Abstract
Key message
Oxidative stress and the antioxidant enzymes’ activity are higher in damaged than in healthy Juniperus procera trees, in summer than in winter, and in dry than in wet condition.
Abstract
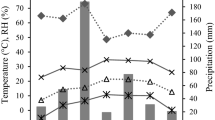
Many of the small stands of Juniperus procera in Saudi Arabia, confined mainly to Aseer Mountains in the southern part of the country, are suffering from branch dieback. As a part of the project on the structural and functional responses of healthy and dieback-affected trees to local environmental conditions of Al-Ghalab, Al-Yazeed, and Saodah locations, this study quantifies the oxidative stress generated and the consequent modulation of proline accumulation and antioxidant enzymes’ activity, as determined by chemical analysis of needle tissues from samples collected in summer and winter seasons. The level of TBARS, which indicated the extent of oxidative stress, was minimum (10.1 nM g−1 f w) at Al-Ghalab and maximum (28.1 nM g−1 f w) at Al-Yazeed, being relatively higher in summer than in winter. Healthy trees had a lower level of TBARS than those suffering from dieback. Proline content showed 147–54 µg g−1 in healthy trees and 460–99 µg g−1 f w in affected ones. Variation in the activity of superoxide dismutase, ascorbate peroxidase, glutathione reductase, and catalase was around 0.7–3.6, 0.01–0.09, 0.02–0.08, and 0.6–3.0 U mg−1 min−1, respectively, in healthy trees, whereas 2.3–6.1, 0.04–0.3, 0.04–0.3, and 2–5.8 U mg−1 min−1, respectively, in the dieback-affected trees of the different locations. Thus, the oxidative stress and the enzymatic stimulation were higher in damaged than in healthy trees and in summer than in winter season. Water-harvesting efforts at the collection sites showed ameliorative effects. Our observations suggest that J. procera tree can be made more tolerant toward stressful condition, and even the risk of dieback can be avoided or minimized by improving soil–water availability through adequate water-harvesting strategies in the drought-affected areas.





Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- DDW:
-
Double distilled water
- EDTA:
-
Ethylene-diamine-tetra-acetic acid
- GR:
-
Gluththione reductase
- GSSG:
-
Glutathione disulfide
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroethanoic acid
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Ghamdi AAM, Jais HM (2012) Study of Juniperus procera and arbuscular mycorhizal fungi (AMF) in Saudi Arabia. Int J Adv Biol Res 2(2):177–183
Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Gonzales P, Hogg T, Rigling A, Breshears D et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manag 259:660–684
Anjum NA, Umar S, Iqbal M, Khan NA (2008) Growth characteristics and antioxidant metabolism of mungbean genotypes differing in photosynthetic capacity subjected to water deficit stress. J Plant Interact 3:127–136
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MNV (2012) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids—a review. Environ Exp Bot 75:307–324
Anjum NA, Aref IA, Pereira E, Ahmad A, Iqbal M (2014) Glutathione and proline can coordinately make plants withstand the joint attack of osmotic and metal(loid) stresses. Front Plant Sci 5(662):4. doi:10.3389/fpls.2014.00662
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res 22:4099–4121. doi:10.1007/s11356-014-3917-1
Anonymous (2011) Methods manual. Soil testing in India. Ministry of Agriculture, Govt of India, New Delhi
Aref MI, Ahmed AI, Khan PR, El-Atta H, Iqbal M (2013a) Drought-induced adaptive changes in the seedling anatomy of Acacia ehrenbergiana and Acacia tortilis subsp. raddiana. Trees Struct Funct 27(4):959–971. doi:10.1007/s00468-013-0848-2
Aref IM, El-Atta H, Al-Shahrani T, Alazba A, Ahmed AI (2013b) Evaluation of the physiological and growth response of Juniperus procera Hochst. ex Endlicher to some types of microcatchments. Int J Plant Anim Environ Sci 3(1):234–241
Aref MI, El-Atta H, El-Obeid M, Ahmed AI, Khan PR, Iqbal M (2013c) Effect of water stress on relative water and chlorophyll contents of Juniperus procera Hochst. ex Endlicher in Saudi Arabia. Life Sci J 10(4):681–685
Aref MI, Khan PR, Al-Mefarrej H, Al-Shahrani T, Ahmed AI, Iqbal M (2014) Cambial periodicity and wood production in Acacia ehrenbergiana Hayne growing on dry sites of Saudi Arabia. J Environ Biol 35(2):301–310
Arshi A, Ahmad A, Aref IM, Iqbal M (2010) Effect of calcium against salinity-induced inhibition in growth, ion accumulation and proline contents in Cichorium intybus L. J Environ Biol 31:939–944
Arshi A, Ahmad A, Aref IM, Iqbal M (2012) Comparative studies on antioxidant enzyme action and ion accumulation in soybean cultivars under salinity stress. J Environ Biol 33:9–20
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51(2):163–190
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Bhattacharjee S (2012) The language of reactive oxygen species signaling in plants. J Bot 2012:22. doi:10.1155/2012/985298
Bloedner C, Majcherczyk A, Kues U, Polle A (2007) Early drought-induced changes to the needle proteome of Norway spruce. Tree Physiol 27:1423–1431
Cakmak I, Horst J (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2(53):13. doi:10.3389/fenvs.2014.00053
