Abstract
Background
Gamma-aminobutyric acid is a major inhibitory neurotransmitter in mammalian brains, and has several well-known physiological functions. Lactic acid bacteria possess special physiological activities and are generally regarded as safe. Therefore, using lactic acid bacteria as cell factories for gamma-aminobutyric acid production is a fascinating project and opens up a vast range of prospects for making use of GABA and LAB. We previously screened a high GABA-producer Lactobacillus brevis NCL912 and optimized its fermentation medium composition. The results indicated that the strain showed potential in large-scale fermentation for the production of gamma-aminobutyric acid. To increase the yielding of GABA, further study on the fermentation process is needed before the industrial application in the future. In this article we investigated the impacts of pyridoxal-5'-phosphate, pH, temperature and initial glutamate concentration on gamma-aminobutyric acid production by Lactobacillus brevis NCL912 in flask cultures. According to the data obtained in the above, a simple and effective fed-batch fermentation method was developed to highly efficiently convert glutamate to gamma-aminobutyric acid.
Results
Pyridoxal-5'-phosphate did not affect the cell growth and gamma-aminobutyric acid production of Lb. brevis NCL912. Temperature, pH and initial glutamate concentration had significant effects on the cell growth and gamma-aminobutyric acid production of Lb. brevis NCL912. The optimal temperature, pH and initial glutamate concentration were 30-35°C, 5.0 and 250-500 mM. In the following fed-batch fermentations, temperature, pH and initial glutamate concentration were fixed as 32°C, 5.0 and 400 mM. 280.70 g (1.5 mol) and 224.56 g (1.2 mol) glutamate were supplemented into the bioreactor at 12 h and 24 h, respectively. Under the selected fermentation conditions, gamma-aminobutyric acid was rapidly produced at the first 36 h and almost not produced after then. The gamma-aminobutyric acid concentration reached 1005.81 ± 47.88 mM, and the residual glucose and glutamate were 15.28 ± 0.51 g L-1 and 134.45 ± 24.22 mM at 48 h.
Conclusions
A simple and effective fed-batch fermentation method was developed for Lb. brevis NCL912 to produce gamma-aminobutyric acid. The results reveal that Lb. brevis NCL912 exhibits a great application potential in large-scale fermentation for the production of gamma-aminobutyric acid.
Similar content being viewed by others
Background
Gamma-aminobutyric acid (GABA) is a non-protein amino acid that is widely distributed in nature from microorganisms to plants and animals [1]. It acts as the major inhibitory neurotransmitter in the mammalian central nervous system. In addition, GABA has hypotensive, tranquilizing and diuretic effects, and can prevent diabetes [2–5]. Also, GABA may improve the concentration of plasma growth hormone and the rate of protein synthesis in the brain [6] and inhibit small airway-derived lung adenocarcinoma [7]. Therefore, GABA has potential as a bioactive component in foods and pharmaceuticals [8]. However, the direction addition of chemical GABA to food is considered unnatural and unsafe [8–10]. So it is necessary to find a natural method to produce and increase GABA in food.
Recent studies have shown that some lactic acid bacteria (LAB) can produce GABA [9, 11–19]. LAB possess special physiological activities and are generally regarded as safe (GRAS), and have been extensively utilized in food industries for a long time [20–23]. It is clear that the GABA production by LAB is natural and safe. In addition, the bio-synthetic production of natural GABA produced by LAB for the manufacturing of food can make full use of the health-promoting properties of GABA and LAB themselves. In recent years, the GABA production by using LAB as bacterial cell factories has therefore been a focus of research [8]. Some fermented products enriched in GABA using GABA-producing LAB as starters such as dairy products [2, 3, 24, 25], black raspberry juice [9], soymilk [26], kimchi [10], and cheese [27] have been developed. The GABA-producing ability varies widely among the strains of LAB, and some GABA-producing LAB strains have shown a great promise potential in large-scale fermentation for the production GABA [11, 13, 15, 16, 19, 28–30].
