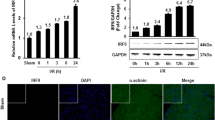
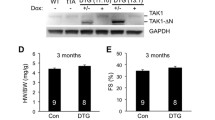
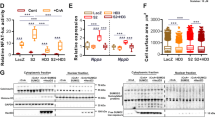
Abstract
Interferon regulatory factor (IRF) 3, a member of the highly conserved IRF family transcription factors, plays a pivotal role in innate immune response, apoptosis, and oncogenesis. Recent studies have implicated IRF3 in a wide range of host defense. However, whether IRF3 induces defensive responses to hypertrophic stresses such as biomechanical stress and neurohumoral factors remains unclear. Herein, we employed an IRF3-deficient mouse model, cardiac-specific IRF3-overexpression mouse model and isolated cardiomyocytes to investigate the role of IRF3 in cardiac hypertrophy induced by aortic banding (AB) or isoproterenol (ISO). The extent of cardiac hypertrophy was quantitated by echocardiography as well as by pathological and molecular analysis. Our results demonstrate that IRF3 deficiency profoundly exacerbated cardiac hypertrophy, whereas overexpression of IRF3 in the heart significantly blunted pathological cardiac remodeling induced by pressure overload. Similar results were also observed in cultured cardiomyocytes upon the treatment with ISO. Mechanistically, we discovered that IRF3 interacted with ERK2 and thereby inhibited the ERK1/2 signaling. Furthermore, inactivation of ERK1/2 by U0126 offset the IRF3-deficient-mediated hypertrophic response induced by aortic banding. Altogether, these data demonstrate that IRF3 plays a protective role in AB-induced hypertrophic response by inactivating ERK1/2 in the heart. Therefore, IRF3 could be a new target for the prevention and therapy of cardiac hypertrophy and failure.







Similar content being viewed by others
References
Berk BC, Fujiwara K, Lehoux S (2007) ECM remodeling in hypertensive heart disease. J Clin Invest 117:568–575. doi:10.1172/JCI31044
Bian Z, Cai J, Shen DF, Chen L, Yan L, Tang Q, Li H (2009) Cellular repressor of E1A-stimulated genes attenuates cardiac hypertrophy and fibrosis. J Cell Mol Med 13:1302–1313. doi:10.1111/j.1582-4934.2008.00633.x
Bian ZY, Huang H, Jiang H, Shen DF, Yan L, Zhu LH, Wang L, Cao F, Liu C, Tang QZ, Li H (2010) LIM and cysteine-rich domains 1 regulates cardiac hypertrophy by targeting calcineurin/nuclear factor of activated T cells signaling. Hypertension 55:257–263. doi:HYPERTENSIONAHA.109.135665
Cai J, Yi FF, Bian ZY, Shen DF, Yang L, Yan L, Tang QZ, Yang XC, Li H (2009) Crocetin protects against cardiac hypertrophy by blocking MEK-ERK1/2 signalling pathway. J Cell Mol Med 13:909–925. doi:10.1111/j.1582-4934.2008.00620.x
Chattopadhyay S, Fensterl V, Zhang Y, Veleeparambil M, Yamashita M, Sen GC (2012) Role of Irf-3-mediated apoptosis in the establishment and maintenance of persistent infection by sendai virus. J Virol. doi:10.1128/JVI.01853-12
Chattopadhyay S, Yamashita M, Zhang Y, Sen GC (2011) The IRF-3/Bax-mediated apoptotic pathway, activated by viral cytoplasmic RNA and DNA, inhibits virus replication. J Virol 85:3708–3716. doi:10.1128/JVI.02133-10
Esposito G, Perrino C, Cannavo A, Schiattarella GG, Borgia F, Sannino A, Pironti G, Gargiulo G, Di Serafino L, Franzone A, Scudiero L, Grieco P, Indolfi C, Chiariello M (2011) EGFR trans-activation by urotensin II receptor is mediated by beta-arrestin recruitment and confers cardioprotection in pressure overload-induced cardiac hypertrophy. Basic Res Cardiol 106:577–589. doi:10.1007/s00395-011-0163-2
