Abstract
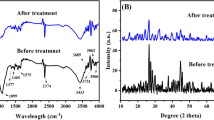
In this work, a novel three dimensional graphene oxide sponge composite material was synthesized by functionalized GO sheets with phytic acid (PA). The as-synthesized samples were characterized and employed to investigate the removal of U(VI) from aqueous solution. Results show that higher pH favored the sorption of uranium on PA–GO. Ionic strength puts insignificant influence on the sorption. The maximum adsorption capacity is 124.3 mg g−1 at pH 5.5. The adsorption isotherms can be well described by Langmuir isotherm model and the sorption kinetics has been successfully modeled by pseudo-second-order kinetic model.









Similar content being viewed by others
References
Craft ES, Abu-Qare AW, Flaherty MM, Garofolo MC, Rincavage HL, Abou-Donia MB (2004) Depleted and natural uranium: chemistry and toxicological effects. J Toxicol Environ Health Part B 7:297–317
Sheppard SC, Sheppard MI, Gallerand M, Sanipelli BJ (2005) Derivation of ecotoxicity thresholds for uranium. Environ Radioact 79:55–83
Aziz A, Jan S, Waqar F, Mohammad B, Hakim M, Yawar W (2010) Selective ion exchange separation of uranium from concomitant impurities in uranium materials and subsequent determination of the impurities by ICP-OES. J Radioanal Nucl Chem 284:117–121
Stoliker DL, Kaviani N, Kent DB, Davis JA (2013) Evaluating ion exchange resin efficiency and oxidative capacity for the separation of uranium(IV) and uranium(VI). Geochem Trans 14:1–9
Chou CL, Moffatt JD (2000) A simple co-precipitation inductively coupled plasma mass spectrometric method for the determination of uranium in seawater. Fresenius J Anal Chem 368:59–61
Jyothi A, Rao GN (1990) Solvent extraction behaviour of lanthanum (III), cerium (III), europium (III), thorium (IV) and uranium (VI) with 3-phenyl-4-benzoyl-5-isoxazolone. Talanta 37:431–433
Ramadevi G, Sreenivas T, Navale AS, Padmanabhan NPH (2012) Solvent extraction of uranium from lean grade acidic sulfate leach liquor with alamine 336 reagent. J Radioanal Nucl Chem 294:13–18
Giridhar P, Venkatesan KA, Subramaniam S (2008) Extraction of uranium (VI) by 1.1 M tri- n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase. J Alloy Compd 448:104–108
Kim GN, Kim SS, Park HM, Kim WS, Park UR, Moon JK (2013) Remediation of soil/concrete contaminated with uranium and radium by biological method. J Radioanal Nucl Chem 297:71–78
St John AM, Cattrall RW, Kolev SD (2010) Extraction of uranium (VI) from sulfate solutions using a polymer inclusion membrane containing di-(2-ethylhexyl) phosphoric acid. J Membr Sci 364:354–361
Sifniades S, Largman T, Tunick AA (1981) Recovery of uranium from phosphoric acid by means of supported liquid membranes. Hydrometallurgy 7:201–212
McCleskey TM, Ehler DS, Young JS (2002) Asymmetric membranes with modified gold films as selective gates for metal ion separations. J Membr Sci 210:273–278
Mishra S, Maity S, Bhalke S, Pandit GG, Puranik VD, Kushwaha HS (2012) Thermodynamic and kinetic investigations of uranium adsorption on soil. J Radioanal Nucl Chem 294:97–102
Zareh MM, Aldaher A, Hussein AEM, Mahfouz MG, Soliman M (2013) Uranium adsorption from a liquid waste using thermally and chemically modified bentonite. J Radioanal Nucl Chem 295:1153–1159
Gupta VK, Saleh TA (2013) Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—an overview. Environ Sci Pollut Res 20:2828–2843
Ma Y, Gao NY, Chu WH, Li C (2013) Removal of phenol by powdered activated carbon adsorption. Front Environ Sci Eng 7:158–165
Qu J, Zhang Q, Xia YS, Cong Q, Luo CQ (2015) Synthesis of carbon nanospheres using fallen willow leaves and adsorption of Rhodamine B and heavy metals by them. Environ Sci Pollut Res 22:1408–1419
Guryanov VV, Petukhova GA, Dubinina LA, Lu SS (2010) Determination of parameters of porous structure of carbon adsorbent during analysis of vapor adsorption isotherms in region of polymolecular adsorption. Prot Met Phys Chem 46:191–196
Yavari R, Huang YD, Mostofizadeh A (2010) Sorption of strontium ions from aqueous solutions by oxidized multiwall carbon nanotubes. J Radioanal Nucl Chem 285:703–710
