Abstract
The biotechnological potential of microalgae has gained considerable importance in many applied fields: biomass production for food and feed, cosmeceutical and pharmaceutical products, energy and phytoremediation. The driving force that inspires the progress in microalgae production is the need for new cultivation systems to obtain simultaneously the maximum yield, reduction of water and nutrients use, and production of economically interesting molecules, such as pigments, fatty acids and polysaccharides. We aim to test, for the first time, the co-cultivation in saline medium of Tisochrysis lutea (Haptophyta) and Nannochloropsis oculata (Ochrophyta) to obtain valuable compounds, i.e. pigments and lipids characteristic of each species, using a single culture process. Mono-cultures of each strain were used as controls. The two strains showed an increase in the concentration of chlorophylls and carotenoids in co-culture. At the end of the experiment, the fatty acid profile was analysed by gas chromatography–mass spectrometry. The lipids in the co-cultivated cell extracts were mainly attributable to N. oculata, which represented 97% of the total cells (ca. 83% of the total biomass) at the end of the experiment. Nevertheless, the ω-3 characteristic of T. lutea (DHA and SDA, absent in N. oculata) was also detectable. Although the co-cultivation of these two phylogenetically different species of microalgae did not show positive effects on the growth and on the total lipid production, however, this process resulted in a reduction of the production costs and a lower consumption of water and nutrients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, the biotechnological potential of microalgae has gained considerable importance because of their wide application range: production of biomass for food and feed, synthesis of high-value compounds (e.g. polyunsaturated fatty acids, pigments, polysaccharides) for cosmetic and pharmaceutical sectors, employment in renewable green-energy and in phytoremediation systems (Sabia et al. 2015; D'Amato et al. 2017; Rizwan et al. 2018; Alam et al. 2020). The evolutionary and phylogenetic diversity of microalgae results in an interesting variety in their biochemical composition. They are extremely attractive as sources of a wide range of biomolecules, such as vitamins, essential amino acids, polyunsaturated fatty acids (PUFA), minerals, carotenoids, enzymes and fibres (Borowitzka 2013; Matos 2017; Rahman 2020). The production of microalgae may be of interest also because it does not require arable land, does not compete with food crops, and can use waste as nutrients for their growth (da Silva Vaz et al. 2016). Moreover, microalgae can act as a natural sink for the reduction of atmospheric CO2 and some of them can also utilize the CO2 derived from industrial exhaust gases (Yen et al. 2015). According to the World Water Development Report 2018 of the United Nations, water use has increased worldwide by about 1% per year since the 1980s, and the global water demand is expected to continue to rise. However, as microalgae cultivation is a water intensive process, water consumption and loss must be managed efficiently during cultivation (Mayers et al. 2016). The use of saline algae could help to reduce freshwater consumption destined to microalgal cultures as reported in Ishika et al. (2017). A current driving force that inspires the progress in microalgae production is the search for novel cultivation systems to obtain a reduction of the costs and a lower consumption of water and nutrients. In this context, several studies have been focused on the co-cultivation of different organisms, a strategy based on the concept of ecological community: no species lives isolated in nature (Smith and Crews 2014; Zhu 2015; Das et al. 2021). In co-cultivation, two or more different microorganisms are grown together; in fact, this cultivation mode can involve different microalgal species or microalgae and other microorganisms such as fungi and bacteria (Zhu et al. 2017). With this perspective, the main purpose of this study was to test a co-cultivation strategy in saline medium of the algae Nannochloropsis oculata (Ocrophyta) and Tisochrysis lutea (Haptophyta), species particularly interesting for their composition in pigments and fatty acids (Ryckebosch et al. 2014; Cavalier-Smith et al. 2018). Natural pigments are used as additives and colourants in aquaculture, and in nutraceutical, pharmaceutical and cosmeceutical industries (Alam et al. 2020); consequently, their demand is increasing over the years. In this context, microalgae are a valuable solution, also considering that they contain a different pattern of pigments, but also of lipids and other valuable molecules (e.g. polysaccharides), in relation to their phylogenetic position (Barsanti and Gualtieri 2014; Begum et al. 2016). Nannochloropsis sp. contains chlorophyll a, β-carotene and the accessory pigments, violaxanthin, diadinoxanthin and zeaxanthin (Ryckebosch et al. 2014; Sukarni et al. 2014). Tisochrysis lutea is characterized by chlorophyll a, c1 and c2, β-carotene, diadinochrome, diatoxanthin, and fucoxanthin (Barsanti and Gualtieri 2014; Ryckebosch et al. 2014). In microalgae, PUFAs usually account for 25–60% of the total lipids (Richmond 2004). The most common are arachidonic acid (AA; 20:4n6, ω-6), docosahexaenoic (DHA; 22:6n3, ω-3) and eicosapentaenoic acid (EPA; 20:5n3, ω-3) (Pulz and Gross 2004; Mata et al. 2010), which are the most nutritionally significant PUFAs present in marine food and fish oil (Ramesh Kumar et al. 2019; Zhang et al. 2019). In N. oculata, the lipid content is usually about 30% of the dry weight biomass, but it can increase or decrease as a consequence of growth or stress conditions, e.g. inoculum, irradiance, salinity and nitrogen availability (Su et al. 2011). As regards ω-3 fatty acids, N. oculata does not contain DHA, while it accumulates high levels of EPA, about 193 mg g−1 of extracted oil (Ryckebosch et al. 2014). Tisochrysis lutea is characterized by a total lipid content of approximately 20% of the dry weight biomass. High levels of DHA are detectable, about 46 mg g−1 of extracted oil; conversely, the levels of EPA are low, about 2.8 mg g−1 (Ryckebosch et al. 2014). Taken together, N. oculata and T. lutea could represent a valid alternative to the use of fish oil and fishmeal, both in aquaculture and in human nutrition, because of their complementarity of the biochemical composition, especially in ω-3 PUFAs (Becker 2004, Shah et al. 2018). Moreover, it should be considered that, currently, fish- and animal-derived products find less and less consensus among consumers, especially vegetarians or vegans (Wang et al. 2020). Thus, the aim of this research is focused to ascertain if the co-cultivation of N. oculata and T. lutea could allow the production of high-value molecules, in particular ω-3 fatty acids, for obtaining in a single cultivation system a microalgal biomass rich in all ω-3 fatty acids typical of fish oil and fishmeal. Furthermore, the co-cultivation represents an added value in the perspective of reducing (in this case, halving) water consumption for algae cultivation.
