Abstract
Background
This is the fourth updated Enhanced Recovery After Surgery (ERAS®) Society guideline presenting a consensus for optimal perioperative care in colorectal surgery and providing graded recommendations for each ERAS item within the ERAS® protocol.
Methods
A wide database search on English literature publications was performed. Studies on each item within the protocol were selected with particular attention paid to meta-analyses, randomised controlled trials and large prospective cohorts and examined, reviewed and graded according to Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system.
Results
All recommendations on ERAS® protocol items are based on best available evidence; good-quality trials; meta-analyses of good-quality trials; or large cohort studies. The level of evidence for the use of each item is presented accordingly.
Conclusions
The evidence base and recommendation for items within the multimodal perioperative care pathway are presented by the ERAS® Society in this comprehensive consensus review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Enhanced Recovery After Surgery (ERAS®) Society care pathways include evidence-based items designed to reduce perioperative stress, maintain postoperative physiological function and accelerate recovery after surgery. Using such a multimodal stress-minimising approach has been shown repeatedly to reduce rates of morbidity, improve recovery and shorten length of stay (LOS) after major colorectal surgery [1,2,3,4,5,6,7].
Since the first guidelines were published in 2005 [8], more colorectal operations are being performed using minimally invasive techniques. Furthermore, the evidence base underpinning all perioperative care items is in continuous development, which necessitates frequent updates of the knowledge base. This article represents the joint efforts of the ERAS® Society (www.erassociety.org) and authors from other international ERAS chapters to present an updated consensus review of perioperative care for colorectal surgery based on best current evidence.
Methods
Literature search
Starting from our previous guidelines in colon [9] and rectal [10] surgery published in 2013 the first and last author identified topics for inclusion. International authors known for their expertise in each item, respectively, and in overall perioperative care were invited to participate in the work. All invited authors accepted participation and received instructions for the literature search. PubMed, Embase and Cochrane databases were used to identify relevant contributions from January 2012 (end date for the search in the previously published guidelines [9]) and October 2017. Keywords included “colon”, “rectum”, “enhanced recovery”, “ERAS” and “fast track”. Meta-analyses randomised controlled trials (RCTs) and prospective/retrospective cohort studies were considered for each perioperative item. The individual authors screened titles and abstracts in order to identify relevant articles. The first and last author then repeated this procedure.
Quality assessment and data analyses
The Cochrane checklist [11] was used to assess methodological quality of the included studies. Quality of evidence and recommendations were evaluated according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. Quoting from the GRADE statement [12,13,14], the recommendations are given as follows:
Strong recommendations: The panel is confident that the desirable effects of adherence to a recommendation outweigh the undesirable effects.
Weak recommendations: The desirable effects of adherence to a recommendation probably outweigh the undesirable effects, but the panel is less confident.
Recommendations are based on quality of evidence (high, moderate, low) but also on the balance between desirable and undesirable effects; and on values and preferences of practitioners. Thus, strong recommendations may be reached from low-quality data and vice versa.
One or two authors covered the evidence base for each item. The quality of evidence for each item was then reviewed and crosschecked by several other authors in the author list.
Presentation
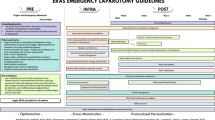
The evidence and recommendations for ERAS items are presented in four different headings: preadmission, preoperative, intraoperative and postoperative and are numbered in the order they are to be used in clinical practice. A summary figure (Figs. 1, 2, 3, 4) shows an overview of the quality of evidence and grade of recommendation for each phase of the perioperative course. Table 1 shows all the ERAS items.
Evidence base and recommendations
Preadmission items
See Fig. 1.
1. Preadmission information, education and counselling
Comprehensive preoperative counselling has several important goals. First, as patients fear the unknown, proper and complete information may reduce anaesthesia- and surgery-related anxiety and subsequent pain [15,16,17,18,19]. Secondly, the patient’s preparedness, satisfaction and overall surgical experience may be improved considerably by detailed, procedure-specific and patient-centred information giving sessions [20,21,22]. As a consequence of this psychological support, a positive impact of preoperative information on LOS and postoperative outcomes has been reported in an RCT and a Cochrane analysis [23, 24]. Modern education strategies including multimedia or virtual reality experiences may be considered [15, 25]. Patients and relatives/carers should meet with a multidisciplinary team comprising a surgeon, anaesthesiologist and most importantly a nurse or allied health professional, all whom have a role in guiding the patient through the surgery-related experience before admission to the hospital [26].
-
Summary and recommendation:
-
Patients should receive dedicated preoperative counselling routinely.
-
Quality of evidence: Moderate (study quality, heterogeneous endpoints)
-
Recommendation grade: Strong
2. Preoperative optimisation
Risk assessment
There are several examples of preoperative risk assessment scores proposed in the literature [27,28,29,30] but due to the low level of evidence of these scores, their use is limited. For instance, while it is generally believed that a multidisciplinary team should evaluate patients with a high risk of cardiac disease undergoing major surgery, the level of evidence for this intervention is very low [29]. While nutritional assessment and intervention seem to be useful for the high-risk malnourished patients, there is only one prospective study available [28]. For more general preoperative risk assessment tools, prospective data showing any effect on outcomes are lacking [27]. Most commonly tools describe control of systemic diseases such as optimisation of heart disease, lung disease, kidney disease, hypertension, diabetes, correction of derangements such as anaemia and malnutrition, and cessation of excessive alcohol use and smoking. This section refers to the latter two aspects, which are mainly under the control of the patient.
Smoking cessation
Patients who smoke have an increased risk of intra- and postoperative complications [31]. There are many methods of achieving smoking cessation in different subsets of patients, utilising pharmacologic versus behavioural therapy. In the preoperative setting, there are several meta-analyses [32,33,34] of preoperative smoking cessation, evaluating types of intervention and postoperative complications. In the preoperative setting, intense counselling and nicotine replacement therapy are most likely to be effective [33]. Although the optimal preoperative intervention, duration and intensity are unknown, 4–8 weeks of abstinence appear necessary to reduce respiratory and wound-healing complications [32, 34]. Even at the level of these meta-analyses, it is unclear whether short-term (< 4 weeks) smoking cessation reduces the risk of postoperative respiratory complications.
