GW, Herskovits AZ, Wall JE, Behar KL, Rothman DL, Shulman RG (2001) In vivo (13)C NMR measurement of neurotransmitter glutamate cycling, anaplerosis and TCA cycle flux in rat brain during. J Neurochem 76:975–989
van den Berg CJ, Garfinkel D (1971) A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J 123:211–218
Waelsch H, Berl HW, Rossi CA, Clarke DD, Purpura DP (1964) Quantitative aspects of CO2 fixation in mammalian brain in vivo. J Neurochem 11:717–728
Yu AC, Drejer J, Hertz L, Schousboe A (1983) Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 41:1484–1487
Zwingmann C, Leibfritz D (2003) Regulation of glial metabolism studied by 13C-NMR. NMR Biomed 16:370–399
Zwingmann C, Butterworth RF (2005) An update on the role of brain glutamine synthesis and its relation to cell-specific energy metabolism in the hyperammonemic brain: further studies using NMR spectroscopy. Neurochem Int 47:19–30
Zwingmann C, Brand A, Richter-Landsberg C, Leibfritz D (1998) Multinuclear NMR spectroscopy studies on NH4Cl-induced metabolic alterations and detoxification processes in primary astrocytes and glioma cells. Dev Neurosci 20:417–426
Zwingmann C, Chatauret N, Leibfritz D, Butterworth RF (2003) Selective increase of brain lactate synthesis in experimental acute liver failure: results of a [H–C] nuclear magnetic resonance study. Hepatology 37:420–428
Acknowledgements
This work was supported by the Canadian Instituts for Health Research (CIHR). C. Zwingmann has received fellowships from the Quebec Ministry of Education and the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zwingmann, C. The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis 22, 235–249 (2007). https://doi.org/10.1007/s11011-007-9069-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-007-9069-y