Abstract
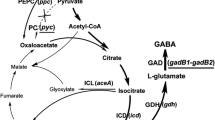
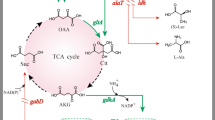
γ-Aminobutyric acid (GABA), a non-protein amino acid, is a bioactive component in the food, feed and pharmaceutical fields. To establish an effective single-step production system for GABA, a recombinant Corynebacterium glutamicum strain co-expressing two glutamate decarboxylase (GAD) genes (gadB1 and gadB2) derived from Lactobacillus brevis Lb85 was constructed. Compared with the GABA production of the gadB1 or gadB2 single-expressing strains, GABA production by the gadB1–gadB2 co-expressing strain increased more than twofold. By optimising urea supplementation, the total production of l-glutamate and GABA increased from 22.57 ± 1.24 to 30.18 ± 1.33 g L−1, and GABA production increased from 4.02 ± 0.95 to 18.66 ± 2.11 g L−1 after 84-h cultivation. Under optimal urea supplementation, l-glutamate continued to be consumed, GABA continued to accumulate after 36 h of fermentation, and the pH level fluctuated. GABA production increased to a maximum level of 27.13 ± 0.54 g L−1 after 120-h flask cultivation and 26.32 g L−1 after 60-h fed-batch fermentation. The conversion ratio of l-glutamate to GABA reached 0.60–0.74 mol mol−1. By co-expressing gadB1 and gadB2 and optimising the urea addition method, C. glutamicum was genetically improved for de novo biosynthesis of GABA from its own accumulated l-glutamate.






Similar content being viewed by others
References
Asakura Y, Kimura E, Usuda Y, Kawahara Y, Matsui K, Osumi T, Nakamatsu T (2007) Altered metabolic flux due to deletion of odhA causes l-glutamate overproduction in Corynebacterium glutamicum. Appl Environ Microbiol 73:1308–1319
Coleman ST, Fang TK, Rovinsky SA, Turano FJ, Moye-Rowley WS (2001) Expression of a glutamate decarboxylase homologue is required for normal oxidative stress tolerance in Saccharomyces cerevisiae. J Biol Chem 276:244–250
Hara Y, Kadotani N, Izui H, Katashkina JI, Kuvaeva TM, Andreeva IG, Golubeva LI, Malko DB, Makeev VJ, Mashko SV, Kozlov YI (2012) The complete genome sequence of Pantoea ananatis AJ13355, an organism with great biotechnological potential. Appl Microbiol Biotechnol 93:331–341
Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y (2004) Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar–Kyoto rats. Br J Nutr 92:411–417
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172
Hiraga K, Ueno Y, Oda K (2008) Glutamate decarboxylase from Lactobacillus brevis: activation by ammonium sulfate. Biosci Biotechnol Biochem 72:1299–1306
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62:99–109
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Kramer R, Linke B, McHardy AC, Meyer F, Mockel B, Pfefferle W, Puhler A, Rey DA, Ruckert C, Rupp O, Sahm H, Wendisch VF, Wiegrabe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of l-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Karatzas KA, Brennan O, Heavin S (2010) Intracellular accumulation of high levels of gamma-aminobutyrate by Listeria monocytogenes 10403S in response to low pH: uncoupling of gamma-aminobutyrate synthesis from efflux in a chemically defined medium. Appl Environ Microbiol 76:3529–3537
Kennerknecht N, Sahm H, Yen MR, Patek M, Saier MH, Eggeling L (2002) Export of l-isoleucine from Corynebacterium glutamicum: a two-gene-encoded member of a new translocator family. J Bacteriol 184:3947–3956
Kirchner O, Tauch A (2003) Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J Biotechnol 104:287–299
Koros A, Varga ZS, Molnar P (2008) Simultaneous analysis of amino acids and amines as their o-phthalaldehyde-ethanethiol-9-fluorenylmethyl chloroformate derivatives in cheese by high-performance liquid chromatography. J Chromatogr A 1203:146–152
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Li HX, Cao YS (2010) Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39:1107–1116
Li HX, Qiu T, Gao DD, Cao YS (2010) Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids 38:1439–1445
Li HX, Qiu T, Huang GD, Cao YS (2010) Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Fact 9:85