Avoiding Alcohol Abuse
Observational studies suggest that alcohol abuse increases postoperative morbidity [35, 36]. A systematic review and meta-analysis identified thirteen observational studies and five RCTs [37] and showed that consumption of more than two units (equal to a total of 50 ml spirits 40%, 150 ml wine 13%, 500 ml 4% beer or alcopop (a ready-mixed drink containing alcohol) of alcohol per day increases the rate of postoperative infections, but not mortality. In the same paper [37], a separate meta-analysis of the RCTs also confirmed that interventions to reduce alcohol intake reduce infections but not mortality. The impact on patients with lesser alcohol intake is unknown. Preoperative abstinence of 4 weeks is recommended [37]. Another review [38] found only two RCTs evaluating the effect of intensive alcohol cessation interventions (69 patients). Intensive preoperative alcohol cessation interventions, including pharmacological strategies for prophylaxis of relapse and withdrawal symptoms, may reduce postoperative complication rates significantly. No effect was found on mortality rates and LOS [38].
Summary and recommendation:
General preoperative medical assessment and optimisation is intuitively important, but for specified risk assessment tools, the evidence of their clinical accuracy remains low.
Smoking increases the risk of postoperative complications. Smoking should cease preoperatively for at least 4 weeks to reduce respiratory and wound-healing complications; shorter periods may still yield lesser benefits. Intense counselling and nicotine replacement therapy are most likely to be effective. Although meta-analyses show the impact of alcohol abuse on postoperative outcomes, only 2 small RCTs show a benefit of alcohol cessation on outcomes.
-
Quality of evidence:
-
Medical risk assessment: Low
-
Smoking: High
-
Alcohol: Low
-
Recommendation:
-
Risk assessment: Strong
-
Smoking: Strong
-
Alcohol: Strong
3. Prehabilitation
Poor preoperative physical status has been shown to be a risk factor for serious postoperative complications and prolonged disability [39]. The preoperative period may provide an opportunity to increase the physiologic reserve in the anticipation of surgery with the intention to improve outcomes and accelerate recovery. Therefore, preoperative optimisation or “prehabilitation” can be a compelling strategy to address modifiable risk factors that impact cancer treatment outcomes [40].
Prehabilitation is defined as “A process in the continuum of care that occurs between the time of diagnosis and the beginning of acute treatment (surgery, chemotherapy, radiotherapy) and includes physical, nutritional and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments” [41]. The initial introduction of prehabilitation programmes using intense exercise showed poor compliance and modest changes in postoperative functional capacity [42]. A follow-up RCT using multimodal structured prehabilitation protocols, which included aerobic and resistance exercises together with protein supplementation and relaxation strategies, demonstrated a positive impact on preoperative physiologic reserve with sustained levels of functional capacity after surgery [43]. In this study, more than 80% of patients who received the multimodal prehabilitation programme returned to baseline values of functional walking capacity by 8 weeks. In contrast, only 40% of patients who did not receive prehabilitation returned to baseline values. With regard to postoperative complications, one RCT demonstrated a 51% reduction in postoperative medical complications using a 4-week prehabilitation programme, thus showing an association between increase in preoperative aerobic capacity and reduction in complications [44].
Summary and recommendation:
Prehabilitation shows promising results in recovery of functional capacity and may reduce complications after colorectal surgery. Patients who are less fit may be more likely to benefit. Further research is required before considering this as a mandatory item in an ERAS protocol.
-
Quality of evidence:
-
Impact of multimodal prehabilitation to increase functional capacity: Moderate
-
Impact of multimodal prehabilitation on postoperative clinical outcome: Low
-
Recommendation: Prehabilitation: Weak
4. Preoperative nutritional care
Preoperative nutritional screening
Preoperative malnutrition has been associated with increased postoperative morbidity and mortality as well as poor oncologic outcomes in surgery for gastrointestinal cancer [45,46,47,48]. Preoperative nutritional assessment to detect overt or subtle malnutrition offers the opportunity to improve nutritional status and correct specific deficits [28]. There is no consensus on how to assess preoperative nutritional status accurately [49]. However, nutritional risk determined using the Nutritional Risk Screening score (NRS 2002) has been associated with increased risk of complications [50]. Preoperative serum albumin concentration has been suggested to be a risk factor of morbidity and mortality in two large studies [51, 52] and may be considered part of the preoperative nutritional assessment [53]. Several more comprehensive assessment tools both subjective and objectives have been proposed. Poor scores on the Subjective Global Assessment (SGA), the Patient Generated Subjective Global Assessment (PG-SGA) and the Malnutrition Universal Screening Tool (MUST) have been associated with both morbidity and mortality after major abdominal surgery and have been considered to be the reference standard for nutritional screening [54,55,56,57,58].
Preoperative nutrition
The risk of complications is increased in patients with unintentional weight loss of 5–10% or more [59], and patients with higher nutritional risk benefit from preoperative nutritional treatment [28]. For malnourished patients, oral nutritional supplementation (or additional parenteral nutrition when indicated) has the best effect if started 7–10 days preoperatively and is associated with a reduction in the prevalence of infectious complications and anastomotic leaks [60].
Summary and recommendation:
Preoperative routine nutritional assessment offers the opportunity to correct malnutrition and should be offered. Preoperatively, patients at risk of malnutrition should receive nutritional treatment preferably using the oral route for a period of at least 7–10 days.
-
Quality of evidence:
-
Preoperative screening: Low
-
Preoperative nutrition: Moderate
-
Recommendation grade:
-
Preoperative screening: Strong
-
Preoperative nutrition: Strong
5. Management of Anaemia
The World Health Organisation definition of anaemia is a haemoglobin (Hb) concentration of < 130 g/L for men and < 120 g/L for women but recently it has been proposed that women should be considered anaemic if Hb < 130 g/L as most attain this figure if not iron deficient [61, 62]. Twenty-five percentage of women with subnormal Hb (120 g/L) are iron deficient [63]. This has significant implications for the potential to restore haemoglobin rapidly through haemopoiesis after blood loss. Anaemia is common in patients presenting for surgery. In a large study with data reported from all surgical specialties showed a prevalence of 31.1% in men and 26.5% in women [64]. Patients scheduled for surgery may have many factors causing anaemia: acute or chronic blood loss; vitamin B12 or folate deficiency; anaemia of chronic disease related or unrelated to their reason for surgery, or a combination of these [63]. All causes of anaemia should be investigated appropriately and corrected. Most patients presenting for colorectal surgery will have iron deficiency because of blood loss or chronic inflammation [62].
