Abstract
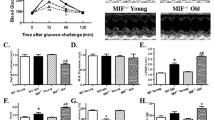
Aging is often accompanied with geometric and functional changes in the heart, although the underlying mechanisms remain unclear. Recent evidence has described a potential role of Akt and autophagy in aging-associated organ deterioration. This study was to examine the impact of cardiac-specific Akt activation on aging-induced cardiac geometric and functional changes and underlying mechanisms involved. Cardiac geometry, contractile and intracellular Ca2+ properties were evaluated using echocardiography, edge-detection and fura-2 techniques. Level of insulin signaling and autophagy was evaluated by western blot. Our results revealed cardiac hypertrophy (enlarged chamber size, wall thickness, myocyte cross-sectional area), fibrosis, decreased cardiac contractility, prolonged relengthening along with compromised intracellular Ca2+ release and clearance in aged (24–26 month-old) mice compared with young (3–4 month-old) mice, the effects of which were accentuated by chronic Akt activation. Aging enhanced Akt and mTOR phosphorylation while reducing that of PTEN, AMPK and ACC with a more pronounced response in Akt transgenic mice. GSK3β phosphorylation and eNOS levels were unaffected by aging or Akt overexpression. Levels of beclin-1, Atg5 and LC3-II-to-LC3-I ratio were decreased in aged hearts, the effect of which with the exception of Atg 5 was exacerbated by Akt overactivation. Levels of p62 were significantly enhanced in aged mice with a more pronounced increase in Akt mice. Neither aging nor Akt altered β-glucuronidase activity and cathepsin B although aging reduced LAMP1 level. In addition, rapamycin reduced aging-induced cardiomyocyte contractile and intracellular Ca2+ dysfunction while Akt activation suppressed autophagy in young but not aged cardiomyocytes. In conclusion, our data suggest that Akt may accentuate aging-induced cardiac geometric and contractile defects through a loss of autophagic regulation.










Similar content being viewed by others
References
Alvers AL, Fishwick LK, Wood MS, Hu D, Chung HS, Dunn WA Jr, Aris JP (2009) Autophagy and amino acid homeostasis are required for chronological longevity in Saccharomyces cerevisiae. Aging Cell 8:353–369. doi:10.1111/j.1474-9726.2009.00469.x
Baudhuin P (1966) Lysosomes and cellular autophagy. Brux Med 46:1059–1070
Birnbaum Y, Long B, Qian J, Perez-Polo JR, Ye Y (2011) Pioglitazone limits myocardial infarct size, activates Akt, and upregulates cPLA2 and COX-2 in a PPAR-gamma-independent manner. Basic Res Cardiol 106:431–446. doi:10.1007/s00395-011-0162-3
Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L (2010) Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab 11:35–46. doi:10.1016/j.cmet.2009.11.010
Blagosklonny MV (2008) Aging: ROS or TOR. Cell Cycle 7:3344–3354. doi:10.4161/cc.7.21.6965
Boengler K, Buechert A, Heinen Y, Roeskes C, Hilfiker-Kleiner D, Heusch G, Schulz R (2008) Cardioprotection by ischemic postconditioning is lost in aged and STAT3-deficient mice. Circ Res 102:131–135. doi:10.1161/CIRCRESAHA.107.164699
Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, Schulz R (2007) Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol 292:H1764–H1769. doi:10.1152/ajpheart.01071.2006
Boengler K, Schulz R, Heusch G (2009) Loss of cardioprotection with ageing. Cardiovasc Res 83:247–261. doi:10.1093/cvr/cvp033
Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK (2011) Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol 106:135–145. doi:10.1007/s00395-010-0133-0
Carneiro-Ramos MS, Diniz GP, Nadu AP, Almeida J, Vieira RL, Santos RA, Barreto-Chaves ML (2010) Blockage of angiotensin II type 2 receptor prevents thyroxine-mediated cardiac hypertrophy by blocking Akt activation. Basic Res Cardiol 105:325–335. doi:10.1007/s00395-010-0089-0
Ceylan-Isik AF, Zhao P, Zhang B, Xiao X, Su G, Ren J (2010) Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis. J Mol Cell Cardiol 48:367–378. doi:10.1016/j.yjmcc.2009.11.003
Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140. doi:10.4161/auto.1.3.2017
Davidoff AJ, Mason MM, Davidson MB, Carmody MW, Hintz KK, Wold LE, Podolin DA, Ren J (2004) Sucrose-induced cardiomyocyte dysfunction is both preventable and reversible with clinically relevant treatments. Am J Physiol Endocrinol Metab 286:E718–E724. doi:10.1152/ajpendo.00358.2003
De Meyer GR, De Keulenaer GW, Martinet W (2010) Role of autophagy in heart failure associated with aging. Heart Fail Rev 15:423–430. doi:10.1007/s10741-010-9166-6
Diniz GP, Carneiro-Ramos MS, Barreto-Chaves ML (2009) Angiotensin type 1 receptor mediates thyroid hormone-induced cardiomyocyte hypertrophy through the Akt/GSK-3beta/mTOR signaling pathway. Basic Res Cardiol 104:653–667. doi:10.1007/s00395-009-0043-1
Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J (2009) Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation 119:1941–1949. doi:10.1161/CIRCULATIONAHA.108.823799
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502. doi:10.1016/j.mam.2006.08.005
Fujita K, Maeda D, Xiao Q, Srinivasula SM (2011) Nrf2-mediated induction of p62 controls Toll-like receptor-4-driven aggresome-like induced structure formation and autophagic degradation. Proc Natl Acad Sci USA 108:1427–1432. doi:10.1073/pnas.1014156108
Ghaboura N, Tamareille S, Ducluzeau PH, Grimaud L, Loufrani L, Croue A, Tourmen Y, Henrion D, Furber A, Prunier F (2011) Diabetes mellitus abrogates erythropoietin-induced cardioprotection against ischemic-reperfusion injury by alteration of the RISK/GSK-3beta signaling. Basic Res Cardiol 106:147–162. doi:10.1007/s00395-010-0130-3
Goswami SK, Das DK (2006) Autophagy in the myocardium: dying for survival? Exp Clin Cardiol 11:183–188
Gottlieb RA, Finley KD, Mentzer RM Jr (2009) Cardioprotection requires taking out the trash. Basic Res Cardiol 104:169–180. doi:10.1007/s00395-009-0011-9
Ha SD, Ham B, Mogridge J, Saftig P, Lin S, Kim SO (2010) Cathepsin B-mediated autophagy flux facilitates the anthrax toxin receptor 2-mediated delivery of anthrax lethal factor into the cytoplasm. J Biol Chem 285:2120–2129. doi:10.1074/jbc.M109.065813
Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N (2005) Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem 280:32081–32089. doi:10.1074/jbc.M502876200
Hamacher-Brady A, Brady NR, Gottlieb RA (2006) Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281:29776–29787. doi:10.1074/jbc.M603783200
Hands SL, Proud CG, Wyttenbach A (2009) mTOR’s role in ageing: protein synthesis or autophagy? Aging (Albany NY) 1:586–597
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395. doi:10.1038/nature08221
Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, Liu LF (2007) Autophagy regulates ageing in C. elegans. Autophagy 3:93–95
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18:1926–1945. doi:10.1101/gad.1212704
Iida RH, Kanko S, Suga T, Morito M, Yamane A (2011) Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol Cell Biochem 348:89–98. doi:10.1007/s11010-010-0642-z
Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, Shioi T (2009) Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120:1695–1703. doi:10.1161/CIRCULATIONAHA.109.871137
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296. doi:10.4161/auto.7.3.14487
Kanaan NM, Kordower JH, Collier TJ (2007) Age-related accumulation of Marinesco bodies and lipofuscin in rhesus monkey midbrain dopamine neurons: relevance to selective neuronal vulnerability. J Comp Neurol 502:683–700. doi:10.1002/cne.21333
Koldovsky O (1971) Developmental changes of beta-galactosidase and beta-glucuronidase in the rat liver and kidney. Arch Biochem Biophys 142:378–381
Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR (2003) Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem 278:39422–39427. doi:10.1074/jbc.M305371200
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD (1995) High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270:17513–17520
Lakatta EG (1999) Cardiovascular aging research: the next horizons. J Am Geriatr Soc 47:613–625
Li Q, Ceylan-Isik AF, Li J, Ren J (2008) Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res 11:725–733. doi:10.1089/rej.2008.0717
Li Q, Ren J (2007) Influence of cardiac-specific overexpression of insulin-like growth factor 1 on lifespan and aging-associated changes in cardiac intracellular Ca2+ homeostasis, protein damage and apoptotic protein expression. Aging Cell 6:799–806. doi:10.1111/j.1474-9726.2007.00343.x
Li Q, Wu S, Li SY, Lopez FL, Du M, Kajstura J, Anversa P, Ren J (2007) Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol 292:H1398–H1403. doi:10.1152/ajpheart.01036.2006
Ma H, Guo R, Yu L, Zhang Y, Ren J (2011) Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J 32:1025–1038. doi:10.1093/eurheartj/ehq253
Matarrese P, Nencioni L, Checconi P, Ciarlo L, Gambardella L, Ascione B, Sgarbanti R, Garaci E, Malorni W, Palamara AT (2011) Pepstatin a alters host cell autophagic machinery and leads to a decrease in influenza a virus production. J Cell Physiol. doi:10.1002/jcp.22696
Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A (2002) Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem 277:22896–22901. doi:10.1074/jbc.M200347200
Matsui T, Nagoshi T, Hong EG, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A (2006) Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Endocrinol Metab 290:E789–E797. doi:10.1152/ajpendo.00564.2004
