Abstract
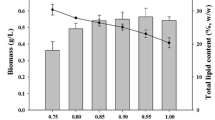
The green microalga Neochloris oleoabundans is able to grow in both low and high salinity media and is largely studied for its capability to accumulate lipids under starvation. Moreover, N. oleoabundans is a mixotrophic alga, and then organic carbon addition can promote its growth. This research aims to study the morpho-physiological aspects, with a particular attention on the photosynthetic response, both during mixotrophic growth and starvation in brackish media, more sustainable than freshwater cultivation. In the first step, the alga was cultivated mixotrophically in a brackish medium added with an apple waste product; in the second one, cells were starved also to verify lipid induction. Results indicate that growth is highly promoted during the first week of mixotrophic cultivation, while photosynthetic pigments and lipids are over-produced during the following three weeks of cultivation. In parallel, in mixotrophic cultures the maximum PSII quantum yield was enhanced during the exponential phase of growth. Interesting changes affected the mixotrophic cultures with respect to the partitioning of absorbed light energy. Starvation of both 7-day-grown mixotrophic and autotrophic cultures caused growth inhibition, pigments and photosynthesis downshifting, and concomitantly promoted evident lipid synthesis.






Similar content being viewed by others
Abbreviations
- A :
-
Absorbance
- AWP:
-
Apple waste product
- Chl:
-
Chlorophyll
- Car:
-
Carotenoids
- F M :
-
Maximum fluorescence in the dark-adapted state
- F M ′ :
-
Maximum fluorescence in the light-adapted state
- F s :
-
Steady-state fluorescence
- F 0 :
-
Minimum fluorescence
- F V :
-
Variable fluorescence
- PAM:
-
Pulse amplitude modulated fluorimetry
- PAR:
-
Photosynthetically active radiation
- PSII:
-
Photosystem II
- TEM:
-
Transmission electron microscopy
- Y(NO):
-
Combined quantum yield of fluorescence and constitutive thermal dissipation
- Y(NPQ):
-
Quantum yield of regulated energy dissipation
- Y(PSII):
-
Actual PSII quantum yield
References
Akazawa K, Okamoto K (1980) Biosynthesis of sucrose. In: Preiss J (ed) The biochemistry of plants, vol 3. Academic Press, New York, pp 199–218
Baker NR (2008) Chlorophyll fluorescence. A probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Baldisserotto C, Ferroni L, Giovanardi M, Pantaleoni L, Boccaletti L, Pancaldi S (2012) Salinity promotes growth of freshwater Neochloris oleoabundans UTEX 1185 (Sphaeropleales, Neochloridaceae): morpho-physiological aspects. Phycologia 51:700–710
Band CJ, Arredondo-Vega BO, Vazquez-Duhalt R, Greppin H (1992) Effect of a salt-osmotic upshock on the edaphic microalga Neochloris oleoabundans. Plant Cell Environ 15:129–133
Beckmann J, Lehr F, Finazzi G, Hankamer B, Posten C, Wobbe L, Kruse O (2009) Improvement of light to biomass conversion by de-regulation of light-harvesting protein translation in Chlamydomonas reinhardtii. J Biotechnol 142:70–77
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of Photosystems I and II in microalgae. Plant Physiol 110:689–696
Brennan L, Owende P (2010) Biofuels from microalgae - A review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 14:557–577
Chantanachat S, Bold HC (1962) Phycological studies. II. Some algae from arid soils. University of Texas No. 6218, p 74
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Ferroni L, Baldisserotto C, Giovanardi M, Pantaleoni L, Morosinotto T, Pancaldi S (2011) Revised assignment of room-temperature chlorophyll fluorescence emission bands in single living cells of Chlamydomonas reinhardtii. J Bioenerg Biomembr 43:163–173
García MCC, Camacho FG, Mirón AS, Sevilla JMF, Chisti Y, Grima EM (2006) Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J Microbiol Biotechnol 16:689–694
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Giovanardi M, Ferroni L, Baldisserotto C, Tedeschi P, Maietti A, Pantaleoni L, Pancaldi S (2013) Morpho-physiological analyses of Neochloris oleoabundans (Chlorophyta) grown mixotrophically in a carbon-rich waste product. Protoplasma 250:161–174
