Abstract
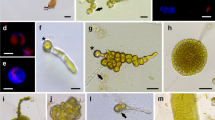
This paper reports the first successful regeneration of whole plants from protoplasts of Gracilaria gracilis (Stackhouse) Steentoft, Irvine and Farnham. Protoplasts were isolated and purified using a previously optimized protocol. Protoplasts with regenerated cell walls divided to produce callus-like cell masses which showed the presence of uniseriate, filamentous outgrowths. Bud outgrowth from callus masses was associated with a distinct change in colour intensity at the point of outgrowth. Ultimately, whole plants were regenerated from the callus-like cell masses with overall yields of approximately two to three whole plants per 104 protoplasts seeded. Two distinctive patterns of regeneration were observed. In the first case, protoplasts regenerated slowly to produce plants which resembled the parent plants, exhibiting slender, branched thalli. Growth rates of regenerated seaweed were similar to that of wild-type G. gracilis cultured under the same conditions with 115 g of seaweed cultured from 15 individually regenerated plants over a year. In the second case, protoplasts regenerated rapidly to produce plants which remained small with thalli that were thick and unbranched and had a limited life span. These results provide an important foundation for the development of a successful tissue culture system for G. gracilis.








Similar content being viewed by others
References
Aguirre-Lipperheide M, Estrada-Rodríguez FJ, Evans LV (1995) Facts, problems, and needs in seaweed tissue culture: an appraisal. J Phycol 31:677–688
Baweja P, Sahoo D, Garcia-Jiménez P, Robaina RR (2009) Seaweed tissue culture as applied to biotechnology: problems, achievements and prospects. Phycol Res 57:45–58
Beer S, Bjork M (1994) Photosynthetic properties of protoplasts, as compared with thalli, of Ulva fasciata (Chlorophyta). J Phycol 30:633–637
Benet H, Bruss U, Duval JC, Kloareg B (1994) Photosynthesis and photoinhibition in protoplasts of the marine brown alga Laminaria saccharina. J Exp Bot 45:211–220
Benet H, Gall E, Asensi A, Kloareg B (1997) Protoplast regeneration from gametophytes and sporophytes of some species in the order Laminariales (Phaeophyceae). Protoplasma 199:39–48
Bjork M, Gomez-Pinchetti JL, Garcia-Reina G, Pederson M (1992) Protoplast isolation from Ulva rigida (Chlorophyta). Eur J Phycol 27:401–407
Bradley PM, Cheney DP (1990) Some effects of plant growth regulators on tissue cultures of the marine red alga Agardhiella subulata (Gigartinales, Rhodophyta). Hydrobiologia 204/205:353–360
Chen YC, Chiang YM (1994) Development of protoplasts from Grateloupia sparsa and G. filicinia (Halymeniaceae, Rhodophyta). Bot Mar 37:361–366
Cheney DP (1990) Genetic improvement of seaweeds through protoplast fusion. In: Yarish C, Penniman CA, Van Patten P (eds) Economically important marine plants of the Atlantic: their biology and cultivation. Connecticut Sea Grant College Program, USA, pp 15–25
Davey MR, Anthony P, Power JB, Lowe KC (2005) Plant protoplasts: status and biotechnological perspectives. Biotech Adv 23:131–171
Davison IR, Polne-Fuller M (1990) Photosynthesis in protoplasts of Macrocystis pyrifera (Phaeophytae). J Phycol 26:384–387
De Nys R, Jameson PE, Brown MT (1991) The influence of cytokinins on the growth of Macrocystis pyrifera. Bot Mar 34:465–467
De Oliveira EC, Alveal K, Anderson RJ (2000) Mariculture of the agar-producing Gracilariod red algae. Rev Fish Sci 8:345–377