Materials and methods
Algal culture condition and growth
Nannochloropsis oculata (Droop, Hibberd 1981; strain CCAP 849/1) and Tisochrysis lutea (Bendif et al. 2013; strain CCAP 927/14) were obtained from the Culture Collection of Algae and Protozoa of Scottish Marine Institute (Scotland, UK; www.ccap.ac.uk). The two algae were co-cultivated and mono-cultures of each strain were used as controls. Both mono- and co-cultures were inoculated at least in triplicate and grown in 300-mL Erlenmeyer flasks (150 mL of total culture volume). Axenic cultures were grown and maintained in liquid f/2 medium (Guillard and Ryther 1962; Guillard 1975) in artificial seawater ESAW (Berges et al. 2001) in a growth chamber (25 ± 1 °C; 60 μmolphotons m−2 s−1 photosynthetically active radiation (PAR); 16:8 h light-dark photoperiod), without shaking, and no additional CO2 was supplied. For mono-cultures, T. lutea cells were inoculated at a density of 0.6 ± 0.1 × 106 cells mL−1, and N. oculata cells at a density of 5 ± 0.1 × 106 cells mL−1, corresponding respectively to a dry biomass (DW) of 0.066 ± 0.008 and 0.069 ± 0.006 g L−1. For dry biomass (DW), aliquots of samples were filtered through pre-dried and pre-weighed glass-fibre filters (1.2 μm pore size; Whatman GF/C). Filters with cells were rinsed with 20 mL of distilled water, dried for 72 h at 60 °C, and weighted until they reached a constant weight (Popovich et al. 2012a). The dry biomass data was used to calculate the biomass yield in cultures (g L−1). For co-cultures, T. lutea and N. oculata cells were inoculated together at the same densities used in mono-culture. Growth was estimated by measuring the optical density at 750 nm and by counting the cells with a Thoma’s chamber (HBG, Germany) sampling 1 mL of culture at days 0, 3, 7, 10, 14, 21. To make the cell counting of T. lutea easier, 100 μL of Lugol’s iodine was added to 1 mL of culture to stop cell movements. For DW, aliquots of co-cultures were treated as described above for mono-cultures. For a better interpretation of final data, an estimation of DW derived from T. lutea and N. oculata in co-cultures was obtained using data of biomass yield vs cell density of mono-cultures.
Light and fluorescence microscopy
Cell samples were observed at 0, 3, 7, 10, 14 and 21 days, under a Zeiss Axiophot microscope equipped with conventional or fluorescent attachments. The light source for chlorophyll fluorescence examinations was an HBO 100 W pressure mercury vapour lamp (filter set, BP436/10 FT 460, LP470). According to Hillebrand et al. (1999) images were employed to calculate the cell volume using “ImageJ” software (https://imagej.nih.gov/ij/index.html). To highlight the presence of acidic mucopolysaccharides, 40 μL of Alcian Blue (1% in 3% acetic acid) was added to an algal pellet obtained after the centrifugation of 2 mL of culture (Mowry and Scott 1967; Discart et al. 2015). After incubation at room temperature for 30 min, the samples were rinsed twice with culture medium to remove the stain excess. Cells were then observed under white light with the microscope described above. In order to highlight the intracellular lipid accumulation, cells were stained with Nile Red (Sigma) according to Giovanardi et al. (2013), with some modifications. Aliquots of 10 μL Nile Red (0.5 mg dissolved in 100 mL acetone) were added to 1.9 mL of a cell suspension with 4 × 106 cells for N. oculata and 0.5 × 106 cells for T. lutea. After incubation at 37 °C in darkness for 15 min, cells were observed with excitation at 485 nm (filter set BP485, LP520). All pictures were taken with a Canon Powershot S40 digital camera.
Transmission and scanning electron microscopy
For transmission electron microscopy (TEM), cells were harvested weekly by centrifugation and prepared as reported in Baldisserotto et al. (2012) with minor modifications: the phosphate buffer was substituted with 0.1 M sodium cacodylate buffer (pH 7.2) for N. oculata and 0.1 M sodium cacodylate buffer 0.25 M with sucrose for T. lutea. Ultra-thin sections were observed with a Hitachi H800 electron microscope (Electron Microscopy Centre, University of Ferrara). For scanning electron microscopy (SEM), co-cultivated cells were harvested by centrifugation at the 21st day of cultivation. Samples were fixed with 2.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.4), post-fixed overnight with 2% (m/v) OsO4 in the same fixation buffer and dehydrated in a graded ethanol series. Samples were then mounted on a metal holder, covered with gold with a sputter Edwards S150, and observed with a Zeiss Evo 40 electron microscope at 20 kV (Electron Microscopy Centre, University of Ferrara).
Pigment extraction and analysis
Cells were collected by centrifugation after 0, 3, 10 and 21 days of experiment and treated with 100% methanol for 10 min at 80 °C (Baldisserotto et al. 2012). Absorption of extracts was measured at 665 nm for chlorophyll a (Chl a), 632 nm for chlorophyll c (Chl c) and 470 nm for carotenoids (Cars) (Wellburn 1994; Ritchie 2006). Extracts were manipulated under dim light to avoid photo-degradation. Chlorophyll concentration in T. lutea was evaluated using the equations of Ritchie (2006). Otherwise, the equations of Wellburn (1994) were employed to determine the chlorophyll concentration in N. oculata and the carotenoid concentration in both samples. Cells in co-culture were separated through a sucrose gradient prepared in f/2 medium in ESAW (20-30-40%). An aliquot of co-culture was placed in a 50-mL tube containing the sucrose gradient. After 10 min centrifugation at 5300×g cells of N. oculata are separated from those of T. lutea. In particular, the cells of N. oculata and T. lutea stratified at the 20–30% and 30–40% interface, respectively. The two cell fractions were recovered using a Pasteur pipette and washed with f/2 medium in ESAW before analysis.
Chlorophyll fluorescence measurements
At days 0, 3, 7, 10, 14 and 21, fluorescence measurements were performed on cell pellets prepared as described by Ferroni et al. (2011). After 15 min of dark incubation, initial fluorescence F0 and maximum fluorescence FM were used to calculate the maximum quantum yield of PSII (FV/FM ratio). Fifteen minutes were found to be the optimal adaptation time for both microalgae after testing a range between 5- and 40-min dark adaptation time. For analyses, a pulse amplitude modulation fluorometer (PAM; Junior-PAM, Walz, Germany) was used with the following setting: measuring light (ML) with intensity and frequency at level 1; 0.6 s saturation pulse at level 6. Before PAM analysis, cells in co-culture were separated through a sucrose gradient as described above. To verify the possible effects of the sucrose gradient procedure on FV/FM values, mono-cultivated cells have been tested using the same procedure.
Proteins extraction and quantification
At days 0 and 21, the total proteins were extracted. Aliquots of mono-cultivated and co-cultivated cells (about 100 mL with optical density of 0.5 at 750 nm) were centrifuged at 500 g for 10 min and treated according to Ivleva and Golden (2007), with some modifications as described in Baldisserotto et al. (2016). Pellets were resuspended in a small quantity (2 mL) of washing buffer [2 mM Na2EDTA, 5 mM ε-aminocaproic acid, 5 mM MgCl2, 5 mM dithiothreitol dissolved in 1× PBS buffer; PBS buffer (1 L, stock solution ×10): 80 g NaCl, 2 g KCl,14.4 g Na2HPO4·2H2O, 2.4 g KH2PO4 dissolved in distilled water], transferred into 2-mL tubes and then centrifuged (10 min, 2000 g). Subsequently, the pellets were resuspended in the extraction buffer (0.1 M NaOH, 1% sodium dodecyl sulphate, 0.5% β-mercaptoethanol dissolved in water). Samples were frozen three times in liquid N2 for 2 min each and subsequently heated at 80 °C for another 2 min, then rapidly frozen in liquid N2 and kept at −20 °C overnight. The subsequent day, glass beads (0.40–0.60 μm diameter; Sartorius, Germany) were added to the samples followedby vigorous vortexing for 10 min (mixing cycles of 30 s, each followed by 30 s cooling on ice). After centrifugation (1500×g,10 min) the supernatants were harvested (extract I). Pellets were re-extracted by resuspending with 0.5 mL of extraction buffer, vortexing in tubes for 2 min, and finally keeping tubes at 60 °C for 15 min. Samples were then centrifuged (1500×g, 10 min) and the supernatant (extract II) was added to the extract I. Finally, the total extract was rapidly frozen in liquid N2 and stored at −20 °C until quantification. Proteins were quantified following the Lowry’s method (Lowry et al. 1951).