Anaemia—Risks of Complications & Mortality
Anaemia may be a risk factor for all complications and mortality [64, 65]. However, the administration of blood products peri-operatively may also increase complications and have a long-term impact on survival in patients with colorectal cancer [66]. One retrospective series of 23,388 patients undergoing colorectal surgery showed that 7.9% of patients received blood transfusions. Statistically, there was no increase in superficial or deep wound infection but there was an increase in organ space surgical site infection and septic shock [67]. In elective orthopaedic surgery, transfusion of blood products increased 4-year mortality by 10% [65]. In liver resection for metastatic colorectal cancer blood transfusion is an independent risk for poor short and long-term outcomes [68, 69]. It is therefore essential to optimise the patient’s Hb concentration preoperatively. The time frame to do this will vary according to the indication and urgency for surgery and how rapidly blood loss is occurring.
Optimal Perioperative Haemoglobin targets
Significant perioperative blood loss can lead to the question of whether to transfuse blood products. The American Society of Anaesthesiologists recommend that a minimum Hb concentration of 60–100 g/L is maintained through the perioperative period individualised to a patient depending on their comorbidities and type of surgery [70]. Patients with, cardiac, renal and pulmonary problems are at higher risk as haemoglobin declines acutely and in these groups a target Hb of > 80 g/L may be better to avoid complications.
Preoperative Interventions to increase haemoglobin
Anaemia of Chronic Disease
In anaemia of chronic disease, such as that encountered in inflammatory bowel disease, the iron regulatory protein hepcidin is activated in response to inflammation. It inhibits absorption of iron from the gastrointestinal tract and reduces bioavailability of iron stores for red cell production in the marrow, making oral iron therapy not very effective. Intravenous iron infusions can overcome this problem in some instances [63].
Oral Iron Therapy
Oral iron is cheap and administered easily but may be tolerated poorly. Absorption of iron may be better by using lower doses between the range of 40–60 mg per day or alternate day with 80–100 mg [63]. Many colorectal surgical patients will either not respond to oral iron due to chronic illness or because of ongoing blood loss. Intravenous iron infusion may be worth considering in this group and is discussed below.
Intravenous Iron Infusions
There are now several different iron infusions available in clinical practice with a low serious adverse reaction rate of 38 incidents per million episodes of administration [71]. Acute reactions are normally mediated via complement activation due to nanoparticles rather than an IgE-mediated response [72]. Timing of and the number of infusions depends on the urgency of surgery; 1–1.5 g usually restores iron stores back to normal and can be given in single or divided doses. One study reports a mean Hb increase of 8 g/L over 8 days following IV ferric carboxymaltose 15 mg/kg, max 1000 mg, given as a single dose over 15 min [73]. A reticulocytosis occurs at 3–5 days after administration. The addition of erythropoietin is not recommended. Timing of infusions and effectiveness in different colorectal populations has still to be determined by large-scale studies although the preoperative target of 130 g/L should be pursued. Serum ferritin concentration < 30 μg/L is the most sensitive and specific test used for the identification of absolute iron deficiency. However, in the presence of inflammation (C-reactive protein > 5 mg/L) and/or transferrin saturation < 20%, a serum ferritin concentration < 100 μg/L is indicative of iron deficiency [62].
Summary and recommendation:
Anaemia is common in patients presenting for colorectal surgery and increases all cause morbidity. Attempts to correct it should be made prior to surgery. Newer preparations of intravenous iron have a low risk of adverse reactions and are more effective than oral iron at restoring haemoglobin concentrations in both iron deficiency anaemia and anaemia of chronic disease. Blood transfusion has long-term effects and should be avoided if possible.
-
Quality of evidence: Screening and treatment of anaemia before surgery: High
-
Recommendation: Strong
-
Quality of evidence: Using a restrictive blood transfusion practice: High
-
Recommendation: Strong
Preoperative items
See Fig. 2.
6. Prevention of nausea and vomiting (PONV)
The prevention of postoperative nausea and vomiting (PONV) is fundamental for patients undergoing colorectal surgery. PONV when severe may result in dehydration, delayed return of adequate nutrition intake, or may require the placement of a nasogastric tube, increase intravenous fluid administration postoperatively, prolong hospital stay, and increase healthcare costs.
PONV affects 30% (vomiting) to 50% (nausea) of all surgical patients and up to 80% of patients who are at high risk for developing these complications [74]. It is also a leading cause of patient dissatisfaction [75]. The aetiology of postoperative nausea and vomiting is multifactorial and is generally divided into patient-related, anaesthesia-related and surgery-related factors [76]. Female gender, those with a past history of PONV or motion sickness and non-smokers, are at particular risk [77]. Volatile anaesthetic gases, nitrous oxide (both of which can be mitigated in part by the use of total intravenous anaesthesia (TIVA) with propofol) and the liberal use of opioids increase the risk significantly [78]. The type and duration of surgery and the gastrointestinal pathology are also important. While opioid use cannot necessarily be avoided, analgesia is best provided by opioid-sparing multimodal techniques. Some studies suggest that carbohydrate loading may also reduce PONV [79].
Several scoring systems have been described for the prediction of PONV, with simpler ones appearing to provide better discrimination [80]. The most commonly used are the Koivuranta score and Apfel’s simplification of this score. These scores are useful when combined with specific therapeutic interventions, especially in high-risk patients [81, 82]. An alternative strategy employed in many practices but not yet studied may be to administer antiemetic prophylaxis (between one and three medications) to all patients who are having inhalational anaesthesia, opiates or major abdominal surgery. This approach is gaining popularity among anaesthetists given that the cost and side-effect profiles of commonly used antiemetic drugs are small [83].
There are several classes of first-line antiemetic drugs, including dopamine (D2) antagonists (e.g. droperidol), serotonin (5HT3) antagonists (e.g. ondansetron) and corticosteroids (e.g. dexamethasone). In one study of 5199 patients, when these classes of drugs were given individually, they were demonstrated to contribute a relative risk reduction of about 25% [84], while multimodal administration of antiemetic drugs reducing PONV even further [85]. If rescue PONV treatment is required, a different class of antiemetic should be administered than the one administered for prophylaxis [74]. For dexamethasone, the dose administered may vary, but a recent meta-analysis with 6696 patients showed that a 4–5 mg dose had clinical effects similar to the 8–10 mg dose [86]. The use of dexamethasone for open or laparoscopic bowel surgery was further confirmed in the recently published Dexamathasone Reduces Emesis After Major Gastrointestinal Surgery (DREAMS) Trial in which 1350 patients were studied. A single 8 mg dose of dexamethasone reduced PONV at 24 h and reduced the need for rescue antiemetics for up to 72 h, without an increase in adverse events [87]. However, the immunosuppressive effects of dexamethasone on long-term oncological survival are still unknown. Other, second-line drugs, such as antihistamines (e.g. promethazine), anticholinergics (e.g. scopolamine) and other D2 antagonists such as metoclopramide may also be used, but their use may be limited by common side effects such as sedation, dry mouth, blurred vision and dyskinesia.
