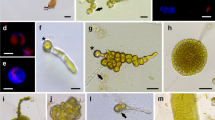
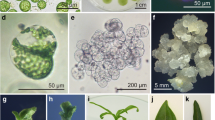
Summary
Protoplasts were isolated from sporophytes and from gametophyte cultures of several species in the order Laminariales. For each example, the isolation and culture procedures were investigated systematically, to identify conditions leading to plant regeneration. After dedifferentiation through a filamentous stage, protoplasts isolated from adultLaminaria saccharina sporophytes regenerated polystichous bladelets. In contrast, cells isolated fromLaminaria digitata sporophytes proved recalcitrant in culture, except when the donor plants were undifferentiated sporelings. The most critical factors for protoplast development were the origin of explants, the osmoticum used for cell isolation, cultivation in plain seawater, and the absence of stress during the first two weeks of culture. We also found that protoplast isolation from the sporophytes of members of the Laminariales results in the release of hydrogen peroxide, up to 5–120 μM final concentration in the macerating medium, a characteristic which may be related to protoplast recalcitrance. Protoplasts isolated from the gametophytic phase readily regenerated into normal gametophytes, capable of gametogenesis and producing sporophytes by fertilization.
Similar content being viewed by others
Abbreviations
- F.W.:
-
fresh weight
- MES:
-
(2-N-morpholino)ethane-sulfonic acid
References
Ar Gall E, Chiang YM, Kloareg B (1993) Isolation and regeneration of protoplasts fromPorphyra dentata andPorphyra crispata. Eur J Phycol 28: 277–283
—, Asensi A, Marie D, Kloareg B (1996) Parthenogenesis and apospory in the Laminariales: a flow cytometry analysis. Eur J Phycol 31: 369–380
Benet H, Bruss H, Duval J-C, Kloareg B (1994) Photosynthesis and photoinhibition in protoplasts of the marine brown algaLaminaria saccharina. J Exp Bot 271: 211–220
Björk M, Haglund K, Ramazanov Z, Garcia-Reina G, Perdersén M. (1992) Inorganic-carbon assimilation in the green seaweedUlva rigida C. Ag. (Chlorophyta). Planta 187: 152–156
Boyen C, Bertheau Y, Barbeyron T, Kloareg B (1990a) Preparation of guluronate lyase fromPseudomonas alginovora for protoplast isolation inLaminaria. Enzyme Microb Technol 12: 885–890
—, Kloareg B, Polne-Fuller M, Gibor A (1990b) Preparation of alginate lyase from marine molluscs for protoplasts isolation in brown algae. Phycologia 29: 173–181
Butler DM, Ostgaard K, Boyen C, Evans LV, Jensen A, Kloareg B (1989) Isolation conditions for high yields of protoplasts fromLaminaria saccharina andLaminaria digitata (Phaeophyceae). J Exp Bot 40: 1237–1246
Chen LCM (1986) Cell development ofPorphyra miniata (Rhodophyceae) under axenic culture. Bot Mar 29: 435–439
Collén J, Pedersén M (1994) Stress induced oxidative burst byEuchema platycladum (Rhodophyta). Physiol Plant 92: 417–422
Criqui MC, Jamet E, Parmentier Y, Marbach J, Durr A, Fleck J (1992a) Isolation and characterization of a plant cDNA showing homology to animal glutathione peroxidases. Plant Mol Biol 18: 623–627
Criqui MC, Plesse B, Durr A, Marbach J, Parmentier Y, Jamet E, Fleck J (1992b) Characterization of genes expressed in mesophyll protoplasts ofNicotiana sylvestris before the re-initiation of the DNA replicational activity. Mech Dev 38: 121–132
Cutler AJ, Saleem M, Coffey MA, Loewen MK (1989) Role of oxidative stress in cereal protoplast recalcitrance. Plant Cell Tissue Organ Culture 18: 113–127
De Marco A, Roubelakis-Angelakis KA (1996) The complexity of enzymic control of hydrogen peroxide concentration may affect the regeneration potential of plant protoplasts. Plant Physiol 110: 137–145
Ducreux G, Kloareg B (1988) Plant regeneration from protoplasts ofSphacelaria (Phaeophyceae). Planta 174: 25–29
Fleck J, Durr A, Lett M, Hirth L (1979) Changes in protein synthesis during the initial stage of life of tobacco protoplasts. Planta 145: 279–285
— — — — (1980) Comparison of proteins synthesized in vivo and in vitro by mRNA from isolated protoplasts. Planta 148: 453–454