Fujimura T, Kajiwara T (1990) Production of bioflavor by regeneration from protoplasts of Ulva pertusa (Ulvales, Chlorophyta). Hydrobiologia 204/205:143–149
Garcia-Reina GL, Gomez-Pinchetti JL, Robledo DR, Sosa P (1991) Actual potential and speculative applications of seaweed cellular biotechnology: some specific comments on Gelidium. Hydrobiologia 221:181–194
Gusev MV, Tambiev AH, Kirikova NN, Shelyastina NN, Aslanyan RR (1987) Callus formation in seven species of agarophyte marine algae. Mar Biol 95:593–597
Hagen Rødde RS, Larsen B (1997) Protoplasts of Laminaria digitata and Laminaria saccharina (Phaeophyta) cultivation and biosynthesis of alginate. Bot Mar 40:391–395
Haglund K, Bjork M, Ramazanov Z, Garcia-Reina G, Pederson M (1992) Role of carbonic anhydrase in photosynthesis and inorganic carbon assimilation in the red alga Gracilaria tenuistipitata. Planta 187:275–281
Huang W, Fujita Y (1997a) Callus induction and thallus regeneration in some species of red algae. Phycol Res 45:105–111
Huang W, Fujita Y (1997b) Callus induction thallus regeneration of the red alga Meristotheca papulosa (Rhodophyta, Gigartinales). Bot Mar 40:55–61
Huddy SM, Meyers AE, Coyne VE (2013) Protoplast isolation optimization and regeneration of cell wall in Gracilaria gracilis (Gracilariales, Rhodophyta). J Appl Phycol 25:433–443
Kaczyna F, Megnet R (1993) The effects of glycerol and plant growth regulators on Gracilaria verrucosa (Gigartinales, Rhodophyceae). Hydrobiologia 268:57–64
Kawashima Y, Tokuda H (1993) Regeneration from callus of Undaria pinnatifida (Harvey) Suringar (Laminariales, Phaeophyta). Hydrobiologia 260/261:385–389
Kim GH, Klochkova TA, Yoon KS, Song YS, Lee KP (2005) Purification and characterization of a lectin, bryohlealin, involved in the protoplast formation of a marine green alga Bryopsis plumosa (Chlorophyta). J Phycol 42:86–95
Millner PA, Callow ME, Evans LV (1979) Preparation of protoplasts from the green alga Enteromorpha intestinalis (L.). Planta 147:174–177
Polne-Fuller M, Gibor A (1984) Developmental studies in Porphyra. I. Blade differentiation in Porphyra perforata as expressed by morphology, enzymatic digestion and protoplast regeneration. J Phycol 20:609–616
Polne-Fuller M, Gibor A (1987) Calluses and callus-like growth in seaweeds: induction and culture. Hydrobiologia 151/152:131–138
Provasoli L (1968) Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Proceedings of the U.S.-Japan Conference, Hakone, pp 63–75
Provasoli L, Pintner IJ (1980) Bacteria induced polymorphism in an axenic laboratory strain of Ulva lactuca (Chlorophyceae). J Phycol 32:479–482
Qin S, Jiang P, Tseng C (2005) Transforming kelp into a marine bioreactor. Trends Biotech 23:264–268
Rajahrishna Kumar G, Reddy CRK, Jha B (2007) Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J Appl Phycol 19:15–25
Reddy CRK, Dipakkore S, Kumar GK, Jha B, Cheney DP, Fujita Y (2006) An improved enzyme preparation for rapid mass production of protoplasts as seed stock for aquaculture of macrophytic marine green algae. Aquaculture 260:290–297
Reddy CRK, Jha B, Fujita Y, Ohno M (2008a) Seaweed micropropagation techniques and their potentials: an overview. J Appl Phycol 20:609–617
Reddy CRK, Gupta MK, Mantri VA (2008b) Seaweed protoplasts: status, biotechnological perspectives and needs. J Appl Phycol 20:619–632
Reddy CRK, Migita S, Fujita Y (1989) Protoplast isolation and regeneration of three species of Ulva in axenic culture. Bot Mar 32:483–490