Gouveia L, Marques AE, Lopes da Silva T, Reis A (2009) Neochloris oleabundans UTEX #1185: a suitable renewable lipid source for biofuel production. J Ind Microbiol Biotechnol 36:821–826
Hendrickson L, Furbank RT, Chow WS (2004) A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth Res 82:73–81
Heredia-Arroyo T, Wei W, Hu B (2010) Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl Biochem Biotechnol 162:1978–1995
Ip PF, Wong KH, Chen F (2004) Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem 39:1761–1776
Klughammer C, Schreiber U (2008) Complementary PSII quantum yields calculated from simple fluorescence parameters measured by PAM fluorimetry and the Saturation Pulse methods. PAM Appl Notes 1:27–35
Levine RB, Costanza-Robinson MS, Spatafora GA (2011) Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenerg 35:40–49
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effect on nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresource Technol 102:123–129
Liu X, Duan S, Li A, Xu N, Cai Z, Hu Z (2009) Effects of organic carbon sources on growth, photosynthesis, and respiration of Phaeodactylum tricornutum. J Appl Phycol 21:239–246
Martinez F, Orus MI (1991) Interactions between glucose and inorganic carbon metabolism in Chlorella vulgaris strain UAM101. Plant Physiol 95:1150–1155
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Merzlyak MN, Chivkunova OB, Gorelova OA, Reshetnikova IV, Solovchenko AE, Khozin-Goldberg I, Cohen Z (2007) Effect of nitrogen starvation on optical properties, pigments, and arachidonic acid content of the unicellular green alga Parietochloris incisa (Trebouxiophyceae, Chlorophyta). J Phycol 43:833–843
Miller GL (1959) Modified DNS method for reducing sugars. Anal Chem 31:426–428
Popovich CA, Damiani MC, Constenla D, Martínez AM, Giovanardi M, Pancaldi S, Leonardi PI (2012) Neochloris oleoabundans grown in natural enriched seawater for biodiesel feedstock: evaluation of its growth and biochemical composition. Bioresource Technol 114:287–293
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43
Simionato D, Sforza E, Corteggiani Carpinelli E, Bertucco A, Giacometti GM, Morosinotto T (2011) Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Bioresource Technol 102:6026–6032
Tornabene TG, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microbiol Technol 5:435–440
Van Donk E, Lurling M, Hessen DO, Lokhorst GM (1997) Altered cell wall morphology in nutrient-deficient phytoplankton and its impact on grazer. Limnol Oceanogr 42:357–364
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometer of different resolution. Plant Physiol 144:307–313
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresource Technol 102:1675–1682
Wijffel RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Xu H, Miao X, Wu Q (2006) High-quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yamane YI, Utsunomiya T, Watanabe M, Sasaki K (2001) Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetics. Biotechnol Lett 23:1223–1228
Yang Y, Xu J, Vail D, Weathers P (2011) Ettlia oleoabundans growth and oil production on agricultural anaerobic waste effluents. Bioresource Technol 102:5076–5082
Young EB, Beardall J (2003) Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and a recovery cycle. J Phycol 39:897–905
Acknowledgments
This work was financially supported by grants from the Consorzio Universitario Italiano per l’Argentina (CUIA) and from the University of Ferrara.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Zheng.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baldisserotto, C., Giovanardi, M., Ferroni, L. et al. Growth, morphology and photosynthetic responses of Neochloris oleoabundans during cultivation in a mixotrophic brackish medium and subsequent starvation. Acta Physiol Plant 36, 461–472 (2014). https://doi.org/10.1007/s11738-013-1426-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1426-3