Garlie JB, Hamid T, Gu Y, Ismahil MA, Chandrasekar B, Prabhu SD (2011) Tumor necrosis factor receptor 2 signaling limits β-adrenergic receptor-mediated cardiac hypertrophy in vivo. Basic Res Cardiol 106:1193–1205. doi:10.1007/s00395-011-0196-6
Heineke J, Molkentin JD (2006) Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7:589–600. doi:10.1038/nrm1983
Heusch G (2009) Diastolic heart failure: a misNOmer. Basic Res Cardiol 104:465–467. doi:10.1007/s00395-009-0025-3
Heusch G (2011) SCIPIO brings new momentum to cardiac cell therapy. Lancet 378:1827–1828. doi:10.1016/S0140-6736(11)61648-6
Heusch G, Kleinbongard P, Skyschally A, Levkau B, Schulz R, Erbel R (2012) The coronary circulation in cardioprotection: more than just one confounder. Cardiovasc Res 94:237–245. doi:10.1093/cvr/cvr271
Heusch G, Neumann T (1998) Calcium responsiveness in canine pacing-induced heart failure. J Mol Cell Cardiol 30:1605–1613. doi:10.1006/jmcc.1998.0726
Heusch G, Schulz R (2006) Pathophysiology of the renin–angiotensin-system in atrial fibrillation. Dtsch Med Wochenschr 131:817–820. doi:10.1055/s-2006-939853
Heusch G, Schulz R (2011) A radical view on the contractile machinery in human heart failure. J Am Coll Cardiol 57:310–312. doi:10.1016/j.jacc.2010.06.057
Heusch P, Canton M, Aker S, van de Sand A, Konietzka I, Rassaf T, Menazza S, Brodde OE, Di Lisa F, Heusch G, Schulz R (2010) The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol 160:1408–1416. doi:10.1111/j.1476-5381.2010.00793.x
Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M (2004) The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428:341–345. doi:10.1038/nature02405
Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J (2011) Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 106:1173–1191. doi:10.1007/s00395-011-0222-8
Hwang S, Kim KS, Flano E, Wu TT, Tong LM, Park AN, Song MJ, Sanchez DJ, O’Connell RM, Cheng G, Sun R (2009) Conserved herpesviral kinase promotes viral persistence by inhibiting the IRF-3-mediated type I interferon response. Cell Host Microbe 5:166–178. doi:10.1016/j.chom.2008.12.013
Kim TK, Lee JS, Oh SY, Jin X, Choi YJ, Lee TH, Lee E, Choi YK, You S, Chung YG, Lee JB, DePinho RA, Chin L, Kim H (2007) Direct transcriptional activation of promyelocytic leukemia protein by IFN regulatory factor 3 induces the p53-dependent growth inhibition of cancer cells. Cancer Res 67:11133–11140. doi:10.1158/0008-5472.CAN-07-1342
Kim Y, Phan D, van Rooij E, Wang DZ, McAnally J, Qi X, Richardson JA, Hill JA, Bassel-Duby R, Olson EN (2008) The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J Clin Invest 118:124–132. doi:10.1172/JCI33255
Kovacic-Milivojevic B, Roediger F, Almeida EAC, Damsky CH, Gardner DG, Ilic D (2001) Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol Biol Cell 12:2290–2307
Krusche CA, Holthofer B, Hofe V, van de Sandt AM, Eshkind L, Bockamp E, Merx MW, Kant S, Windoffer R, Leube RE (2011) Desmoglein 2 mutant mice develop cardiac fibrosis and dilation. Basic Res Cardiol 106:617–633. doi:10.1007/s00395-011-0175-y
Li HL, Huang Y, Zhang CN, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC (2006) Epigallocatechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and -independent signal pathways. Free Radic Biol Med 40:1756–1775
Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH, Yang Q, Huang Y, Wei YS, Liu PP, Liu DP, Liang CC (2007) Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation 115:1885–1894. doi:10.1161/CIRCULATIONAHA.106.656835
Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD (2001) The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem 276:30245–30253. doi:10.1074/jbc.M102174200
Liang Q, Wiese RJ, Bueno OF, Dai YS, Markham BE, Molkentin JD (2001) The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol Cell Biol 21:7460–7469. doi:10.1128/MCB.21.21.7460-7469.2001
Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ (2009) A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med 15:75–83. doi:10.1038/nm.1893
Maelfait J, Beyaert R (2012) Emerging role of ubiquitination in antiviral RIG-I signaling. Microbiol Mol Biol Rev 76:33–45. doi:10.1128/MMBR.05012-11
Molkentin JD (2004) Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 63:467–475. doi:10.1016/j.cardiores.2004.01.021
Mraiche F, Oka T, Gan XT, Karmazyn M, Fliegel L (2011) Activated NHE1 is required to induce early cardiac hypertrophy in mice. Basic Res Cardiol 106:603–616. doi:10.1007/s00395-011-0161-4
Miller CL, Cai Y, Oikawa M, Thomas T, Dostmann WR, Zaccolo M, Fujiwara K, Yan C (2011) Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart. Basic Res Cardiol 106:1023–1039. doi:10.1007/s00395-011-0228-2
Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD (2006) Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res 98:837–845. doi:10.1161/01.RES.0000215985.18538.c4
Piya S, Moon AR, Song PI, Hiscott J, Lin R, Seol DW, Kim TH (2011) Suppression of IRF4 by IRF1, 3, and 7 in Noxa expression is a necessary event for IFN-gamma-mediated tumor elimination. Mol Cancer Res 9:1356–1365. doi:10.1158/1541-7786.MCR-11-0185
Remy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C (2009) Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol 182:1877–1884. doi:10.4049/jimmunol.0802436
Sanna B, Bueno OF, Dai YS, Wilkins BJ, Molkentin JD (2005) Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth. Mol Cell Biol 25:865–878. doi:10.1128/MCB.25.3.865-878.2005
Savitsky D, Tamura T, Yanai H, Taniguchi T (2010) Regulation of immunity and oncogenesis by the IRF transcription factor family. Cancer Immunol Immunother 59:489–510. doi:10.1007/s00262-009-0804-6
Sen N, Sommer M, Che X, White K, Ruyechan WT, Arvin AM (2010) Varicella-zoster virus immediate-early protein 62 blocks interferon regulatory factor 3 (IRF3) phosphorylation at key serine residues: a novel mechanism of IRF3 inhibition among herpesviruses. J Virol 84:9240–9253. doi:10.1128/JVI.01147-10
Shen DF, Tang QZ, Yan L, Zhang Y, Zhu LH, Wang L, Liu C, Bian ZY, Li H (2010) Tetrandrine blocks cardiac hypertrophy by disrupting reactive oxygen species-dependent ERK1/2 signalling. Br J Pharmacol 159:970–981. doi:10.1111/j.1476-5381.2009.00605.x
Stein SC, Falck-Pedersen E (2012) Sensing adenovirus infection: activation of interferon regulatory factor 3 in RAW 264.7 cells. J Virol 86:4527–4537. doi:10.1128/JVI.07071-11
Takaoka A, Tamura T, Taniguchi T (2008) Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci 99:467–478. doi:10.1111/j.1349-7006.2007.00720.x
Tang Q, Cai J, Shen D, Bian Z, Yan L, Wang YX, Lan J, Zhuang GQ, Ma WZ, Wang W (2009) Lysosomal cysteine peptidase cathepsin L protects against cardiac hypertrophy through blocking AKT/GSK3beta signaling. J Mol Med (Berl) 87:249–260. doi:10.1007/s00109-008-0423-2
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N (2001) IRF family of transcription factors as regulators of host defense. Annu Rev Immunol 19:623–655. doi:10.1146/annurev.immunol.19.1.623