Okada T, Sugishita T, Murakami T, Murai H, Saikusa T, Horino T, Onoda A, Kajimoto O, Takahashi R, Takahashi T (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J Jpn Soc Food Sci 47:596–603
Park KB, Oh SH (2007) Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour Technol 98:312–319
Rehm N, Burkovski A (2011) Engineering of nitrogen metabolism and its regulation in Corynebacterium glutamicum: influence on amino acid pools and production. Appl Microbiol Biotechnol 89:239–248
Sanchez B, Champomier-Verges MC, Collado Mdel C, Anglade P, Baraige F, Sanz Y, de los Reyes-Gavilan CG, Margolles A, Zagorec M (2007) Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl Environ Microbiol 70:6450–6459
Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J (1998) A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol 27:299–310
Schuller HM, Al-Wadei HA, Majidi M (2008) Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis 29:1979–1985
Shi F, Li YX (2011) Synthesis of gamma-aminobutyric acid by expressing Lactobacillus brevis-derived glutamate decarboxylase in the Corynebacterium glutamicum strain ATCC13032. Biotechnol Lett 33:2469–2474
Shiio I, Ozaki H (1970) Regulation of nicotinamide adenine dinucleotide phosphate-specific glutamate dehydrogenase from Brevibacterium flavum, a glutamate-producing bacterium. J Biochem 68:633–647
Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M (2007) Synthesis of γ-amino butyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol 73:7283–7290
Takahashi C, Shirakawa J, Tsuchidate T, Okai N, Hatada K, Nakayama H, Tateno T, Ogino C, Kondo A (2012) Robust production of gamma-amino butyric acid using recombinant Corynebacterium glutamicum expressing glutamate decarboxylase from Escherichia coli. Enzym Microb Tech 51:171–176
Tamura T, Noda M, Ozaki M, Maruyama M, Matoba Y, Kumagai T, Sugiyama M (2010) Establishment of an efficient fermentation system of gamma-aminobutyric acid by a lactic acid bacterium, Enterococcus avium G-15, isolated from carrot leaves. Bio Pharm Bull 33:1673–1679
Tramonti A, De Canio M, Delany I, Scarlato V, De Biase D (2006) Mechanisms of transcription activation exerted by GadX and GadW at the gadA and gadBC gene promoters of the glutamate-based acid resistance system in Escherichia coli. J Bacteriol 188:8118–8127
Tujioka K, Ohsumi M, Horie K, Kim M, Hayase K, Yokogoshi H (2009) Dietary gamma-aminobutyric acid affects the brain protein synthesis rate in ovariectomized female rats. J Nutr Sci Vitaminol 55:75–80
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal 10:67–79
Wong CG, Bottiglieri T, Snead OC (2003) GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol 54:S3–S12
Xu DQ, Tan YZ, Huan XJ, Hu XQ, Wang XY (2010) Construction of a novel shuttle vector for use in Brevibacterium flavum, an industrial amino acid producer. J Microbiol Methods 80:86–92
Xu DQ, Tan YZ, Shi F, Wang XY (2010) An improved shuttle vector constructed for metabolic engineering research in Corynebacterium glutamicum. Plasmid 64:85–91
Yao WJ, Deng XZ, Zhong H, Liu M, Zheng P, Sun ZH, Zhang Y (2009) Double deletion of dtsR1 and pyc induce efficient l-glutamate overproduction in Corynebacterium glutamicum. J Ind Microbiol Biotechnol 36:911–921
Zhang Y, Song L, Gao Q, Yu SM, Li L, Gao NF (2012) The two-step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Appl Microbiol Biotechnol 94:1619–1627
Zhao Z, Ding JY, Ma WH, Zhou NY, Liu SJ (2012) Identification and characterization of γ-aminobutyric acid uptake system GabP Cg (NCgl0464) in Corynebacterium glutamicum. Appl Environ Microbiol 78:2596–2601
Acknowledgments
The authors thank the Program of State Key Laboratory of Food Science and Technology (contract no. SKLF-TS-201103) and the Fundamental Research Funds for the Central Universities (contract no. JUSRP 21109) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Shi and J. Jiang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, F., Jiang, J., Li, Y. et al. Enhancement of γ-aminobutyric acid production in recombinant Corynebacterium glutamicum by co-expressing two glutamate decarboxylase genes from Lactobacillus brevis . J Ind Microbiol Biotechnol 40, 1285–1296 (2013). https://doi.org/10.1007/s10295-013-1316-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1316-0