Total lipid content
At the 21st day of cultivation, lipid extraction was performed according to Ryckebosch et al. (2012) with some modifications. Duplicate freeze-dried samples containing 100 mg of biomass were vortexed for 30 s at room temperature in 6 mL chloroform:methanol (1:1, v:v) and 0.4 mL of distilled water. The solvent mixture (2 mL) and water (0.4 mL) were then added and the tube was mixed again with a vortex and subsequently centrifuged. The aqueous layer was removed, and the solvent layer was transferred into a clear tube. The pellet was re-extracted with 4 mL of chloroform:methanol (1:1, v:v). The combined solvent layers were passed through a layer of anhydrous Na2SO4 using Whatman No. 1 filter paper in a funnel. After removal of the solvent by rotary evaporation at 40 °C, the lipid content was determined gravimetrically.
Lipid fractionation and fatty acid profile
Lipid fractionation in neutral lipids, glycolipids and phospholipids was performed using a silica Sep–Pak (SP) of 700 mg (Waters) according to Popovich et al. (2012b) with some modifications. Adsorbent conditioning was performed with 10 mL of chloroform, and samples were loaded by 1 mL of chloroform/oil solution containing 15 mg of oil in 100 mL of chloroform. Elution of neutral lipids from the adsorbent bed was performed with 15 mL of chloroform, glycolipids were recovered by elution with 10 mL of acetone/methanol (9:1, v:v) and phospholipids by elution with 10 mL of methanol. Each fraction was collected into a conical vial and evaporated to dryness under nitrogen. The efficiency of SP was verified by thin layer chromatography (Silicagel G 60 70–230 mesh, Merck, Germany). Concentrated solutions of each fraction of lipids were applied to the bottom of the plates and the plates were processed with chloroform:methanol (2:1, v:v). After solvent evaporation, plates were sprayed with phosphomolybdic acid and heated at 120–130 °C. Fatty acid profiles were determined by methyl ester derivation and gas chromatographic (GC) analysis, with a Varian GC-3800 gas chromatograph, equipped VF-5 ms column with stationary phase bound poly-5% phenyl-95% dimethyl-siloxanes (internal diameter, 0.25 mm, length, 30 m, film thickness, 0.25 μm) and a Varian MS-4000 mass spectrometer with ionization for electronic impact and ion trap, equipped with a NIST library. Fatty acid methyl esters (FAMEs) have been identified comparing their retention time and the mass spectrum with that of a mixture of 37 pure compounds (Supelco, Sigma-Aldrich).
Statistical analysis
Data were processed and analysed using Excel (Microsoft Office 2016). When necessary, statistical tests such as Student t-test, one-way ANOVA and two-way ANOVA were performed using Origin 2018 analysis software, followed by Tukey post-hoc test (p < 0.05).
Results
Growth kinetics
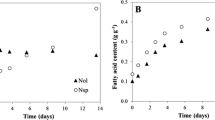
Growth kinetics of N. oculata and T. lutea in mono- and in co-cultivation are reported in Fig. 1. In both systems of cultivation, N. oculata cells remained in the logarithmic phase of growth up to the tenth day, then the cells entered the stationary phase. However, the mono-cultures showed a higher cell density, and the final cell density was about 26 ± 1.5 × 106 cells mL−1 in co-culture and 35 ± 5.2 × 106 cells mL−1 in mono-culture. In contrast, both mono- and co-cultures of T. lutea showed a rapid growth during the first 3 days. Subsequently, while the growth of the mono-cultures continued slowly until the end of the experiment, the growth of the co-cultivated cells entered the stationary phase already on the third day. Final cell density was about 1 ± 0.3 × 106 cells mL−1 in co-cultures and 6 ± 0.6 × 106 cells mL−1 in mono-cultures. As shown in Fig. 1, both microalgae species had a lower growth in co-cultivation than in mono-cultivation. Furthermore, at the end of the experiment, biomass, evaluated for all cultures, yielded 0.95 and 0.93 g L−1 for mono-cultivated T. lutea and N. oculata, respectively, and 0.99 g L−1 for co-cultures (ANOVA, p > 0.05). For co-cultures, it was estimated that T. lutea, which was about only 3% of total cells, yielded about 0.165 g L−1 (17% of total biomass), while N. oculata yielded 0.821 g L−1 (83% of total biomass).
Cell morphology
Figure 2 shows light and fluorescence microscope observations of mono- and co-cultivated N. oculata and T. lutea cells.
Light and epifluorescence microscope observations of mono- and co-cultivated cells of N. oculata and T. lutea at different stage of cultivation. N. oculata (a–f); T. lutea (g–n); co-cultivated cells (o–t). Most figures consist of a collage of photos exemplifying the observations made. Empty arrows indicate translucent globules; filled arrows indicate vacuoles. Scale bars = 6 μm
Observed under the light microscope, both mono- and co-cultivated cells of N. oculata had a spheroid shape, with a variable diameter between 2 and 3 μm, corresponding to a cell volume between 10 and 14 μm3. Mono- and co-cultivated cells differed in the accumulation of translucent globules: in mono-cultivation, they were visible already at the 7th day and increased during the experiment (Fig. 2b and c); in co-cultivation, some translucent globules were observed only in the final stages of growth, 18–21 days (Fig. 2q). The lipid-specific fluorochrome Nile Red was used to investigate the nature of these translucent globules (Fig. 3). The N. oculata cells grown in mono-culture showed an increase in fluorescent lipid droplets during the period of cultivation, reaching the most intense reaction at the end of the experiment, when the globules were in number of 3–4 per-cell and occupied a large part of the cell volume (Fig. 3c). In contrast, cells grown in co-culture accumulated lipids only at the end of the experiment (Fig. 3i).
Epifluorescence photomicrographs of Nile Red stained cells of mono-cultivated and co-cultivated N. oculata and T. lutea at different stage of cultivation: 7 (a N. oculata; d T. lutea; g co-cultivated cells), 14 (b N. oculata; e T. lutea; h co-cultivated cells) and 21 days (c N. oculata; f T. lutea; i co-cultivated cells) day. T T. lutea cells, N N. oculata cells. In co-cultivated N. oculata cells the reaction is negative in the early stages of experimentation (g, h). Scale bars = 6 μm
Tisochrysis lutea cells, typically golden brown in colour, had a shape from spherical to ovate or oblong, with two apical flagella. In some cells, the loss or rupture of one or both flagella were observed in the in the late stages of cultivation. The volume of the microalga in co-cultures was smaller than in mono-cultures, 80 and 190 μm3, respectively (mean values at 21st day). The cup-shaped plastid laid peripherally in the cell. From the 7th day, vacuoles of variable size appeared in both mono- (Fig. 2h and i) and co-cultivated T. lutea cells (Fig. 2p and q). Moreover, from the first days of cultivation, translucent globules were visible, and their number increased over time in both mono- and co-cultivated cells; since the beginning of the experiment, 2–3 lipid globules were present in most of the cells (Fig. 3d and g). In the 10–14 days interval, the number and volume of globules increased (Fig. 3e and h) and, at the final stages of cultivation, 4–5 lipid droplets occurred in the cells and were larger than during the previous days (Fig. 3f and i).