Tarassishin L, Bauman A, Suh HS, Lee SC (2012) Anti-viral and anti-inflammatory mechanisms of the innate immune transcription factor interferon regulatory factor 3: relevance to human CNS diseases. J Neuroimmune Pharmacol. doi:10.1007/s11481-012-9360-5
Tenhunen O, Sarman B, Kerkela R, Szokodi I, Papp L, Toth M, Ruskoaho H (2004) Mitogen-activated protein kinases p38 and ERK 1/2 mediate the wall stress-induced activation of GATA-4 binding in adult heart. J Biol Chem 279:24852–24860. doi:10.1074/jbc.M314317200
Tsushima K, Osawa T, Yanai H, Nakajima A, Takaoka A, Manabe I, Ohba Y, Imai Y, Taniguchi T, Nagai R (2011) IRF3 regulates cardiac fibrosis but not hypertrophy in mice during angiotensin II-induced hypertension. FASEB J 25:1531–1543. doi:10.1096/fj.10-174615
Wang F, Ma Y, Barrett JW, Gao X, Loh J, Barton E, Virgin HW, McFadden G (2004) Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nat Immunol 5:1266–1274. doi:10.1038/ni1132
Wenzel S, Henning K, Habbig A, Forst S, Schreckenberg R, Heger J, Maxeiner H, Schlüter KD (2010) TGF-beta1 improves cardiac performance via up-regulation of laminin receptor 37/67 in adult ventricular cardiomyocytes. Basic Res Cardiol 105:621–629. doi:10.1007/s00395-010-0108-1
Xu Z, Desai M, Philip J, Sivsubramanian N, Bowles NE, Vallejo JG (2011) Conditional transgenic expression of TIR-domain-containing adaptor-inducing interferon-β (TRIF) in the adult mouse heart is protective in acute viral myocarditis. Basic Res Cardiol 106:1159–1171. doi:10.1007/s00395-011-0226-4
Xiao J, Moon M, Yan L, Nian M, Zhang Y, Liu C, Lu J, Guan H, Chen M, Jiang D, Jiang H, Liu PP, Li H (2012) Cellular FLICE-inhibitory protein protects against cardiac remodelling after myocardial infarction. Basic Res Cardiol 107:239. doi:10.1007/s00395-011-0239-z
Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR (2006) Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J 25:3869–3879. doi:10.1038/sj.emboj.7601252
Zhou H, Shen DF, Bian ZY, Zong J, Deng W, Zhang Y, Guo YY, Li H, Tang QZ (2011) Activating transcription factor 3 deficiency promotes cardiac hypertrophy, dysfunction, and fibrosis induced by pressure overload. PLoS ONE 6:e26744. doi:10.1371/journal.pone.0026744
Zhu Y, Li T, Song J, Liu C, Hu Y, Que L, Ha T, Kelley J, Chen Q, Li C, Li Y (2011) The TIR/BB-loop mimetic AS-1 prevents cardiac hypertrophy by inhibiting IL-1R-mediated MyD88-dependent signaling. Basic Res Cardiol 106:787–799. doi:10.1007/s00395-011-0182-z
Acknowledgments
We thank Dr. Tadatsugu Taniguchi for providing IRF3-knockout mice and Dr. Kensuke Tsushima for discussion by Email. This study was supported Funds for Distinguished Young Scientists of Hubei (2010CDA092) and by the National Natural Science Foundation of China (Grants 30900524 and 81170086, 3087451 and 30801351), and National Science and Technology Support Project (NO. 2011BAI15B02 and No. 2012BAI39B05), National Basic Research Program of China (No. 2011CB503902).
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Lu, Z.-Y. Bian and R. Zhang are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, J., Bian, ZY., Zhang, R. et al. Interferon regulatory factor 3 is a negative regulator of pathological cardiac hypertrophy. Basic Res Cardiol 108, 326 (2013). https://doi.org/10.1007/s00395-012-0326-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0326-9