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Miyamoto S, Murphy AN, Brown JH (2009) Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerg Biomembr 41:169–180. doi:10.1007/s10863-009-9205-y
Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings BA, Kass DA, Champion HC, Rosenzweig A (2005) PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115:2128–2138. doi:10.1172/JCI23073
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624. doi:10.1038/nm1574
Oudit GY, Penninger JM (2009) Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res 82:250–260. doi:10.1093/cvr/cvp014
Penna C, Tullio F, Perrelli MG, Moro F, Abbadessa G, Piccione F, Carriero V, Racca S, Pagliaro P (2011) Ischemia/reperfusion injury is increased and cardioprotection by a postconditioning protocol is lost as cardiac hypertrophy develops in nandrolone treated rats. Basic Res Cardiol 106:409–420. doi:10.1007/s00395-010-0143-y
Qian J, Ren X, Wang X, Zhang P, Jones WK, Molkentin JD, Fan GC, Kranias EG (2009) Blockade of Hsp20 phosphorylation exacerbates cardiac ischemia/reperfusion injury by suppressed autophagy and increased cell death. Circ Res 105:1223–1231. doi:10.1161/CIRCRESAHA.109.200378
Ronnebaum SM, Patterson C (2010) The FoxO family in cardiac function and dysfunction. Annu Rev Physiol 72:81–94. doi:10.1146/annurev-physiol-021909-135931
Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC (2009) Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ 16:46–56. doi:10.1038/cdd.2008.110
Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K (2011) Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50:117–127. doi:10.1016/j.yjmcc.2010.10.018
Simonsen A, Cumming RC, Brech A, Isakson P, Schubert DR, Finley KD (2008) Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy 4:176–184
Sussman MA, Anversa P (2004) Myocardial aging and senescence: where have the stem cells gone? Annu Rev Physiol 66:29–48. doi:10.1146/annurev.physiol.66.032102.140723
Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Shirasawa T, Mizushima N, Otsu K (2010) Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6:600–606. doi:10.4161/auto.6.5.11947
Toth ML, Sigmond T, Borsos E, Barna J, Erdelyi P, Takacs-Vellai K, Orosz L, Kovacs AL, Csikos G, Sass M, Vellai T (2008) Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4:330–338
Vettor R, Fabris R, Serra R, Lombardi AM, Tonello C, Granzotto M, Marzolo MO, Carruba MO, Ricquier D, Federspil G, Nisoli E (2002) Changes in FAT/CD36, UCP2, UCP3 and GLUT4 gene expression during lipid infusion in rat skeletal and heart muscle. Int J Obes Relat Metab Disord 26:838–847. doi:10.1038/sj.ijo.0802005
Wei JY (1992) Age and the cardiovascular system. N Engl J Med 327:1735–1739. doi:10.1056/NEJM199212103272408
Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA Jr (2007) Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res 10:281–292. doi:10.1089/rej.2006.0535
Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J (2006) Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. FASEB J 20:1024–1026. doi:10.1096/fj.05-5288fje
Yang X, Sreejayan N, Ren J (2005) Views from within and beyond: narratives of cardiac contractile dysfunction under senescence. Endocrine 26:127–137. doi:10.1385/ENDO:26:2:127
Yitzhaki S, Huang C, Liu W, Lee Y, Gustafsson AB, Mentzer RM Jr, Gottlieb RA (2009) Autophagy is required for preconditioning by the adenosine A1 receptor-selective agonist CCPA. Basic Res Cardiol 104:157–167. doi:10.1007/s00395-009-0006-6
Zhang C, Cuervo AM (2008) Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med 14:959–965. doi:10.1038/nm.1851
Zhang Y, Ren J (2010) Autophagy in ALDH2-elicited cardioprotection against ischemic heart disease: slayer or savior? Autophagy 6:1212–1213. doi:10.4161/auto.6.8.13652
Zhang Y, Zeng Y, Wang M, Tian C, Ma X, Chen H, Fang Q, Jia L, Du J, Li H (2011) Cardiac-specific overexpression of E3 ligase Nrdp1 increases ischemia and reperfusion-induced cardiac injury. Basic Res Cardiol 106:371–383. doi:10.1007/s00395-011-0157-0
Acknowledgments
The authors wish to acknowledge the technical assistance of Ms. Lisa Li from the University of Wyoming. This work was supported in part by NIH/NCRR P20 RR016474.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Hua and Y. Zhang contributed equally.
Rights and permissions
About this article
Cite this article
Hua, Y., Zhang, Y., Ceylan-Isik, A.F. et al. Chronic akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol 106, 1173–1191 (2011). https://doi.org/10.1007/s00395-011-0222-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-011-0222-8