More recently, the use of preoperative administration of gabapentin and pregabalin has been examined for a range of operations. Recent meta-analyses confirm that both drugs significantly reduce nausea and vomiting, although there is a significantly increased risk of visual disturbance (pregabalin) [88] and sedation (gabapentin and pregabalin) [89]. A neurokinin-1 (NK1) receptor antagonist (e.g. aprepitant) may be used in high-risk patients, although it has not been shown to be superior to ondansetron in PONV prevention [90].
In addition, the use of prophylactic analgesia such as intravenous paracetamol (acetaminophen) (i.e. before the onset of pain) in a meta-analysis of 2364 patients reduced the incidence of nausea and correlated with the reduction in pain [91]. There is also some evidence for the use of alternative therapies to reduce PONV, which include music therapy, aromatherapy, acupuncture, hypnosis and relaxation techniques [92]. Finally, there are also reports of a small beneficial effect of high-inspired oxygen concentration on reducing the incidence of nausea [93], although one meta-analysis show no benefit of the treatment [94].
Summary and recommendation:
A multimodal approach to PONV prophylaxis should be considered in all patients and incorporated into ERAS protocols. Patients with 1–2 risk factors should ideally receive a two-drug combination prophylaxis using first-line antiemetics. Patients with ≥ 2 risk factors undergoing colorectal surgery should receive 2–3 antiemetics. If nausea and or vomiting still occur, despite prophylaxis, salvage therapy should be provided using a multimodal approach using different classes of drugs from those used for prophylaxis.
-
Quality of evidence:
-
Multimodal PONV prophylaxis: High
-
PONV rescue with different class of antiemetic: High
-
Recommendation grade: Strong
7. Pre-anaesthetic medication
Psychological distress (pre- and postoperative anxiety) may increase perioperative analgesic requirements [95] and postoperative complication rates [96]. Given that high levels of anxiety occur days prior to hospital admission, and only in a minority of patients peaks on the day of surgery, it is imperative that anxiolytic strategies are employed that exceed the mere administration of anxiolytic-sedatives (benzodiazepines) in the immediate preoperative period. Effective communication strategies, including attending a preoperative educational session (‘Surgery School’) with information for patients on the intent of ERAS pathways, can successfully reduce patient anxiety and improve their perioperative experience [18].
The adverse side effects of drugs, such as benzodiazepines, opioids or beta-blockers, can limit their use as anxiolytic pre-anaesthetic medications [97]. In particular, benzodiazepines, even after single-dose administration, may cause psychomotor and cognitive impairment and exhibit sedative effects. The American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication (PIM) use in older patient populations (aged 65 years and older) [98] provide a strong recommendation, with moderate quality of evidence, that due to their increased sensitivity to all benzodiazepines and due to their decreased metabolism of long-acting agents, that benzodiazepines should be avoided in older patients where possible to offset the risk of cognitive impairment, delirium and falls. While there are data against the use of pre-medication especially in the elderly, studies from day surgery report minimal impact on time to discharge, less nausea and headaches with the use of lower doses of benzodiazepines. However, there remains a risk of impaired motor function in higher doses [99, 100].
Given the disadvantages of benzodiazepines, alternate anxiolytics should be explored when pre-anaesthetic medication is needed. A meta-analysis, with high-grade quality of evidence, reported that melatonin (tablets or sublingually) provided effective preoperative anxiolysis with few side effects compared with placebo; with low-grade quality of evidence that melatonin is equally effective to midazolam and that melatonin may also provide postoperative anxiolysis [101].
Pre-anaesthetic medication can also be employed as part of the ERAS strategy to achieve multimodal, opioid-sparing analgesia to decrease opioid-related adverse effects (e.g. nausea, vomiting, sedation, ileus and respiratory depression) and to expedite recovery after surgery. Pre-anaesthetic medication may therefore include a combination of paracetamol, a NSAID and a gabapentinoid (such as gabapentin and pregabalin, originally used for the treatment of chronic neuropathic pain). Paracetamol, NSAIDS and gabapentinoids administered as oral formulations prior to surgery are very cost-effective. All should be age and dose adjusted. It is important that the timing of dosing should achieve an optimal pharmacodynamic effect that coincides with the onset of surgery to ensure a maximal multi-modal opioid-sparing effect.
Gabapentinoids are only available in an oral form and are increasingly used as oral pre-anaesthetic medications for their opioid-sparing effects. Meta-analyses indicate that a single dose of gabapentin or pregabalin, administered preoperatively, associates with decreased postoperative pain and opioid consumption; however, these benefits are offset by increased postoperative sedation, dizziness and visual disturbances [102, 103]. All doses of pregabalin (≤ 75, 100–150 and 300 mg) resulted in an opioid sparing at 24 h after surgery. Importantly, there were no significant differences in acute pain outcomes between single preoperative dosing regimens and those including additional doses repeated after surgery [103]. To limit the adverse effects, including synergistic effects with opioids, sedation, dizziness and peripheral oedema, gabapentinoid dosing should be limited to a single and lowest preoperative dose, unless indicated for postoperative neuropathic pain. In elderly patients and patients with renal dysfunction the dose of these agents should be adjusted accordingly and be used with further caution.
Summary and recommendation:
Preoperative education can reduce patient anxiety to an acceptable level without the need for anxiolytic medication. Pharmacologic anxiolysis with long- or short-acting sedative medication (especially benzodiazepines and especially in the elderly) should be avoided if possible before surgery. Opioid-sparing multimodal re-anaesthetic medication can be used with a combination of acetaminophen, NSAIDS and [70] gabapentanoids. All should be dose adjusted according to age and renal function. Gabapentinoids should preferably be limited to a single lowest dose to avoid sedative side effects.
-
Quality of Evidence:
-
Avoiding routine sedative medication: Moderate
-
Recommendation grade: Strong
8. Antimicrobial prophylaxis and skin preparation
A Cochrane review published in 2014 underpinned the mandatory use of oral or intravenous antibiotic prophylaxis before colorectal surgery with a consecutive reduction of surgical site infections (SSI) from 39 to 13% [104]. Standard oral or intravenous antibiotics covering aerobic and anaerobic bacteria was the preferred option, with current preference for a cephalosporin in combination with metronidazole. Intravenous antibiotic prophylaxis should be administered within 60 min before incision. No benefit was shown for repeated administration [104, 105]. These conclusions are made on studies where patients are treated with bowel preparation.