Since the primary goal of fermentation is the cost-effective and simple production of bio-products, it is important to select a proper process that allows of the highest yielding of the target product. In batch fermentation, substrate should be put in the tank once only. The thing is that the higher initial concentration of fermentation substrate can inhibit the cell growth or waste material resource, and the lower concentration of substrate can not meet the need of high production. Fed-batch culture can make up the weakness and has been widely applied in the production of various bioproducts [31–34]. During fed-batch cultivation, one or more components are supplied to the fermentor while cells and products remain in the tank until the end of operation [31]. A proper initial substrate concentration not inhibiting cell growth can be selected in a fed-batch fermentation, and the limitation component can be added with feeding in the fermentation course. It may help to obtain a high yield and productivity. We previously screened a high GABA-producing Lb. brevis NCL912 [11] and the GABA concentration reached 345.83 mM in the optimized fermentation medium [35]. To further increase the yielding of GABA, the effects of pyridoxal-5'-phosphate (PLP), pH, temperature and initial glutamate concentration on the GABA production by Lactobacillus brevis NCL912 were firstly determined using flask fermentation in this work. Then a simple process for efficient production of GABA by fed-batch fermentation using Lb. brevis NCL912 was developed.
Results and discussion
Effect of PLP on GABA production and bacterial growth
Glutamic acid decarboxylase (GAD, EC 4.1.1.15) catalyzes the irreversible α-decaboxylation of glutamate to produce GABA. GAD uses PLP as coenzyme. Therefore, from the theoretical point of view, an addition of PLP to medium may be a workable method to increase GAD activity to enhance synthetic capacity of GABA. Previous studies had shown that the addition of PLP to medium could effectively increase the GABA production of LAB [15, 19]. In our present study, however, the addition of PLP neither affected the cell growth (Figure 1A) nor increased GABA content (Figure 1B) in the fermentation. The possible reason was that NCL912 cells could synthesize sufficient PLP for themselves.
Effect of temperature on GABA synthesis and bacterial growth
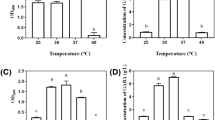
Figure 2 shows that the considerable variation in the yields of both bacterial growth and GABA production under different fermentation temperatures. The bacterial growth increased with the increase of temperature and peaked at 35°C, then decreased with the increase of temperature. For GABA production, a trend similar to the bacterial growth was observed. It was clear that high cell density was required for effective synthesis of GABA. On the other hand, GABA concentration at 30°C was almost the same to that at 35°C but biomass at 30°C was less than that at 35°C. In addition, NCL912 could not produce GABA at 45°C while the strain could grow under this temperature. These data indicated that appropriate temperature was beneficial to produce GABA, and excessively high temperature was unfavorable to the GABA production. The above results indicated that high efficient conversion glutamate to GABA needed not only high cell density but also appropriate temperature. By comprehensive consideration of the above data, 32°C was selected for the following tests.
Effect of pH on GABA synthesis and bacterial growth
It was reported that the GABA biosynthesis in LAB was strictly pH regulated [15, 19, 36]. To investigate the effect of different pH levels on the production of biomass and GABA of NCL912 in the course of fermentation, the initial pHs of the media were adjusted to 3, 4, 5 and 6, respectively. During the fermentation process, the pHs were adjusted to corresponding initial values immediately after sampling, respectively. As shown in Figure 3, pH has a significant effect on the production of biomass and GABA. The strain could hardly grow at pH3.0 and almost no GABA produced. A highest yield of GABA was obtained at pH5.0 in the fermentation course even if the biomass was less than that of pH6.0 after 24 h. The optimal pH value for the GABA production was 5.0 that accorded with the previous reports about the optimal pH values for maintaining the activity of LAB GADs were in the range of 4.0 to 5.0 [14, 37–39]. The higher or lower pH may lead to the partial loss of the GAD activity.
pH 5.0 was also the optimal pH for the cell growth, though the biomass decreased drastically after 24 h. However, the biomass kept increasing during the fermentation course when pH values were maintained at 4.0 or 6.0. It might be the combined inhibitory effect of high concentration of GABA and H2SO4. For pH5.0, more GABA was produced than pH4.0 and 6.0. The decarboxylation of glutamate to GABA catalyzed by GAD takes the following general form [1, 8]:
Decarboxylation of glutamate occurred in LAB results in the stoichiometric release of the end product GABA and the consumption of a proton. The net effect of this reaction is to increase the alkalinity of the cytoplasm and environment. For pH5.0, more H2SO4 was therefore supplemented into the fermentation broth in order to offset pH increase arising from the decarboxylation.