Grosset J, Meyer Y, Chartier Y, Kauffmann S, Legrand M, Frittig B (1990) Tobacco mesophyll protoplasts synthesize 1,3-β-glucanase, chitinases and “osmotins” during in vitro culture. Plant Physiol 92: 520–527
Hahne G, Lörz H (1988) Release of phytotoxic factors from plant cell walls during protoplast isolation. J Plant Physiol 132: 345–350
Ishii S (1987) Generation of active oxygen species during enzymic isolation of protoplasts from oat leaves. In Vitro Cell Dev Biol 239: 653–658
— (1988) Factors influencing protoplast viability of suspension-cultured rice cells during isolation process. Plant Physiol 88: 26–29
Kloareg B, Quatrano R (1987) Enzymatic removal of the cell walls from zygotes ofFucus distichus (L.) Powell (Phaeophyta). Hydrobiologia 151/152: 123–129
—, Polne-Fuller M, Gibor A (1989) Mass production of viable protoplasts fromMacrocystis pyrifera (L.) C. Ag. (Phaeophyta). Plant Sci 62: 105–112
Kuhlenkamp R, Muller DG (1994) Isolation and regeneration of protoplasts from healthy and virus-infected gametophytes ofEctocarpus siliculosus (Phaeophyceae). Bot Mar 37: 525–530
Mejjad M, Loiseaux de Goer S, Ducreux G (1992) Protoplast isolation, development and regeneration in different strains ofPilayella littoralis (L.) Kjellm (Phaeophyceae). Protoplasma 169: 42–48
Meyer Y, Chartier Y (1981) Hormonal control of mitotic development in tobacco protoplasts: two-dimensional distribution of newly-synthetised proteins. Plant Physiol 68: 1273–1278
— —, Grosset J, Marty I, Brugidou C, Marinho P, Rivera R (1993) Gene expression in mesophyll protoplasts. In: Roubelakis-Angelakis KA, Tranh Tan Van K (eds) Morphogenesis in plants: molecular approaches. Plenum, New York, pp 221–236
Mieth H, Speth V, Ebel J (1986) Phytoalexin production by isolated soybean protoplasts. Z Naturforsch 41: 193–201
Polne-Fuller M, Gibor A (1984) Developmental studies inPorphyra. I Blade differentiation inPorphyra perforata as expressed by morphology, enzymatic digestion and protoplast regeneration. J Phycol 20: 609–616
— — (1986) Calluses, cells, and protoplasts in studies toward genetic improvement of seaweeds. Aquaculture 57: 117–123
Provasoli L (1986) Media and prospects for cultivation of marine algae. In: Watanabe A, Hattori A (eds) Cultures and collections of algae. Proceedings U.S.-Japan Conference, Hakone. Japanese Society for Plant Physiologists, Kyoto, pp 63–75
Reddy CRK, Fujita Y (1991) Regeneration of plantlets fromEnteromorpha (Ulvales, Chlorophyta) protoplasts in axenic culture. J Appl Phycol 3: 265–275
—, Migita S, Fujita Y (1989) Protoplasts isolation and regeneration of three species ofUlva in axenic culture. Bot Mar 32: 483–490
Roubelakis-Angelakis KA (1993) An assessment of possible factors contributing to recalcitrance of plant protoplasts. In: Roubelakis-Angelakis KA, Tranh Tan Van K (eds) Morphogenesis in plants: molecular approaches. Plenum, New York, pp 201–219
Rusig AM, Le Guyader H, Ducreux G (1994) Dedifferentiation and microtubule reorganization in the apical cell protoplast ofSphacelaria (Phaeophyceae). Protoplasma 179: 83–94
Saga N, Kudo T (1989) Isolation and culture of protoplasts from the green algaMonostroma angicava. J Appl Phycol 1: 25–30
Sawabe T, Ezura Y (1996) Regeneration fromLaminaria japonica Areschoug (Laminariales, Phaeophyceae) protoplasts isolated with bacterial alginase. Plant Cell Rep 15: 892–895
Siminis CI, Kanellis AK, Roubelakis-Angelakis K (1994) Catalase is differentially expressed in dividing and non-dividing protoplasts. Plant Physiol 105: 1375–1383
Wolff SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233: 182–189
Wu CY, Lin GH (1987) Progress in the genetics and breeding of economic seaweeds in China. Hydrobiologia 151/152: 57–61
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benet, H., Ar Gall, E., Asensi, A. et al. Protoplast regeneration from gametophytes and sporophytes of some species in the order Laminariales (Phaeophyceae). Protoplasma 199, 39–48 (1997). https://doi.org/10.1007/BF02539804
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02539804