SEM and TEM observations
TEM observations are shown in Fig. 4. In the early stages of mono-cultivation (3–10 days), the N. oculata chloroplast occupied more than half of the cell volume. Large mitochondria indicated an intense cellular activity during the early stages of cultivation. Small globules, probably corresponding to lipid droplets, were visible (Fig. 4a). After 10 days of cultivation, the chloroplast still occupied a significant portion of the total cell volume, but the thylakoids were less numerous and locally appressed. Several large lipid globules were always present (Fig. 4b). At the 21st day, the thylakoids were fewer and more appressed than in previous days; moreover, the stroma appeared darker. On the other hand, the lipid globules increased in size and number, consistent with Nile Red analysis (Fig. 4c). No morphological differences in the co-cultivated cells were noted in the early phases of cultivation (Fig. 4d). After 10 days of co-cultivation, the thylakoids looked appressed (Fig. 4e) and even more after 21 days; nevertheless, well-structured mitochondria were visible (Fig. 4f). Lipid globules only occurred at the last stages of cultivation, confirming the observations in epifluorescence with Nile Red (Fig. 4f).
Transmission electron micrographs of mono-cultivated and co-cultivated cells of N. oculata and T. lutea at different stage of cultivation. Mono-cultivated (a–c) and co-cultivated (d–f) N. oculata cells; mono-cultivated (g–i) and co-cultivated (l–n) T. lutea cells. N N. oculata cells; T T. lutea cells; C chloroplast; L lipid globules; M mitochondrion; P pyrenoid; V vacuole. Scale bars: a, b, d, l, h, i, m and n = 1 μm; c, e and f = 0.5 μm; g = 2 μm
In mono-cultured T. lutea cells, the cup-shaped plastid and pyrenoid were visible (Fig. 4i). Already from the third day of cultivation, some lipid globules were visible in mono-cultivated cells (Fig. 4g). After 10 and 21 days from the inoculation, the cells showed the first signs of vacuolisation, and the lipids droplets appeared darker than at the early stages (Fig. 4h, i). Moreover, from the 10th day, a great secretion activity was visible and characterized by the presence of many vesicles releasing their contents outside the cells (Fig. 5a). During the early phases of co-cultivation, T. lutea cells showed no substantial differences compared to those mono-cultivated (Fig. 4l). On the other hand, from the 10th day, all organelles and membranes were altered (Fig. 4m, n). The extrusion of vesicles was also found in co-cultures and Alcian Blue staining highlighted on the surface of T. lutea cells the presence of acid mucopolysaccharides which were probably responsible for the cell aggregation phenomena observed in co-cultures (Fig. 5b, insert): N. oculata cells adhered to the surface of T. lutea cells (Fig. 5b).
Production and extrusion of acidic mucopolysaccharides in T. lutea cells. T. lutea cell secretory vesicles (arrows) at 10 days of cultivation (a). Scanning electron micrographs of N. oculata and T. lutea cells at the 21st day of co-cultivation (b); insert shows light microscopy observation of co-cultivated cells at the 21st day after Alcian Blue staining: N N. oculata T T. lutea
Pigment content
The time-course of pigment content is shown in Fig. 6. In order to compare the pigment content in mono- and co-cultivated cells, N. oculata and T. lutea from co-cultures were separated on a sucrose gradient. An increase in Chl a (Fig. 6a) and total carotenoids (Fig. 6b) occurred when N. oculata cells were co-cultivated. This increase was observed at the 3rd and 10th day of cultivation. In co-cultivated cells Chl a content was 63% higher than that in mono-cultivated algae at the 3rd day (p < 0.05; Student t-test) and 119% higher at the 10th day (p < 0.05; Student t-test). The same trend characterized the carotenoids concentration: 75% higher in co-cultivated N. oculata cells after 3 days (p < 0.05; Student t-test) and 105% after 10 days (p < 0.05; Student t-test). In contrast, after 21 days, carotenoids were significantly less concentrated in co-cultivated cells than in the mono-cultivated ones (p < 0.05; Student t-test), while Chl a content was similar in mono- and co-cultivated N. oculata cells. Likewise, T. lutea co-cultivated cells showed a higher concentration of pigments, which underwent a progressive increase during cultivation and especially in the last phases (Fig. 6c–e). At the 21st day, the co-cultured cells contained 173% higher content of Chl a, 63% higher content of Chl c and 129% higher amount of total carotenoids than in mono-cultivated T. lutea (p < 0.05; Student t-test).
Time-course variations of pigment content (nmol 10−6 cells) in N. oculata and T. lutea cells in mono-culture (light grey) and co-culture (dark grey). N. oculata: Chl a (a) and total carotenoids (b) content; T. lutea: Chl a (c), Chl c (d) and total carotenoids (e) content. Values are means ± SD (n = 3). Asterisks identify significant differences between samples: *p < 0.05; **p < 0.01 (Student t-test)
PSII maximum quantum yield measure
The effects of the co-cultivation process on photosynthetic efficiency were evaluated by PAM fluorimetry. In order to compare the PSII maximum quantum yield, measured as Fv/FM ratio, in mono- and co-cultivated cells, N. oculata and T. lutea from co-cultures were separated in a sucrose gradient. Mono-cultivated cells have been tested using the same procedure to verify the possible effects of the sucrose gradient procedure on FV/FM values. T. lutea cells showed no significant differences (p = 0.1; Student t-test) in Fv/FM values between gradient-treated and no-treated cells. On the contrary, N. oculata cells showed a significant decrease of 13% (p < 0.05; Student t-test) in Fv/FM values after the treatment with sucrose gradient. This difference was considered and used to correct the values recorded from gradient-treated co-cultivated cells. In microalgae, Fv/FM values of about 0.6 indicate an efficient use of light in the photosynthetic processes (White et al. 2013; Sirin and Sillanpää 2015; Bhola et al. 2016). FV/FM was higher in mono-cultivated than in the co-cultivated cells (Fig. 7); this difference was always significant (p < 0.05; Student t-test), except for N. oculata cells at 7th day, when there was no significant difference between mono- and co-culture. Nanochloropsis oculata co-cultivated cells showed low FV/FM values, until they reached an average value of 0.359 ± 0.016 after 21 days of cultivation. However, FV/FM also decreased in mono-cultures, reaching the average 0.431 ± 0.030 at the end of the experiment (Fig. 7a). In T. lutea mono-cultivated cells, the ratio remained almost stable above 0.600 throughout the experiment, with a decrease to 0.540 only after 21 days (Fig. 7b). Co-cultivated T. lutea showed FV/FM values lower than 0.600 already from the 7th day. The ratio decreased to less than 0.450 in the last stages of cultivation (14–21 days).
Protein content
As shown in Fig. 8, both microalgal species, either in mono-cultivation or co-cultivation, showed a significant reduction in their protein content from the day of the inoculation to the 21st day (p < 0.05; Student t-test): in N. oculata it decreased from 22.15 to 3.62 μg protein 10−6 cells (−83%); in T. lutea from 174.18 to 24.76 μg protein 10−6 cells (−85%); in co-cultivated cells from 55.65 to 4.59 μg protein 10−6 cells (−91%).