Addition of oral antibiotic decontamination to preoperative intravenous antibiotics is an ongoing controversy. The additional benefits of administering oral antibiotics, which are usually given 18–24 h before surgery, are attributed to its possible local effects of inhibiting opportunistic pathogens in the colonic lumen before opening the colon, however, with a potential pitfall to disturb the gastrointestinal microbiota. The addition of oral antibiotics to intravenous administration in patients with bowel preparation was shown to reduce the risk for surgical site infections when compared with intravenous coverage alone [RR 0.56 (0.43, 0.74]) or oral alone [RR 0.56 (0.40–0.76)] [104]. These results were confirmed in a recent meta-analysis [106] where SSI was significantly reduced in patients who received oral and systemic antibiotics and mechanical bowel preparation compared with patients who received systemic antibiotics alone with mechanical bowel preparation. Similarly, retrospective registry data from the USA suggested largely reduced SSI rates in patients having both, mechanical bowel preparation in combination with oral antibiotics alone [107]. However, oral antibiotic decontamination alone in patients with no bowel preparation has not been studied and any potential effect remains unknown. Also, it remains unknown if the triple combination of intravenous antibiotics, oral decontamination and bowel preparation is superior to only intravenous prophylaxis and bowel without preparation.
For skin decontamination, a randomised trial in colorectal surgery and a recent meta-analysis of 13 RTCs (6997 patients) suggested lower incidence of SSI after preoperative antisepsis using chlorhexidine [108, 109]. In contrast, available evidence does not support the practice of preoperative antiseptic shower or adhesive drapes [110, 111]. Lastly, routine hair removal before surgery does not reduce SSI rates, but should be preferably performed—if deemed necessary—by use of clippers rather than razors immediately before surgery [112].
-
Summary and recommendation:
-
Intravenous antibiotic prophylaxis should be given within 60 min before incision as a single-dose administration to all patients undergoing colorectal surgery. In addition, in patients receiving oral mechanical bowel preparation, oral antibiotics should be given. No recommendation for the use of oral antibiotic decontamination can be given for patients having no bowel preparation. Skin disinfection should be performed using chlorhexidine–alcohol-based preparations. Evidence is insufficient to support advanced measures such as antiseptic showering, routine shaving and adhesive incise sheets.
-
Quality of evidence:
-
Intravenous antibiotic prophylaxis: High
-
Oral antibiotic decontamination: Low
-
Chlorhexidine–alcohol-based skin preparation: High
-
Advanced measures for skin decontamination: Low
-
Patients undergoing resections receiving MBP: Oral and intravenous prophylaxis: Low
-
Recommendation grade:
-
Intravenous antibiotic prophylaxis: Strong
-
Oral antibiotic decontamination: Weak
-
Chlorhexidine–alcohol-based skin preparation: Strong
-
Advanced measures for skin decontamination: Weak
-
Patients undergoing resections receiving MBP: Oral and intravenous prophylaxis: Weak
9. Bowel preparation
In previous ERAS guidelines in colon [9] and rectum [10] surgery, given the universal use of systemic antibiotic prophylaxis, the recommendation has been to avoid the use of mechanical bowel preparation (MBP) in colonic surgery but that it may be advantageous in rectal surgery. The rationale behind this is to avoid preoperative dehydration, electrolyte disturbance and discomfort with no clinical gain for the patient [113].
The role of MPB alone has been evaluated in a meta-analysis of 36 studies comparing adult patients receiving MBP versus with those receiving no MBP [114]. A total of 21,568 patients undergoing elective colorectal surgery were included from 23 RCTs and 13 observational studies. When all studies were considered, MBP versus no MBP was not associated with any significant difference in anastomotic leak rates (OR 0.90, 95% CI 0.74 to 1.10), surgical site infection (OR 0.99, 95% CI 0.80 to 1.24), intra-abdominal collection (OR 0.86, 95% CI 0.63 to 1.17), mortality (OR 0.85, 95% CI 0.57 to 1.27), reoperation (OR 0.91, 95% CI 0.75 to 1.12) or hospital LOS (overall mean difference 0.11 days, 95% CI − 0.51 to 0.73), when compared with no MBP, nor when evidence from RCTs only were analysed. A sub-analysis of MBP versus absolutely no preparation or a single rectal enema similarly revealed no differences in clinical outcomes. Still, in rectal surgery, a diverting stoma is often used and this may be a reason for MBP or an enema to avoid stools remaining in the diverted colon.
Recently the avoidance of MBP has been questioned mainly because of data from retrospective cohort and large database studies from the USA, indicating that the combination of oral antibiotic preparation together with systemic antibiotics and MBP reduces morbidity after colorectal surgery compared with MBP and systemic antibiotics alone [115], but also compared with patients who received no bowel preparation but systemic antibiotics alone [116]. These findings are also supported by a meta-analysis of 1769 patients in randomised trials [106]. Much of these new data have been derived from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP®) targeted colectomy database, with a likely degree of cross-reporting of patient populations. A recent meta-analysis of 23 randomised controlled trials and 8 cohort studies [117] including a total of 63,432 patients undergoing elective colorectal surgery demonstrated that systemic antibiotic used alone was associated with a significant reduction in surgical site infection versus oral antibiotics alone [Odds Ratio (OR) 1.82, 95% CI 1.28 to 2.59], although the combination of oral and systemic antibiotics was superior to oral antibiotics alone (OR 0.44, 95% CI 0.33 to 0.58). The addition of oral antibiotic preparation to MBP in the setting of systemic antibiotics significantly reduced the incidence of surgical site infection (RR 0.48, 95% CI 0.44 to 0.52). However, when studies comparing oral antibiotic preparation and systemic antibiotic versus MBP and systemic antibiotic were compared, no significant difference was seen in the incidence of surgical site infection (RR 0.94, 95% CI 0.73 to 1.20).
The largest observational study to date arising from the ACS NSQIP database [118] included 40,446 patients, with 13,219 (32.7%), 13,935 (34.5%), and 1572 (3.9%) in the no-preparation, mechanical bowel preparation alone, and oral antibiotic preparation alone groups, respectively, and 11,720 (29.0%) in the combined preparation group. Conditional logistic regression following patient matching, oral antibiotic preparation alone was protective of surgical site infection (OR, 0.63; 95% CI, 0.45–0.87), anastomotic leak (OR, 0.60; 95% CI, 0.34–0.97), ileus (OR, 0.79; 95% CI, 0.59–0.98) and major morbidity (OR, 0.73; 95% CI, 0.55–0.96), but not mortality (OR, 0.32; 95% CI, 0.08–1.18). Combined oral antibiotics and MBP conveyed no benefit in any major outcome over oral antibiotics alone in this study. However, to date no RCTs have been performed to support this observation, and as such, further high-quality evidence is necessary to inform the debate.