Effect of initial glutamate concentration on GABA production and bacterial growth
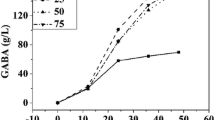
As shown in Figure 4A, a moderate addition of glutamate to the medium resulted in an increase in biomass. LAB can metabolize sugars to produce large amount of low weight molecule organic acids, resulting in an acidic environment that was hard for bacteria growth. Some LAB can employ GAD system for maintaining neutral cytoplasmic pH when the external pH drops because the decarboxylation of glutamate within the LAB cell consumes an intracellular proton. In Figure 4C, the pH value of the culture without glutamate decreased to 3.4 after 24 h of fermentation. pH values of the cultures supplemented with glutamate, however, are generally above 5.0. It implied that GAD system of Lb. brevis NCL912 acted under low pH and resulted in an increase of pH in medium with glutamate, and protected cell survival from acidic condition. The role of GAD conferring acid resistance to microbial cells was further verified in the present study. On the other hand, the cell growth and biomass decreased with the increase of glutamate concentration at the given levels (0.25, 0.5, 0.75 and 1.0 M). It was apparent that extra high concentration of glutamate was harmful to the strain growth. An addition of 0.25-0.5 M of glutamate was suitable for the strain NCL912 growth and production of GABA.
Fed-batch production of GABA with pH control
In the fed-batch process, the initial concentration of glutamate in medium was 400 mM, for the cell growth was strongly inhibited when glutamate exceeded 500 mM. The time courses of GABA production, residual glutamate, DCW, and residual glucose were tested (Figure 5). The cell growth occurred immediately after inoculation and biomass rapidly increased at the first 12 h. And then the biomass dramatically decreased due to the combined inhibitory effect of high concentration of GABA, glutamate and H2SO4. The concentrations of GABA and glutamate were 381.6 mM and 484.6 mM at 12 h, respectively. In addition, about 100 ml of 10 N H2SO4 was supplemented into the fermentor in order to offset pH increase arising from the glutamate addition and the decarboxylation. Such harsh conditions were certainly detrimental to the cells and therefore resulted in a sharp decline in biomass after 12 h. The cell growth was almost completely inhibited after 36 h. Without question, compared to feeding strategies maintaining low level glutamate, the current utilized feeding strategy strongly inhibited the cell growth. This feeding strategy, however, was simple and energy-saving. The most important was that the current fermentation method still exhibited powerful capacity of synthesis of GABA (reaching 1095.63 ± 61.03 mM at the end of the fermentations). The biosynthetic kinetics indicate that the GABA concentration increased rapidly with the fermentation time from 0-36 h, increased slowly from 36-60 h, and kept constant after 60 h. The GABA concentrations at 36, 48 and 60 h were 931.50 ± 29.65, 1005.81 ± 47.88 and 1075.05 ± 82.72 mM, respectively, which were obviously higher than that (345.83 mM) [35] before the optimization. Based on a comprehensive consideration of the GABA concentration, energy conservation and fermentation period, 48 h of fermentation was recommended in the future practical production. The volume of the fermentation broth was increased to about 3.75 L due to the inoculation and feed. Residual glutamate and glucose were 134.45 ± 24.22 mM and15.28 ± 0.51 g L-1 at 48 h. Total 738.24 g glutamate (plus the glutamate in the seed medium) was added into the fermentation medium, in which the converted glutamate was 705.81 ± 33.60 g according to the generated GABA mol number, and the residual glutamate was 94.35 ± 17.00 g. This good balance of glutamate added, converted and residual showed that the added glutamate did not participate in other metabolisms. Complete conversion of substrates was beneficial to save material and purify the end product from culture broth. In further practical applications, an addition of 112.28 g glutamate at 24 h, and 35 g L-1 of glucose in the medium are suggested.