Lipid fraction and fatty acid profile
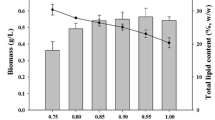
The lipid amount is very important in order to select oleaginous microalgae species. All analyses of lipid fraction were performed at the 21st day of cultivation, when Nile Red staining and TEM observations showed the maximum presence of lipid droplets inside all cells. Figure 9 shows the total lipid content (% DW) in N. oculata and T. lutea mono- and co-cultures: differences between the tested samples were not significant (p > 0.2; one-way ANOVA). However, the fractions of neutral lipids, glycolipids and phospholipids showed significant differences only in the content of neutral lipids (p < 0.05; one-way ANOVA) (Fig. 10). In particular, the percentage content of neutral lipids differed between mono-cultivated T. lutea and N. oculata (55% and 69% respectively; p < 0.05; one-way ANOVA followed by Tukey’s post-hoc test) and between T. lutea and the co-cultivated microalgae, in which the neutral lipids were about 67% of total lipids (p < 0.05; one-way ANOVA followed by Tukey’s post-hoc test).
The fatty acid profile of the neutral lipid fraction was characterized by gas-chromatography coupled to mass spectrometry (GC-MS; Table 1). In mono-cultivated N. oculata, palmitoleic (C16:1) and palmitic (C16:0) acids were the major components (respectively 31.41% and 24.32%), followed by oleic acid (C18:1n9c; 13.68%). The characteristic ω-3 of this species, i.e. EPA (C20:5n3), accounted for 11.1% and the probable presence of eicosatrienoic acid (ETE—C20:3n3) accounted for 2.38%. Furthermore, two ω-6 PUFAs, linoleic (C18:2n6) and arachidonic (C20:4n6) acids, were also present, with 5.55% and 1.34%, respectively. Differently, in mono-cultivated T. lutea, the most abundant fatty acid was oleic acid (C18:1n9; 25.46%), followed by the ω-3 SDA (C18:4n3) and DHA (C22:6n3), 20.23% and 17.59%, respectively. The ω-3 EPA was extremely low (0.43%) (Table 1).
The fatty acid profile of co-cultivated cells was quite similar to that of N. oculata mono-cultivated samples; this was attributable to the low number of T. lutea in co-cultures at the 21st day, i.e. only 3% of total cells (estimated to be approximately 17% of total DW). For example, palmitoleic and palmitic acids, i.e. the major components in mono-cultivated N. oculata, were only slightly reduced in co-cultures. Differently, oleic acid, 25.46% in T. lutea and 13.68% in N. oculata, was reduced to about 15%. Finally, SDA, present only in T. lutea, accounted for about 1.6% (Table 1). Moreover, the co-cultures showed a not significant lower fatty acid percentages than the mono-cultivated N. oculata (about 21% vs. 24%) (Fig. 9). Interestingly, the contribution of T. lutea to the ω-3 profile in the co-cultivated samples was visible owing to the detection of the characteristic DHA and SDA, absent in N. oculata (Ryckebosch et al. 2014), and to the increase, even if small, in EPA percentage.
An estimation of fatty acid profile productivity in the neutral lipid fraction of samples was also calculated (Table S1). Results are consistent with those expressed as percentage of total fatty acids (Table 1). In both cases, it emerges that total ω-3 PUFAs are accumulated the most in T. lutea mono-cultures (38.46% of total fatty acids or 6.442 g (100 gDW)−1 algal biomass), while the lower content was observed for N. oculata (13.48% of total fatty acids or 2.225 g (100 gDW)−1 algal biomass). Interestingly, in co-cultures, characterized by a 97% of N. oculata of total cells (about 83% of total biomass), ω-3 content was improved, due to the even less presence of T. lutea cells in the biomass. In co-cultures, total ω-3 PUFAs accounted for 17.1% of total fatty acids or 2.438 g (100 gDW)−1 algal biomass, i.e. about 10% and 26% more than in mono-cultivated N. oculata, if considering results as total percentage or as yield, respectively.
Discussion
We studied the capability of T. lutea and N. oculata to grow in a co-cultivation batch system in saline medium. This strategy may aim to obtain distinctive compounds of each algae, like pigments (chlorophylls and carotenoids), as well as fatty acids (e.g. DHA and EPA), using a single process of cultivation, with the concomitant advantage to halve water and nutrient use. Typical goals of co-culture are, in fact, overyielding (higher productivity of the desired product(s) than mono-cultures), greater stability under perturbations, and/or more efficient use of inputs (e.g. nutrients or water).
Growth and cell morphology
Under the experimental conditions used in this study, both microalgae grew better in mono-culture than in co-cultivation, as reported in some other studies on microalgae-microalgae co-cultivation systems (Tejido-Nuñeza et al. 2020; Rashid et al. 2019) Recently, Tejido-Nuñeza et al. (2020) reported that Chlorella vulgaris and Tetradesmus obliquus, when co-cultivated in sterile and non-sterile water both derived from an aquaponic system, grew less than in mono-cultures. In our research, although the initial dry biomass used to inoculate was the same for each species, the lower growth, observed in co-culture, might have been depended upon the inoculum ratio used for the two microalgae: at the beginning of the experiment, the ratio between the two algae was about 1:8 (0.6 × 106 cells mL−1 of T. lutea and 5 × 106 cells mL−1 of N. oculata). Indeed, the initial cell density affects the growth and metabolism of microalgae, both in mono- and in co-cultures. As an example, Lu et al. (2012) reported different growth rates and lipid profile in mono-cultures of C. sorokiniana depending on inoculum size, from 10 × 104 to 1 × 107 cells mL−1. Similarly, the co-cultivation of Ettlia sp. with Chlorella sp. starting from different proportions of both algae gave different results in terms of productivity and was related to even very different percentages of the inoculated algae in the cultures at the end of experiment (Rashid et al. 2019). Independent from final cell density, it is noteworthy that mono- and co-cultivated N. oculata entered the stationary phase from the 10th day, sharing same kinetics. Differently, both mono- and co-cultivated T. lutea concluded the logarithmic phase on the 3rd day, consistent with other studies in mono-cultivations, which reported the end of the exponential growth between the 3rd and 6th day (Garnier et al. 2014; Rasdi and Qin 2015; del Pilar Sánchez-Saavedra et al. 2016). In our experiment, while mono-cultivated T. lutea continued to grow slowly after the 3rd day and reached the stationary phase around the end of the experiment, the number of co-cultivated T. lutea cells decreased, pointing to negative competition with N. oculata. As mentioned above, growth of algae in co-cultivation is not obvious and the issue deserves dedicated studies to guarantee good biomass production. The limited growth of co-cultured T. lutea is consistent with decrease in size, alterations of thylakoids and the other cellular structures observed by TEM analyses. Moreover, especially for co-cultivated T. lutea, a stress condition was confirmed by the lower mobility observed under the light microscope. At the best of our knowledge, nowadays no literature data report alterations of size in T. lutea due to stresses, but analogous results are described in several other microalgae with flagella and without a robust cell wall (Goiris et al. 2015; Borowitzka 2018; Wang and Lan 2018). In this research, we propose that the presence in co-culture of abundant small N. oculata cells with a hard wall can have triggered reduction in cell size of the Haptophyta, as an effect linked to a sort of shear or hydrodinamic stress. In addition, already at early stages of co-cultivation T. lutea produced acidic exo-mucopolysaccharides, as shown by Alcian Blue staining and SEM analysis. Extrusion of such polysaccharidic compounds by microalgae often represents a defensive mechanism against stress conditions, also due to the presence of other microorganisms (Wotton 2004). Furthermore, the production of these defensive molecules by T. lutea may in part support the lower growth of N. oculata in co-cultures compared to mono-cultures. The extrusion activity of T. lutea was also observed in mono-cultivated cells, but only in advanced stages of cultivation (about after 18 days from the inoculation; not shown), when microalgae entered the stationary phase.