-
Summary and recommendation:
-
Mechanical bowel preparation alone with systemic antibiotic prophylaxis has no clinical advantage and can cause dehydration and discomfort and should not be used routinely in colonic surgery, but may be used for rectal surgery. There is some evidence from randomised controlled trials to support the use of a combination of MBP and oral antibiotics over MBP alone.
-
MBP Alone:
-
Quality of evidence: High
-
Recommendation grade: Strong
-
Combined MBP and oral antibiotic preparation:
-
Quality of evidence: Low
-
Recommendation grade: Weak
10. Preoperative fluid and electrolyte therapy
It is imperative that the patient should reach the anaesthetic room in as close a state to euvolaemia as possible and any preoperative fluid and electrolyte excesses or deficits must be corrected. Pre-existing comorbidities must be taken into account when assessing fluid status. Avoidance of prolonged preoperative fasting, provision of clear liquids (including carbohydrate drinks) for up to 2 h prior to the induction of anaesthesia and avoidance of mechanical bowel preparation help reduce the incidence of preoperative fluid and electrolyte deficits and substantially reduced intraoperative fluid requirements. However, when mechanical bowel preparation is indicated, patients may lose up to 2 L of total body water as a consequence [113], and fluid and electrolyte derangements may occur even if patients are permitted oral fluids. Hence, some of these patients may require appropriate intravenous fluid therapy to compensate for these deficits and improve outcome [119].
-
Summary and recommendation: Patients should reach the anaesthetic room in as close a state to euvolaemia as possible and any preoperative fluid and electrolyte excesses or deficits should be corrected.
-
Quality of evidence: Moderate
-
Recommendation grade: Strong
11. Preoperative fasting and carbohydrate loading
Several RCTs have demonstrated that non-alcoholic clear fluids can be safely given up to 2 h, and a light meal up to 6 h, before elective procedures requiring general anaesthesia, regional anaesthesia or procedural sedation and analgesia in children and adults [120,121,122].
Preoperative administration of oral carbohydrates (complex CHO-maltodextrin, 12.5%, 285 mOsm/kg, 800 ml in the evening before surgery and 400 ml 2–3 h before induction of anaesthesia) has been shown to attenuate the catabolic response induced by overnight fasting and surgery [123]. CHO in RCTs has been shown to improve preoperative well-being, reduce postoperative insulin resistance, decrease protein breakdown and better maintain lean body mass and muscle strength, as well as beneficial cardiac effects. In a recent large RCT in 880 patients undergoing elective major abdominal surgery, oral CHO administration resulted in lower insulin requirements and less hyperglycaemia (> 180 mg/dl) compared with placebo [124]. Another recent RCT in coronary artery bypass patients, reported that CHO significantly reduced myocardial injury [125].
Faster surgical recovery and better postoperative well-being from CHO still remains controversial, while few data so far support an effect on postoperative morbidity or mortality from this treatment. In a recent Cochrane Review, 27 trials involving 1976 participants were included [126]. Trials were performed in Europe, China, Brazil, Canada and New Zealand and involved patients undergoing elective minor and major abdominal surgery, orthopaedic surgery, cardiac surgery and thyroidectomy. Overall, the administration of preoperative carbohydrate was associated with a small reduction in hospital stay (MD − 0.30 days, 95% CI − 0.56 to − 0.04) compared with the placebo or fasting group. Patients undergoing major abdominal surgery had a greater absolute decrease in LOS (MD − 1.66 days, 95% CI − 2.97 to − 0.34). However, the heterogeneity observed in average LOS, and the variation in study quality makes the interpretation of these results difficult.
Based on two trials including 86 participants, preoperative carbohydrate treatment was also, in this review, associated with shortened time to passage of flatus when compared with placebo or fasting, as well as increased postoperative peripheral insulin sensitivity.
Oral fluids including CHOs may not be administered safely in patients with documented delayed gastric emptying or gastrointestinal motility disorders as well as in patients undergoing emergency surgery. Although gastric emptying has been reported previously to be normal in obese patients [127], diabetics when given with their normal diabetic medication [128], and elderly patients with acute hip fracture [129], studies are still too small and incomplete to allow routine to recommendation of this intervention in such patients. However, both obese and diabetic patients have been increasingly included in recent studies of CHO [130] and no issues with regard to safety have been reported.
-
Summary and recommendation:
-
Patients undergoing elective colorectal surgery should be allowed to eat up until 6 h and take clear fluids including CHO drinks, up until 2 h before initiation of anaesthesia. Patients with delayed gastric emptying and emergency patients should remain fasted overnight or 6 h before surgery. No recommendation can be given for the use of CHO in patients with diabetes.
-
Quality of evidence:
-
In elective colorectal surgery in patients without delayed gastric emptying; 6-h fasting for solids and 2 h for clear fluids including CHO drinks: High
-
CHO drinks improving well-being, insulin resistance: Moderate
-
CHO drinks reducing complications and improving recovery time: Low
-
Recommendation grade: Adherence to fasting guidelines (avoid overnight fasting): Strong
-
Administration of preoperative CHOs: Strong
-
Administration of preoperative CHOs in well-controlled diabetic and obese patients: weak
Intraoperative items
See Fig. 3.
12. Standard Anaesthetic Protocol
Anaesthetic agent and Cerebral Function Monitoring
The avoidance of benzodiazepines and use of short-acting general anaesthetic agents in an opioid-sparing ERAS Pathway allow rapid awakening with minimal residual effects. Propofol for induction of anaesthesia, combined with short-acting opioids such as fentanyl, alfentanil, sufentanil or remifentanil infusions, if opioids are required, minimises residual effects at the end of anaesthesia. There are no strong data to support the recommendation of either anaesthetic gases or total intravenous anaesthesia (TIVA) using propofol infusions to maintain anaesthesia. The use of propofol TIVA may reduce PONV in certain patients and there are data from a large retrospective study suggesting a beneficial effect of propofol on cancer outcomes, but no definitive recommendation can be made for this currently [131]. In intubated patients under general anaesthesia, using short-acting inhalational agents such as sevoflurane or desflurane in oxygen-enriched air is standard practice around much of the world [132]. Nitrous oxide is normally avoided due to its delaying effects on the bowel although the increased risk of PONV can be markedly reduced with standard PONV prophylaxis [133].