Conclusions
In this study we investigated the effects of PLP, temperature, pH and initial glutamate concentration on the GABA production and bacterial growth of Lb. brevis NCL912. PLP did not affect the bacterial growth and the GABA production. In contrast, temperature, pH and initial glutamate concentration had a significant effect on the cell growth and the GABA production, and the conditions were optimized. Then a simple and effective fed-batch fermentation method was developed as follows. The fermentation medium [35] was inoculated with 10% (v/v) seed culture grown to early exponential phase at 32°C. The fed-batch fermentation was carried out under the following conditions: temperature 32°C, agitation speed 100 rpm, pH 5.0, and fermentation time 48 h. 280.70 g and 224.56 g of glutamate were fed into the bioreactor at 12 h and 24 h, respectively. The GABA concentration reached 1005.81 ± 47.88 mM at 48 h. To ensure a complete utilization of glutamate and glucose, 400 mM of glutamate and 35 g L-1 of glucose in the initial medium, and the addition of 280.70 g glutamate at 12 h and 112.28 g glutamate at 24 h into the bioreactor, are suggested.
Methods
Strain, medium and cultivation
GABA-producing Lb. brevis NCL912 was isolated by our laboratory from paocai, a Chinese traditional fermented vegetable [11]. In a previous study, we optimized the fermentation medium for production of GABA by Lb. brevis NCL912, and this medium was theoretically composed of (g L-1) [35]: glucose, 55.25; soya peptone, 30.25; MnSO4·4H2O, 0.0061; and Tween-80 1.38 mL L-1. To facilitate the calculation and preparation of the medium in practice, we adjusted the medium components to (g L-1): glucose, 50; soya peptone, 25; MnSO4·4H2O, 0.01; and Tween 80, 2 mL L-1. Unless otherwise emphasized, the initial sodium L-glutamate concentration in the medium was 500 mM. The seed medium was composed of (g L-1): glucose, 50; soya peptone, 25; MnSO4·4H2O, 0.01; L-glutamate, 150 mM; and Tween 80, 2 mL L-1. Nitrogen sources, glutamate and the other compositions were autoclaved separately at 121°C for 20 min and mixed together prior inoculation. Lb. brevis NCL912 was cultured in the seed medium at 32°C for 10 h till the absorbance value at 600 nm (A600) between 4.0 and 6.0 and then used for seed culture inoculation. Culture condition optimization was conducted in 250-mL Erlenmeyer flasks that contained 100 mL of the medium. The flasks were inoculated with 10% (v/v) seed culture grown to early exponential phase, and incubated under static conditions in an incubator. The fed-batch fermentation was carried out in a 5-L fermentor (Labo-controller MDL-8C; B. E. Marubishi, Tokyo, Japan) under following conditions: medium volume 3 L, inoculum size 10% (v/v), temperature 32°C, pH 5.0, agitation speed 100 rpm, and fermentation time 84 h. The pH was kept constant at 5.0 with addition of 10 N H2SO4. 280.70 g and 224.56 g glutamate were supplemented into the bioreactor at 12 h and 24 h, respectively. Each flask fermentation was performed in two replicates and the fed-batch fermentation was performed in three replicates.
Analytic procedures
Glutamate and GABA concentrations in the culture broths were determined by pre-staining paper chromatography [40]. Cell growth was monitored by measuring A600 on a TU-1901 UV-vis spectrophotometer (Beijing Purkinje General Instrument, China). Dry cell weight (DCW) was calculated by a consistent calibration curve of DCW versus A600. Glucose concentration was determined by 3, 5-dinitrosalicylic acid method [41]. Each sample was analyzed in duplicate and the mean values were calculated.
References
Ueno H: Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B: Enzym. 2000, 10: 67-79. 10.1016/S1381-1177(00)00114-4.
Hayakawa K, Kimura M, Kasaha K, Matsumoto K, Sansawa H, Yamori Y: Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Nutr. 2004, 92: 411-417. 10.1079/BJN20041221.
Inoue K, Shirai T, Ochiai H, Kasao M, Hayakawa K, Kimura M, Sansawa H: Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur J Clin Nutr. 57 (3): 490-495. 003
Jakobs C, Jaeken J, Gibson KM: Inherited disorders of GABA metabolism. J Inherit Metab Dis. 1993, 16: 704-715. 10.1007/BF00711902.