Photosynthetic properties
An early and progressive decline of PSII maximum quantum yield was observed in co-cultivated cells, which showed FV/FM values below 0.6, lower than in the mono-cultured controls. The low FV/FM values is in line with the lower growth observed in co-culture and indicates a stress condition of the microorganisms; in fact, the analysis of the PSII maximum quantum yield by PAM fluorimetry allows an estimate of the photosynthetic efficiency and can be used as a parameter to evidence the physiological stress in microalgae (Cosgrove and Borowitzka 2010; Ramanna et al. 2014; Dao and Beardall 2016). In microalgae, FV/FM values higher than 0.6 indicate good conditions of growth and an efficient use of light for photosynthesis, a condition met in mono-cultures (White et al. 2013; Sirin and Sillanpää 2015; Bhola et al. 2016). Lower FV/FM values were generally reported under nutrient deficiency or very dense cultures or light-stressed cultures (Beardall et al. 2001a, b; Baldisserotto et al. 2014; Benvenuti et al. 2015). In our experiment conducted under non-photo-inhibiting light and leading to not very dense cultures, the lower FV/FM values in co-cultivation could not depend on these two latter factors. While a nutrient deficiency could have occurred, this cause of stress seems not supported by the photosynthetic pigment content. In particular, up to 10 days of cultivation, the strong accumulation of chlorophylls/cell, hosted in more appressed thylakoids, did not justify a nutrient limitation, especially nitrogen (da Silva Ferreira and Sant'Anna 2017). Conversely, the accumulation of chlorophylls could be an attempt to compensate for the decreased photosynthetic efficiency of PSII. The reason why the co-cultivation of N. oculata and T. lutea decreased FV/FM in both algae is still unknown, but strongly suggests that the co-cultivation is a source of stress for both.
On the other hand, the accumulation of pigments can have relevant impact on the biotechnological exploitation of the co-cultivation itself. Not only carotenoids but also chlorophylls have a wide market: carotenoids are mainly used due to their antioxidant properties (García et al. 2017; Mourelle et al. 2017; Sayo et al. 2013), while chlorophylls are employed as food colourant, as cosmetic ingredient, e.g. in toothpaste or in deodorant, and also for human health for their anticancer and antioxidant activities (Timberlake and Henry 1986; Abad 1994; da Silva Ferreira and Sant'Anna 2017; Vesenick et al. 2012).
Lipid and fatty acid profile
At the end of cultivation, the lipid accumulation inside cells was the higher, as highlighted by morphological observations. Total lipids (24, 31 and 21% of algal DW, respectively for mono-cultures of N. oculata and T. lutea, and for the co-cultures) did not differ significantly between mono- and co-cultivated algae (p > 0.2; one-way ANOVA). The results for mono-cultures were substantially in line with those available in the literature for the same algae (Su et al. 2011; Gao et al. 2020). Interestingly, the lower, even if not significant, value obtained for the co-cultures reflected the different proportion of algae in the whole population, where N. oculata was dominant over T. lutea. The obvious influence of different proportion of algae in the co-cultures on lipid content was already observed also in other microalgae-microalgae co-cultivation experiments, e.g. Ettlia with Chlorella (Rashid et al. 2019). From the analysis of neutral lipids, glycolipids and phospholipids, neutral lipids were higher in samples of the co-culture and of the mono-cultivated N. oculata (67% and 69% of total lipids, respectively). For N. oculata cells, our results are in contrast to those by Ryckebosch et al. (2014), who reported that standard cultures of the alga contained about 40% of neutral lipids. Hovewer, our data are in agreement with other papers using the alga under stress conditions (Obeid et al. 2018). In our experiments, the stress conditions could be the age of culture in mono-cultivation and both the age of the culture and other stresses, like hydrodynamic stress or interaction with mucopolysaccharides released by T. lutea. Differently, in mono-cultivated T. lutea, the neutral lipids accounted for about 55% of total lipids, quite similar to that found by Ryckebosch et al. (2014) for the same alga (about 60%).
In co-culture, N. oculata cells were dominant and T. lutea represented only the 3% of the total cells (about 17% of total biomass) at the end of experiment. In fact, the fatty acid profile of co-cultivated cells was similar to that of N. oculata mono-cultivated. In accordance, Rashid et al. (2019) described an analogous effect on fatty acids profile during their research on co-cultivated microalgae Ettlia and Chlorella, both under standard autotrophic condition and under mixotrophy. Despite the low number of T. lutea cells, in co-culture ω-3 DHA and SDA were detectable; probably, the larger cell size of T. lutea compared to N. oculata influenced this result. The obtainment of a mixture of ω-3 fatty acids combining EPA as the main one (1.74 g (100gDW)−1, plus SDA and DHA, even if as minor components (0.223 and 0.139 g (100gDW)−1, respectively), represents a promising starting point to produce a “green” alternative to fish oil for food/feed. Currently fish oil is one of the main sources of long-chain ω-3 fatty acids for human nutritional supplement use and for animal feed (Misund et al. 2017; Chua et al. 2020; Matsui et al. 2020). However, the increasing demand for this material raises issues related to sustainability; in fact, fish oil is a limited resource derived from wild fish (Shepherd and Bachis 2014). Currently, the mixture of ω-3 PUFAs obtained from our co-cultivated N. oculata and T. lutea opens a novel understanding on the co-cultivation of two microalgae phylogenetically very distant, thus with a great diversity in their morpho-physiology, also in terms of molecules they produce. Furthermore, the two microalgae share marine media for their cultivation, making it possible to employ seawater, instead of freshwater, for the cultivation. This, combined with the halving of water consumption, makes more sustainable the whole process. The knowledge acquired in this work can drive future research, for example by modulating environmental parameters (e.g. light quality, temperature) that can help emphasize biomass and lipid production in a new kind of co-cultivation.
Conclusions
The aim of the present work was to test the feasibility to co-cultivate N. oculata and T. lutea, two marine microalgae that, due to their phylogenetic position, produce different and complementary molecules, with the added advantage of a higher environmental sustainability linked to a less consumption of freshwater. The method has still some limitations, but it is noteworthy that the algal biomass from co-cultivation was enriched both in natural pigments and in a promising blend of ω-3 PUFAs, the latter being exploitable as a plant alternative to fish oil. The opportunity to set up a single cultivation, instead of two, can imply a reduction of production costs in terms of culturing facilities, energy and nutrients consumption, and water demand. Through the improvement of some cultivation parameters, the co-cultivation of N. oculata and T. lutea could become a promising method to produce valuable natural fatty acids and pigments in a single cultivation process, potentially using seawater.