Cerebral Function Monitoring using bi-spectral index (BIS) and maintaining a target between 40 and 60 can reduce the risk of awareness in high-risk patients [134]. The use of BIS or newer burst suppression monitoring to avoid overdose of anaesthesia in the elderly may have a role in reducing the risk of postoperative delirium and postoperative cognitive dysfunction in this patient population [135].
Muscle relaxation and Neuromuscular Monitoring
Laparoscopic and robotic surgery requires insufflation of the peritoneum to create space for operating. High intra-abdominal pressure can worsen cardiac function, impede ventilation and reduce renal blood flow [136]. There is some evidence in certain patients suggesting that maintaining muscle relaxation of the abdominal muscles (a term called ‘deep block’) may allow operating at lower pressure while maintaining intra-abdominal space for surgery [137]. Reducing the intra-abdominal pressure below 10–12 mmHg may result in a reduction in the physiological effects of pneumoperitoneum leading to a reduction in aortic afterload, improvement in renal blood flow and lower peak airway ventilator pressures [138].
There is evidence to support that cumulative dosing of intermediate muscle relaxants increases the risk of postoperative pulmonary complications [139]. Neuromuscular monitoring should be a standard of care with acceleromyography (objective monitoring) being more accurate than basic peripheral nerve stimulators. Reversal of neuromuscular block to a train-of-four (TOF) ratio of 90% is important to avoid residual paralysis and risk of postoperative pulmonary complications [140]. Sugammadex reverses rocuronium and vecuronium rapidly and predictably by encapsulating the molecules responsible for paralysis. Neostigmine is an alternative option for reversal due to its anticholinergic effect. If correctly dosed, sugammadex reduces the risk of residual neuromuscular block [141].
-
Summary and recommendation:
-
The use of short-acting anaesthetics, cerebral monitoring to improve recovery and reduce the risk for postoperative delirium, monitoring of the level and complete reversal of neuromuscular block is recommended.
-
Quality of evidence: Short-acting anaesthetics: Low
-
Recommendation grade: High
-
Quality of evidence: Use of Cerebral Monitoring: High
-
Recommendation grade: Strong
-
Quality of evidence: Reducing intra-abdominal pressure during laparoscopic surgery facilitated by neuromuscular block: Low
-
Recommendation grade: Weak
-
Evidence: Monitoring (objective) the level and complete reversal of neuromuscular block: High
-
Recommendation grade: Strong
13. Intraoperative fluid and electrolyte therapy
The aim of intravenous fluid therapy is to maintain intravascular volume, cardiac output and tissue perfusion while avoiding salt and water overload. Most patients require crystalloids at a rate of 1–4 ml/kg/h to maintain homoeostasis [142]. However, some patients require volume therapy where goal-directed boluses of intravenous solutions (usually a colloid) aimed at maintaining central normovolaemia by utilising changes in stroke volume measured by a minimally invasive cardiac output monitor to optimise the patients on their individual Frank–Starling curve [143, 144]. Fluids are administered to treat objective evidence of hypovolaemia, and consequently improve intravascular volume and circulatory flow [145]. Although the earlier studies on goal-directed fluid therapy (GDFT) showed a significant improvement in outcome in terms of reduction in complications, shorter duration of ileus and reduced LOS, more recent studies performed within the context of enhanced recovery programmes showed no difference in outcome [146,147,148]. Using this concept of GDFT in the setting or conventional care versus enhanced recovery protocols, a recent meta-analysis that included 23 studies with 2099 patients has generated interesting results [149]. Overall, GDFT was associated with a significant reduction in morbidity, LOS, intensive care LOS and time to passage of faeces. However, no difference was seen in mortality, return of flatus or risk of paralytic ileus. If patients were managed within enhanced recovery pathways, the only significant reductions were in intensive care LOS and time to passage of faeces. If managed in a traditional care setting, a significant reduction was seen in both overall morbidity and total hospital LOS. Hence, within ERAS programmes, it may not be necessary to offer all patients GDFT, and this should be reserved, after risk stratification, for high-risk patients or for patients undergoing high-risk procedures [142]. Arterial hypotension should be treated with vasopressors when administering intravenous fluid boluses fails to improve the stroke volume significantly (stroke volume > 10%) [150, 151]. Inotropes should be considered in patients with reduced contractility (cardiac index < 2.5 L/min) to achieve adequate oxygen delivery [151].
-
Summary and recommendation: The goal of perioperative fluid therapy is to maintain fluid homoeostasis avoiding fluid excess and organ hypoperfusion. Fluid excess leading to perioperative weight gain more than 2.5 kg should be avoided, and a perioperative near-zero fluid balance approach should be preferred. GDFT should be adopted especially in high-risk patients and in patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift). Inotropes should be considered in patients with poor contractility (CI < 2.5 L/min).
-
Quality of evidence:
-
Perioperative near-zero fluid balance: High
-
GDFT: High
-
Recommendation grade:
-
GDFT: Strong in high-risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift)
-
GDFT: Weak in low-risk patients and in patients undergoing low-risk surgery
-
Zero fluid balance: Strong
-
Use of advanced haemodynamic monitoring: strong in high-risk patients and for patients undergoing surgery with large intravascular fluid loss (blood loss and protein/fluid shift)
14. Preventing intraoperative hypothermia
The importance of maintaining normothermia in patients (a temperature of 36 °C or over) undergoing major surgery including colorectal surgery is well recognised [152]. Both general anaesthesia and neuroaxial anaesthesia affect thermoregulation by impairing vasoconstriction and shivering, causing temperature redistribution from the core to the periphery, leading to heat loss in excess of heat production [153]. Even mild inadvertent perioperative hypothermia (IPH) has been associated with adverse effects: in a meta-analysis with a median temperature of 35.6 °C, blood loss was increased by 16% and blood transfusion rate by 22% [154]. Other effects may include vasoconstriction, increased afterload, myocardial ischaemia and cardiac arrhythmias, reduction in splanchnic blood flow and reduced drug biotransformation. The problems extend well into the postoperative period too, where there may be shivering with a concomitant increase in oxygen consumption, a prolonged stay in the post-anaesthetic care unit (PACU), an increase in rates of infection and a prolonged hospital stay. Patients at higher risk of IPH or its sequalae include ASA 2-5, preoperative hypothermia, those undergoing combined regional and general anaesthesia, major surgery and those at risk of cardiovascular complications [155].