Wong CG, Bottiglieri T, Snead OC: GABA, gamma-hydroxybutyric acid, and neurological disease. Ann Neurol. 2003, 54: S3-12. 10.1002/ana.10696.
Tujioka K, Ohsumi M, Horie K, Kim M, Hayase K, Yokogoshi H: Dietary gamma-Aminobutyric Acid Affects the Brain Protein Synthesis Rate in Ovariectomized Female Rats. J Nutr Vitaminol. 2009, 55: 75-80. 10.3177/jnsv.55.75.
Schuller HM, Al-Wadei HAN, Majidi M: Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008, 29: 1979-1985. 10.1093/carcin/bgn041.
Li H, Cao Y: Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids.
Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT: Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int J Food Microbiol. 2009, 130: 12-16. 10.1016/j.ijfoodmicro.2008.12.028.
Seok JH, Park KB, Kim YH, Bae MO, Lee MK, Oh SH: Production and characterization of kimchi with enhanced levels of gamma-aminobutyric acid. Food Sci Biotechnol. 2008, 17: 940-946.
Li H, Cao Y, Gao D, Xu H: A high γ-aminobutyric acid-producing ability Lactobacillus brevis isolated from Chinese traditional paocai. Ann Microbiol. 2008, 58: 649-653. 10.1007/BF03175570.
Siragusa S, De Angelis M, Di Cagno R, Rizzello CG, Coda R, Gobbetti M: Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol. 2007, 73: 7283-7290. 10.1128/AEM.01064-07.
Yokoyama S, Hiramatsu J, Hayakawa K: Production of gamma-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO-12005. J Biosci Bioeng. 2002, 93: 95-97.
Nomura M, Nakajima I, Fujita Y, Kobayashi M, Kimoto H, Suzuki I, Aso H: Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology. 1999, 145: 1375-1380. 10.1099/13500872-145-6-1375.
Komatsuzaki N, Shima J, Kawamoto S, Momose H, Kimura T: Production of gamma-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005, 22: 497-504. 10.1016/j.fm.2005.01.002.
Cho YR, Chang JY, Chang HC: Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007, 17: 104-109.
Park KB, Oh SH: Isolation and characterization of Lactobacillus buchneri strains with high gamma-aminobutyric acid producing capacity from naturally aged cheese. Food Sci Biotechnol. 2006, 15: 86-90.
Sun TS, Zhao SP, Wang HK, Cai CK, Chen YF, Zhang HP: ACE-inhibitory activity and gamma-aminobutyric acid content of fermented skim milk by Lactobacillus helveticus isolated from Xinjiang koumiss in China. Eur Food Res Technol. 2009, 228: 607-612. 10.1007/s00217-008-0969-9.
Yang SY, Lu FX, Lu ZX, Bie XM, Jiao Y, Sun LJ, Yu B: Production of gamma-aminobutyric acid by Streptococcus salivarius subsp thermophilus Y2 under submerged fermentation. Amino Acids. 2008, 34: 473-478. 10.1007/s00726-007-0544-x.
Karahan AG, Kilic GB, Kart A, Aloglu HS, Oner Z, Aydemir S, Erkus O, Harsa S: Genotypic identification of some lactic acid bacteria by amplified fragment length polymorphism analysis and investigation of their potential usage as starter culture combinations in Beyaz cheese manufacture. J Dairy Sci. 2010, 93: 1-11. 10.3168/jds.2008-1801.
Lee JY, Kim CJ, Kunz B: Identification of lactic acid bacteria isolated from kimchi and studies on their suitability for application of as starter culture in the production fermented sausages. Meat Sci. 2006, 72: 437-445. 10.1016/j.meatsci.2005.08.013.
Leroy F, De Vuyst L: Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol. 2004, 15: 67-78. 10.1016/j.tifs.2003.09.004.
Yan PM, Xue WT, Tan SS, Zhang H, Chang XH: Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control. 2008, 19: 50-55. 10.1016/j.foodcont.2007.02.008.