Caccin M, Giacobbo F, Ros MD, Besozzi L, Mariani M (2013) Adsorption of uranium, cesium and strontium onto coconut shell activated carbon. J Radioanal Nucl Chem 297:9–18
Yue YF, Sun XG, Mayes RT, Kim JS, Fulvio PF, Qiao ZA, Brown S, Tsouris C, Oyola Y, Dai S (2013) Polymer-coated nanoporous carbons for trace seawater uranium adsorption. Sci China Chem 56:1510–1515
Wang MM, Qiu J, Tao XQ, Wu CP, Cui WB, Liu Q (2011) Effect of pH and ionic strength on U(IV) sorption to oxidized multiwalled carbon nanotubes. J Radioanal Nucl Chem 288:895–901
Wu WQ, Yang Y, Zhou HH, Ye TT, Huang ZY, Liu R, Kuang YF (2012) Highly efficient removal of Cu(II) from aqueous solution by using graphene oxide. Water Air Soil Pollut 224:1372–1379
Xu J, Lv H, Yang ST, Luo J (2013) Preparation of grapheme adsorbents and their applications in water purification. Rev Inorg Chem 33:139–160
Zhao LQ, Xue FM, Yu BW, Xie JR, Zhang XL, Wu RH, Wang RJ, Hu ZY, Yang ST, Luo JB (2015) TiO2-graphene sponge for the removal of tetracycline. J Nanopart Res 17:16
Zhao JP, Ren WC, Cheng HM (2012) Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J Mater Chem 22:20197–20202
Bi HC, Xie X, Yin KB, Zhou YL, Wan S, He LB, Xu F, Banhart F, Sun LT, Ruoff RS (2012) Spongy graphene as a highly efficient and recyclable sorbent for oils and organic solvents. Adv Funct Mater 22:4421–4425
Lei YL, Chen F, Luo YJ, Zhang L (2014) Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr(VI) removal. J Mater Sci 49:4236–4245
Tsao GT, Zheng YZ, Lu J, Radioanal Gong J, Nucl Chem CS (1997) Adsorption of heavy metal ions by immobilized phytic acid. Appl Biochem Biotech 63–65:731–741
Ferial D, Georg S (2004) In vitro analysis of binding capacities of calcium to phytic acid in different food samples. Eur Food Res Technol 219:409–415
Williams PAM, Baran EJ (1993) The interaction of the vanadyl(IV) cation with phytic acid. Biol Trace Elem Res 36:143–150
Ulusoy U, Şimşek S, Ceyhan Ö (2003) Investigations for modification of polyacrylamide-bentonite by phytic acid and its usability in Fe3+, Zn2+ and UO2 2+ adsorption. Adsorption 9:165–175
Mishra AK, Ramaprabhu S (2011) Functionalized graphene sheets for arsenic removal and desalination of sea water. Desalination 282:39–45
Gu ZX, Wang Y, Tang J, Yang JJ, Liao JL, Yang YY, Liu N (2015) The removal of uranium(VI) from aqueous solution by graphene oxide–carbon nanotubes hybrid aerogels. J Radioanal Nucl Chem 303:1835–1842
Bonato M, Ragnarsdottir KV, Allen GC (2012) Removal of uranium(VI), lead(II) at the surface of TiO2 nanotubes studied by X-ray photoelectron spectroscopy. Water Air Soil Pollut 223:3845–3857
Liu YL, Yuan LY, Yuan YL, Lan JH, Li ZJ, Feng YX, Zhao YL, Chai ZF, Shi WQ (2012) A high efficient sorption of U(VI) from aqueous solution using amino-functionalized SBA-15. J Radioanal Nucl Chem 292:803–810
Reddad Z, Gerente C, Andres Y, Cloirec LP (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36:2067–2073
Sahiner N, Yu HN, Tan G, He JB, John VT, Blake DA (2012) Highly porous acrylonitrile-based submicron particles for UO2 2+ absorption in an immunosensor assay. ACS Appl Mater Interfaces 4:163–170
Song WC, Shao DD, Lu SS, Wang XK (2014) Simultaneous removal of uranium and humic acid by cyclodextrin modified graphene oxide nanosheets. Sci China Chem 57:1291–1299
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Unlu N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136:272–280
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Acknowledgments
The authors thank the support of financial support from the National Natural Science Foundation of China (Grants 21367001) and Jiangxi Educational Committee Foundation (GJJ13471). Fundamental Science on Radioactive Geology and Exploration Technology Laboratory (2011RGET015) and State Key Laboratory Breeding Base of Nuclear Resources and Environment, East China Institute of Technology, provided technical assistance (NRE1318).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, S., Ma, J., Zhang, W. et al. Three-dimensional graphene oxide/phytic acid composite for uranium(VI) sorption. J Radioanal Nucl Chem 306, 507–514 (2015). https://doi.org/10.1007/s10967-015-4162-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4162-x