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
El-Atta H, Aref IM (2010) Effect of terracing on rainwater harvesting and growth of Juniperus procera Hochst ex Endlicher. Int J Environ Sci Technol 7(1):59–66
Favaretto VF, Martinez CA, Soriani HH, Furriel RPM (2011) Differential responses of antioxidant enzymes in pioneer and late-successional tropical tree species grown under sun and shade conditions. Environ Exp Bot 70:20–28
Fink T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15
Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119:355–364
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. doi:10.1016/j.plaphy.2010.08.016
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signalling networks. Front Plant Sci 5:art 151:10. doi:10.3389/fpls.2014.00151
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: Physiological, biochemical and molecular characterization. Int J Genom. doi:10.1155/2014/701596 (Art. ID 701596)
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments—a review. Plant Signal Behav 7:11. doi:10.4161/psb.21949
Impa SM, Nadaradjan S, Jagadish SVK (2012) Drought stress induced reactive oxygen species and antioxidants in plants. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 131–147
Iqbal M, Bano R, Wali B (2005) Plant growth responses to air pollution. In: Chaturvedi SN, Singh KP (eds) Plant biodiversity, microbial interaction and environmental biology. Avishkar Publishers, Jaipur, pp 166–188
Iqbal M, Mahmooduzzafar, Aref IM, Khan PR (2010) Behavioural responses of leaves and vascular cambium of Prosopis cineraria (L.) Druce to different regimes of coal-smoke pollution. J Plant Interact 5(2):117–133
Iqbal M, Ahmad A, Ansari MKA, Qureshi MI, Aref MI, Khan PR, Hegazy SS, El-Atta H, Husen A, Hakeem KR (2015) Improving the phytoextraction capacity of plants to scavenge metal(loid)-contaminated sites. Environ Rev 23(1):44–65. doi:10.1139/er-2014-0043
Jabeen R, Ahmad A, Iqbal M (2009) Phytoremediation of heavy metals: physiological and molecular aspects. Bot Rev 75:339–364
Jackson ML (1967) Soil chemical analysis, 7th edn. Prentice Hall (India) Pvt Ltd, New Delhi, p 498
Lee BR, Li LS, Jung WJ, Jin YLJC, Avice JC, Ourry A, Kim TH (2009) Water deficit-induced oxidative stress and the activation of antioxidant enzymes in white clover leaves. Biol Plant 53:505–510
Mantry N, Patade V, Penna S, Ford R, Pang E (2012) Abiotic stress responses in plants: present and future. In: Ahmad P, Prasad MNV (eds) Abiotic stress responses in plants: metabolism, productivity and sustainability. Springer, New York, pp 1–19
Mendrey KJ (2013) Metal response of Douglas-fir: a comparison of foliar metals and phytochelatin production in trees planted in soils amended with biosolids or metal salts. MS Thesis, University of Washington, USA, p 185
Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35:299–319
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbatespecific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
NCWCD, JICA (2006) The management plan for conservation of juniper woodlands. The Final Report of the joint study of National Commission of Wildlife Conservation and Development (NCWCD) and Japan International Cooperation Agency (JICA). pp 142 + 2 appendices
Qureshi MI, Israr M, Abdin MZ, Iqbal M (2005) Responses of Artemisia annua L. to lead and salt-induced oxidative stress. Environ Exp Bot 53:185–193
Qureshi MI, Abdin MZ, Qadir S, Iqbal M (2007) Lead-induced oxidative stress and metabolic alterations in Cassia angustifolia Vahl. Biol Plant 51:121–128
Qureshi MI, Abdin MZ, Ahmad J, Iqbal M (2013) Effect of long-term salinity on cellular antioxidants, compatible solute and fatty-acid profile of Sweet annie (Artemisia annua L.). Phytochemistry 95:215–223
Rao MV (1992) Cellular detoxification mechanisms to determine age dependent injury in tropical plant exposed to SO2. J Plant Physiol 140:737–740
Sahu S, Das P, Ray M, Sabat SC (2010) Osmolyte-modulated enhanced rice leaf catalase activity under salt stress. Adv Biosci Biotechnol 1:39–46
Spano C, Bruno M, Bottega S (2013) Calystegia soldanella: dune versus laboratory plants to highlight key adaptive physiological traits. Acta Physiol Plant 35(4):1329–1336
Subbaiah BV, Asija GL (1956) A rapid procedure for determination of available nitrogen in soils. Curr Sci 25:259–260
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Sweetlove LJ, Moller IM (2009) Oxidation of proteins in plants—mechanisms and consequences. Adv Bot Res 52:1–23
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining P in water and NaHCO3 extract from soil. Soil Sci Soc Am Proc 29:677–678
Acknowledgments
Financial support provided by the National Plan for Sciences, Technology and Innovation, Saudi Arabia, for sponsoring this study under the research project #10-AGRI 1310-02 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by W. Bilger.
Rights and permissions
About this article
Cite this article
Aref, I.M., Khan, P.R., Khan, S. et al. Modulation of antioxidant enzymes in Juniperus procera needles in relation to habitat environment and dieback incidence. Trees 30, 1669–1681 (2016). https://doi.org/10.1007/s00468-016-1399-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-016-1399-0