Park KB, Oh SH: Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol. 2007, 98: 1675-1679. 10.1016/j.biortech.2006.06.006.
Skeie S, Lindberg C, Narvhus J: Development of amino acids and organic acids in Norvegia, influence of milk treatment and adjunct Lactobacillus. Int Dairy J. 2001, 11: 399-411. 10.1016/S0958-6946(01)00075-9.
Tsai JS, Lin YS, Pan BS, Chen TJ: Antihypertensive peptides and gamma-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochem. 2006, 41: 1282-1288. 10.1016/j.procbio.2005.12.026.
Nomura M, Kimoto H, Someya Y, Furukawa S, Suzuki I: Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci. 1998, 81: 1486-1491. 10.3168/jds.S0022-0302(98)75714-5.
Choi SI, Lee JW, Park SM, Lee MY, Ji GE, Park MS, Heo TR: Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J Microbiol Biotechnol. 2006, 16: 562-568.
Huang J, Le-He M, Wu H, Lin DQ: Biosynthesis of gamma-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J Microbiol Biotechnol. 2007, 23: 865-871. 10.1007/s11274-006-9311-5.
Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ: Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol Bioprocess Eng. 2007, 12: 707-712. 10.1007/BF02931089.
Krause M, Ukkonen K, Haataja T, Ruottinen M, Glumoff T, Neubauer A, Neubauer P, Vasala A: A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures. Microb Cell Fact. 2010, 9: 11- 10.1186/1475-2859-9-11.
Ihssen J, Kowarik M, Dilettoso S, Tanner C, Wacker M, Thöny-Meyer L: Production of glycoprotein vaccines in Escherichia coli. Microb Cell Fact. 2010, 9: 61- 10.1186/1475-2859-9-61.
Glazyrina J, Materne EM, Dreher T, Storm D, Junne S, Adams T, Greller G, Neubauer P: High cell density cultivation and recombinant protein production with Escherichia coli in a rocking- motion- type bioreactor. Microb Cell Fact. 2010, 9: 42- 10.1186/1475-2859-9-42.
Hahn-Hagerdal B, Karhumaa K, Larsson CU, Gorwa-Grauslund M, Gorgens J, van Zyl WH: Role of cultivation media in the development of yeast strains for large scale industrial use. Microb Cell Fact. 2005, 4: 31- 10.1186/1475-2859-4-31.
Li H, Qiu T, Gao D, Cao Y: Medium optimization for production of gamma-aminobutyric acid by Lactobacillus brevis NCL912. Amino Acids. 2010, 38: 1439-1445. 10.1007/s00726-009-0355-3.
Sanders JW, Leenhouts K, Burghoorn J, Brands JR, Venema G, Kok J: A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol Microbiol. 1998, 27: 299-310. 10.1046/j.1365-2958.1998.00676.x.
Komatsuzaki N, Nakamura T, Kimura T, Shima J: Characterization of glutamate decarboxylase from a high gamma-aminobutyric acid (GABA)-producer, Lactobacillus paracasei. Biosci Biotechnol Biochem. 2008, 72: 278-285. 10.1271/bbb.70163.
Huang J, Mei LH, Sheng Q, Yao SJ, Lin DQ: Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chin J Chem Eng. 2007, 15: 157-161. 10.1016/S1004-9541(07)60051-2.
Ueno Y, Hayakawa K, Takahashi S, Oda K: Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci Biotechnol Biochem. 1997, 61: 1168-1171. 10.1271/bbb.61.1168.
Li H, Qiu T, Cao Y, Yang J, Huang Z: Pre-staining paper chromatography method for quantification of gamma-aminobutyric acid. J chromatogr A. 2009, 1216: 5057-5060. 10.1016/j.chroma.2009.04.044.
Miller GL: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959, 31: 426-428. 10.1021/ac60147a030.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The initiative for this work came from YC. YC and HL designed the experiments; HL and TQ carried out the experimental work; HL and GH analyzed data; YC and HL drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Li, H., Qiu, T., Huang, G. et al. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Fact 9, 85 (2010). https://doi.org/10.1186/1475-2859-9-85
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2859-9-85