Robaina RR, Garcia-Reina G, Luque A (1990) The effects of the physical characteristics of the culture medium on the development of the red seaweeds in tissue culture. Hydrobiologia 204/205:137–142
Rothman MD, Anderson RJ, Boothroyd CJT, Kemp FA, Bolton JJ (2009) The gracilarioids in South Africa: long-term monitoring of a declining resource. J Appl Phycol 21:47–53
Schroeder DC, Jaffer MJ, Coyne VE (2003) Investigation of the role of a β(1–4) agarose produced by Pseudoalteromonas gracilis B9 in eliciting disease symptoms in the red alga Gracilaria gracilis. Microbiology 149:2919–2929
Singh RP, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2011a) Role of bacterial isolates in enhancing the bud induction in the industrially important red algal Gracilaria dura. FEMS Microbiol Ecol 76:381–392
Singh RP, Mantri VA, Reddy CRK, Jha B (2011b) Isolation of seaweed associated bacteria and their morphogenesis inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat Biol 12:13–21
Smit AJ, Bolton JJ (1999) Organismic determinants and their effect on growth and regeneration in Gracilaria gracilis. J Appl Phycol 11:293–299
Smith RG, Bidwell RGS (1989) Inorganic carbon uptake by photosyntheticallly active protoplasts of the red macroalga Chondrus crispus. Mar Biol 102:1–4
Stevens DR, Purton S (1997) Genetic engineering of eukaryotic algae: progress and prospects. J Phycol 33:713–722
Sturz AV, Christie BR, Nowak J (2000) Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 19:1–30
Yan X-H, Wang S-J (1993) Regeneration of whole plants from Gracilaria asiatica Chang et Xia protoplasts (Gracilariaceae, Rhodophyta). Hydrobiologia 260/261:429–436
Yeoman MH (1987) Bypassing the plant. Ann Bot 60:157–174
Yeong H, Khalid N, Phang S (2008) Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta). J Appl Phycol 20:641–651
Yokoya NS (2000) Apical callus formation and plant regeneration controlled by plant growth regulators on axenic culture of the red alga Gracilariopsis tenuifrons (Gracilariales, Rhodophyta). Phycol Res 48:133–142
Yokoya NS, Guimaraes SMPB, Handro W (1993) Development of callus-like structures and plant regeneration in thallus segments of Grateloupia filiformis Kützing (Rhodophyta). Hydrobiologia 260/261:407–413
Yokoya NS, Handro W (1996) Effects of auxins and cytokinins on tissue culture of Grateloupia dichotoma (Gigartinales, Rhodophyta). Hydrobiologia 326/327:393–400
Yokoya NS, West JA, Luchi AE (2004) Effects of plant growth regulators on callus formation, growth and regeneration in axenic tissue cultures of Gracilaria tenuistipitata and Gracilaria perplexa (Gracilariales, Rhodophyta). Phycol Res 52:244–254
Zablackis E, Vreeland V, Kloareg B (1993) Isolation of protoplasts from Kappaphycus alvarezi var. tambalang (Rhodophyta) and secretion of ι-carrageenan fragments by cultured cells. J Exp Bot 44:1515–1522
Acknowledgments
The authors thank Irvine and Johnson Abalone Culture Division, Danger Point, Gansbaai, South Africa for supplying G. gracilis. This work was funded by a National Research Foundation (NRF) grant (GUN 2053564) awarded to VEC and a Medical Research Council of South Africa grant awarded to AEM. SMH was supported by a NRF Scarce Skills Scholarship and a University of Cape Town student bursary.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huddy, S.M., Meyers, A.E. & Coyne, V.E. Regeneration of whole plants from protoplasts of Gracilaria gracilis (Gracilariales, Rhodophyta). J Appl Phycol 27, 427–435 (2015). https://doi.org/10.1007/s10811-014-0278-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-014-0278-6