References
Abad R (1994) Therapeutic and cosmetic composition for treatment of skin. US Patent US005538740A
Adarme-Vega TC, Lim DKY, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Alam MA, Xu J-L, Wang Z (eds) (2020) Microalgae biotechnology for food, health and high value products. Springer Nature, Singapore
Baldisserotto C, Ferroni L, Giovanardi M, Boccaletti L, Pantaleoni L, Pancaldi S (2012) Salinity promotes growth of freshwater Neochloris oleoabundans UTEX 1185 (Sphaeropleales, Chlorophyta): morphophysiological aspects. Phycologia 51:700–710
Baldisserotto C, Giovanardi M, Ferroni L, Pancaldi S (2014) Growth, morphology and photosynthetic responses of Neochloris oleoabundans during cultivation in a mixotrophic brackish medium and subsequent starvation. Acta Physiol Plant 36:461–472
Baldisserotto C, Popovich C, Giovanardi M, Sabia A, Ferroni L, Constenla D, Leonardi P, Pancaldi S (2016) Photosynthetic aspects and lipid profiles in the mixotrophic alga Neochloris oleoabundans as useful parameters for biodiesel production. Algal Res 16:255–265
Barsanti L, Gualtieri P (2014) Algae: anatomy, biochemestry and biotechnology, 2nd edn. CRC Press, Boca Raton
Beardall J, Berman T, Heraud P, Kadiri MO, Light BR, Patterson G, Roberts S, Sulzberger B, Sahan E, Uehlinger U, Wood B (2001a) A comparison of methods for detection of phosphate limitation in microalgae. Aquat Sci 63:107–121
Beardall J, Young E, Roberts S (2001b) Approaches for determining phytoplankton nutrient limitation. Aquat Sci 63:44–69
Becker W (2004) Microalgae in human and animal nutrition. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Science, Oxford, pp 312–351
Begum H, Yusoff HM, Banerjee S, Khatoon H, Shariff M (2016) Availability and utilization of pigments from microalgae. Crit Rev Food Sci Nutr 56:2209–2222
Bendif EM, Probert I, Schroeder DC, de Vargas C (2013) On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J Appl Phycol 25:1763–1776
Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2015) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27:1425–1431
Berges JA, Franklin DJ, Harrison PJ (2001) Evolution of an artificial seawater medium: improvements in enriched seawater, artificial water over the last two decades. J Phycol 37:1138–1145
Bhola VK, Swalaha FM, Nasr M, Kumari S, Bux F (2016) Physiological responses of carbon-sequestering microalgae to elevated carbon regimes. Eur J Phycol 51:401–412
Borowitzka MA (2013) High-value products from microalgae - their development and commercialisation. J Appl Phycol 25:743–756
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Cavalier-Smith T, Chao EE, Lewis R (2018) Multigene phylogeny and cell evolution of chromist infrakingdom Rhizaria: contrasting cell organisation of sister phyla Cercozoa and Retaria. Protoplasma 255:1517–1574
Chua ET, Dal’Molin C, Thomas-Hall S, Netzel ME, Netzel G, Schenk PM (2020) Cold and dark treatments induce omega-3 fatty acid and carotenoid production in Nannochloropsis oceanica. Algal Res 51:102059
Cosgrove J, Borowitzka MA (2010) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
da Silva Ferreira V, Sant'Anna C (2017) Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J Microbiol Biotechnol 33:20
da Silva Vaz B, Botelho Moreira J, de Morais MG, Vieira Costa JA (2016) Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci 7:73–77
D'Amato D, Droste N, Allen B, Kettunen M, Lähtinen K, Korhonen J, Leskinen P, Matthies BD, Toppinen A (2017) Green, circular, bio economy: a comparative analysis of sustainability avenues. J Clean Prod 168:716–734
Dao LH, Beardall J (2016) Effects of lead on growth, photosynthetic characteristics and production of reactive oxygen species of two freshwater green algae. Chemosphere 147:420–429
Das PK, Rani J, Rawat S, Kumar S (2021) Microalgal co-cultivation for biofuel production and bioremediation: current status and benefits. Bioenerg Res. https://doi.org/10.1007/s12155-021-10254-8
del Pilar Sánchez-Saavedra M, Maeda-Martínez AN, Acosta-Galindo S (2016) Effect of different light spectra on the growth and biochemical composition of Tisochrysis lutea. J Appl Phycol 28:839–847
Discart V, Bilad MR, Vankelecom IF (2015) Critical evaluation of the determination methods for transparent exopolymer particles, agents of membrane fouling. Crit Rev Environ Sci Technol 45:167–192
Ferroni L, Baldisserotto C, Giovanardi M, Pantaleoni L, Morosinotto T, Pancaldi S (2011) Revised assignment of room-temperature chlorophyll fluorescence emission bands in single living cells of Chlamydomonas reinhardtii. J Bioenerg Biomembr 43:163–173
Gao F, Teles I (Cabanelas, ITD), Ferrer-Ledo N, Wijffels RH, Barbosa MJ (2020) Production and high throughput quantification of fucoxanthin and lipids in Tisochrysis lutea using single-cell fluorescence. Bioresour Technol 318:124104
García JL, de Vicente M, Galán B (2017) Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol 10:1017–1024
Garnier M, Carrier G, Rogniaux H, Nicolau E, Bougaran G, Saint-Jean B, Cadoret JP (2014) Comparative proteomics reveals proteins impacted by nitrogen deprivation in wild-type and high lipid-accumulating mutant strains of Tisochrysis lutea. J Proteome 105:107–120
Giovanardi M, Ferroni L, Baldisserotto C, Tedeschi P, Maietti A, Pantaleoni L, Pancaldi S (2013) Morphophysiological analyses of Neochloris oleoabundans (Chlorophyta) grown mixotrophically in a carbon-rich waste product. Protoplasma 250:161–174
Goiris K, Van Colen W, Wilches I, León-Tamariz F, De Cooman L, Muylaert K (2015) Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res 7:51–57
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Can J Microbiol 8:229–239
Hibberd DJ (1981) Notes on the taxonomy and nomenclature of the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Bot J Linn Soc 82:93–119
Hillebrand H, Dȕrselen C-D, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Ishika T, Moheimani NR, Bahri PA (2017) Sustainable saline microalgae co-cultivation for biofuel production: a critical review. Renew Sust Energ Rev 78:356–368
Ivleva NB, Golden SS (2007) Protein extraction, fractionation, and purification from Cyanobacteria. In: Rosato E (ed) Circadian rhythms. Methods in molecular biology, vol 362. Humana Press
Lowry OH, Rosebrough NJ, Faee AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu S, Wang J, Niu Y, Yang J, Zhou J, Yuan Y (2012) Metabolic profiling reveals growth related FAME productivity and quality of Chlorella sorokiniana with different inoculum sizes. Biotechnol Bioeng 109:1651–1662
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Matos AP (2017) The impact of microalgae in food science and technology. J Am Oil Chem Soc 94:1333–1350
Matsui H, Intoy MMB, Waqalevu V, Ishikawa M, Kotani T (2020) Suitability of Tisochrysis lutea at different growth phases as an enrichment diet for Brachionus plicatilis sp. complex rotifers. J Appl Phycol 32:3933–3947
Mayers JJ, Ekman Nilsson A, Svensson E, Albers E (2016) Integrating microalgal production with industrial outputs - reducing process inputs and quantifying the benefits. Indust Biotech 12:219–234
Misund B, Oglend A, Mezzalira Pincinato RB (2017) The rise of fish oil: from feed to human nutritional supplement. Aquac Econ Manag 21:185–210
Mourelle ML, Gómez CP, Legido JL (2017) The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 4:4040046
Mowry RW, Scott JE (1967) Observations on the basophilia of amyloids. Histochemie 10:8–32
Obeid S, Beaufils N, Camyd S, Takache H, Ismail A, Pontaliera P-Y (2018) Supercritical carbon dioxide extraction and fractionation of lipids from freeze-dried microalgae Nannochloropsis oculata and Chlorella vulgaris. Algal Res 34:49–56
Popovich CA, Damiani C, Constenla D, Leonardi PI (2012a) Lipid quality of the diatoms Skeletonema costatum and Navicula gregaria from the South Atlantic Coast (Argentina): evaluation of its suitability as biodiesel feedstock. J Appl Phycol 24:1–10
Popovich CA, Damiani C, Constenla D, Martínez AM, Freije H, Giovanardi M, Pancaldi S, Leonardi PI (2012b) Neochloris oleoabundans grown in enriched natural seawater for biodiesel feedstock: evaluation of its growth and biochemical composition. Bioresour Technol 114:287–293
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Rahman A, Kumar S, Nawaz T (2020) Biosynthesis of nanomaterials using algae. in: Yousuf A (ed) Microalgae cultivation for biofuels production. Academic Press. https://doi.org/10.1016/B978-0-12-817536-1.00017-5
Ramanna L, Guldhe A, Rawat I, Bux F (2014) The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour Technol 168:127–135
Ramesh Kumar B, Deviram G, Mathimani T, Duc PA, Pugazhendhi A (2019) Microalgae as rich source of polyunsaturated fatty acids. Biocatal Ag Biotech 17:583–588584
Rasdi NW, Qin JG (2015) Effect of N:P ratio on growth and chemical composition of Nannochloropsis oculata and Tisochrysis lutea. J Appl Phycol 27:2221–2230
Rashid N, Ryu AJ, Jeong KJ, Lee B, Chang Y-K (2019) Co-cultivation of two freshwater microalgae species to improve biomass productivity and biodiesel production. Energy Convers Manag 196:640–648
Richmond A (ed) (2004) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing Ltd, Oxford
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Rizwan M, Mujtabab G, Memonc S, Leed K, Rashid N (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sust Energ Rev 92:394–404
Ryckebosch E, Muylaert K, Foubert I (2012) Optimization of an analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc 89:189–198
Ryckebosch E, Bruneel C, Termote-Verhalle R, Goiris K, Muylaert K, Foubert I (2014) Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chem 160:393–400
Sabia A, Baldisserotto C, Biondi S, Marchesini R, Tedeschi P, Maietti A, Giovanardi M, Ferroni L, Pancaldi S (2015) Re-cultivation of Neochloris oleoabundans in exhausted autotrophic and mixotrophic media: the potential role of polyamines and free fatty acids. Appl Microbiol Biotechnol 99:10597–10609
Sayo T, Sugiyama Y, Inoue S (2013) Lutein: a nonprovitamin a, activates the retinoic acid receptor to induce HAS3-dependent hyaluronan synthesis in keratinocytes. Biosci Biotechnol Biochem 77:1282–1286
Shah MR, Lutzu GA, Alam A, Sarker P, Chowdhury K, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Shepherd J, Bachis E (2014) Changing supply and demand for fish oil. Aquacult Econ Manag 18:395–416
Sirin S, Sillanpää M (2015) Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour Technol 191:79–87
Smith VH, Crews T (2014) Applying ecological principles of crop cultivation in large-scale algal biomass production. Algal Res 4:23–34
Su C, Chien LJ, Gome J et al (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sukarni S, Hamidi N, Yanuhar U, Wardana ING (2014) Potential and properties of marine microalgae Nannochloropsis oculata as biomass fuel feedstock. Int J Energy Environ Eng 5:279–290
Tejido-Nuñeza Y, Aymerich E, Sancho L, Refardt D (2020) Co-cultivation of microalgae in aquaculture water: interactions, growth and nutrient removal efficiency at laboratory- and pilot-scale. Algal Res 49:101940
Timberlake CF, Henry BS (1986) Plant pigments as natural food colours. Endeavour 10:31–36
Vesenick DC, Paula NA, Niwa AM, Mantovani MS (2012) Evaluation of the effects of chlorophyllin on apoptosis induction, inhibition of cellular proliferation and mRNA expression of CASP8, CASP9, APC and b-catenin. Curr Res J Biol Sci 4:315–322
Wang C, Lan CQ (2018) Effects of shear stress on microalgae – a review. Biotechnol Adv 36:986–1002
Wang A, Yan K, Chu D, Nazer M, Lin NT, Samaranayake E, Chang J (2020) Microalgae as a mainstream food ingredient: demand and supply perspective. In: Alam MA, Xu J-L, Wang Z (eds) Microalgae biotechnology for food, health and high value products. Springer Nature, Singapore, pp 29–79
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
White S, Anandraj A, Trois C (2013) The effect of landfill leachate on hydrogen production in Chlamydomonas reinhardtii as monitored by PAM fluorometry. Int J Hydrog Energy 38:14214–14222
Wotton RS (2004) The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Sci Mar 68:13–21
Yen H-W, Chen P-W, Chen L-J (2015) The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour Technol 184:148–152
Zhang T-T, Xu J, Wang Y-M, Xue C-H (2019) Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog Lipid Res 75:1009972
Zhu L (2015) Microalgal culture strategies for biofuel production: a review. Biofuels Bioprod Biorefin 9:801–814
Zhu L, Nugroho YK, Shakeel SR, Li Z, Martinkauppi B, Hiltunen E (2017) Using microalgae to produce liquid transportation biodiesel: what is next? Renew Sust Energ Rev 78:391–400
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement. This work was supported by grants from the University of Ferrara (Fondo di Ateneo per la Ricerca Scientifica, FAR-2018 and Fondo per l’Incentivazione alla Ricerca, FIR-2018 granted to SP).
Author information
Authors and Affiliations
Contributions
Conception or design of the work: Simonetta Pancaldi. Data collection: Michele Maglie, Costanza Baldisserotto and Alessandra Guerrini. Data analysis and interpretation: Simonetta Pancaldi, Michele Maglie, Alessandra Sabia and Costanza Baldisserotto. Drafting the article: Michele Maglie. Critical revision of the article: Simonetta Pancaldi and Lorenzo Ferroni. Final approval of the version to be published: Simonetta Pancaldi, Michele Maglie, Costanza Baldisserotto, Alessandra Guerrini, Alessandra Sabia and Lorenzo Ferroni.
Corresponding author
Ethics declarations
Consent for publication
All authors read and approved the manuscript for the publication in Journal of Applied Phycology.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 529 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maglie, M., Baldisserotto, C., Guerrini, A. et al. A co-cultivation process of Nannochloropsis oculata and Tisochrysis lutea induces morpho-physiological and biochemical variations potentially useful for biotechnological purposes. J Appl Phycol 33, 2817–2832 (2021). https://doi.org/10.1007/s10811-021-02511-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02511-2