Accurate measurement of temperature is fundamental. Core temperature measurements are best carried out directly (or using a direct estimate) rather than using indirect estimate. Various methods are used such as nasopharyngeal measurement (with the probe inserted 10–20 cm) [153]. More recently the zero heat-flux (deep forehead) thermometry is also recommended [155] and has been the subject of a separate recent review, with over 500 patients from 7 studies confirming its reliability [156]. There are many methods described to conserve body temperature, including warming and humidification of anaesthetic gases, warming IV and irrigation fluids and forced air warming blankets and devices. In addition, the ambient temperature should be at least 21 °C while the patient is exposed prior to active warming starting [155].
While heat loss in laparoscopic surgery is reduced when compared with open surgery, hypothermia may still occur due to cold, dry carbon dioxide used for insufflation. A recent meta-analysis analysed 13 studies and demonstrated that the use of warmed and humidified CO2 was associated with a significant increase in intraoperative core temperature (mean change 0.3 °C) [157]. However, a Cochrane review looked at 22 studies with 1428 participants, and while confirming the above preservation of temperature and demonstrating a reduced post-anaesthesia care unit (PACU) stay, commented that the data were heterogeneous and when low risk of bias studies only were included, the PACU stay was not significantly reduced. Overall there was no improvement in patient outcome, reduction in lens fogging, etc., and thus its use was not supported [158].
Another area to minimise IPH is the use of prewarming. Recent reviews supported this with significantly higher temperatures perioperatively [159, 160] unless this would delay emergency surgery, although the practicalities of this may not be easy to overcome.
-
Summary and recommendation: Reliable temperature monitoring should be undertaken in all colorectal surgical patients and methods to actively warm patients to avoid IPH should be employed.
-
Quality of evidence:
-
Maintenance of normothermia: High
-
Monitoring of temperature: High
-
Prewarming: Moderate
-
Recommendation grade: Strong
15. Surgical access (Open and minimally invasive surgery including laparoscopic, robotic and trans-anal approaches)
Minimally invasive surgery (MIS) for both colonic and rectal resection is well established and in many countries, it has become the standard of care. The extent to which it has replaced open surgery varies widely but in European countries where data collection is good such as Denmark (Danish colorectal cancer group 2016) and Holland [161] the reported proportion of colonic and rectal cancer surgery undertaken with minimally invasive techniques is as high as 90% with conversion rates of < 10%. Some countries have achieved this through centralisation of services and others such as the UK have undertaken formalised centrally funded training programmes aimed at safely introducing new technologies while avoiding a rise in complications related to the learning curve [162].
There have been several RCTs [163,164,165,166,167,168,169] of laparoscopic versus open surgery for colorectal cancer, which generally reveal an advantage in favour of laparoscopy for recovery, LOS, blood loss and complications. There is variable evidence of an oncological advantage but no evidence of an oncological disadvantage. Improved survival after laparoscopic surgery has been demonstrated in two trials [168, 169] and a large national audit [170]. Cochrane reviews of the available data concerning short and long-term outcomes also support the results of the trials [171,172,173]. There is no evidence of a difference in survival comparing laparoscopic and robotic surgery [174], but data on long-term survival in robotic surgery are still sparse.
There have been two more recent non-inferiority trials published [175, 176] of laparoscopic versus open surgery for rectal cancer that use similar methodologies and use a composite score of specimen quality as the primary outcome. Non-inferiority of laparoscopic approach was not established in either trial but no long-term oncological results are yet available.
For colonic resection, the options are predominantly standard laparoscopy with no evidence, introducing robotic technology adds any advantage but increases costs considerably [177]. Variations such as single-port surgery also offers little advantage over multiport or reduced port surgery but is practiced effectively by some clinicians who report better cosmesis and reduced postoperative pain [178] although the evidence for this is weak. In both colon and rectal surgery, hand-assisted laparoscopy has been of historical interest but is not necessary in modern surgery.
For rectal resection, robotic surgery and laparoscopy combined with a trans-anal approach to the rectum [179] have developed as alternatives to standard laparoscopy. Robotic surgery for rectal cancer has been subjected to a meta-analysis and a RCT. The meta-analysis [180] showed no significant difference in any outcomes measure compared with standard laparoscopy except conversion rate. An RCT showed no significant difference in the primary outcome measure of conversion and the trial has also confirmed higher cost and that robotic rectal resection was not cost-effective [181]. Several systematic reviews of the trans-anal approach to rectal cancer [182,183,184,185] reveal no difference in specimen quality or anastomotic leak rates compared with laparoscopic and open surgery. A large prospective registry of cases has revealed anastomotic failure rates and specimen quality not dissimilar to databases of standard laparoscopy [186]. An RCT comparing the trans-anal with standard laparoscopic approach (COLOR III) has been initiated [187].
The focus on the different minimally invasive approaches is on improving the cancer-related outcomes, reducing the morbidity of pelvic surgery and reducing conversion rates. However, all have a similar capacity to reduce the trauma and immunological impact of surgery compared with an open approach. MIS is both an important enabling technology for many of the elements of ERAS and an independent predictor of good outcome [188]. It independently has the capacity to reduce complications, which is the ultimate goal of an ERAS programme. MIS enables reduced pain and opiate requirement, early mobilisation, less impact on fluid shifts and reduced ileus.
The relative influences of laparoscopy and enhanced recovery protocols have been compared in several trials [189,190,191]. The LAFA study [191] was a multicentre RCT, which randomised patients to laparoscopic and open segmental colectomy and ‘fast track’ and ‘standard care’ within nine Dutch centres. The median hospital stay was 2 days shorter after laparoscopic resection and the best outcomes with the least impact on the immune system was in the group receiving both minimally invasive surgery and enhanced recovery protocol. Regression analysis showed that laparoscopic surgery was the only predictive factor to reduce hospital stay and morbidity. The EnRol trial [190] randomised between laparoscopic and open colorectal resection within an enhanced recovery protocol and measured physical fatigue at 1 month as its primary outcome. Median hospital stay was 2 days shorter after laparoscopic surgery. A meta-analysis of protocol-driven care and laparoscopic surgery for colorectal cancer concluded that the combination reduced colorectal cancer surgery complications, but not mortality [192].
-
Summary and recommendation:
-
A minimally invasive approach to colon and rectal cancer has clear advantages for improved and more rapid recovery, reduced general complications, reduced wound-related complications including incisional hernia and fewer adhesions. It is also an enabler for successful administration of many of the major components of ERAS such as opiate sparing analgesia and optimised fluid therapy.
-
Quality of evidence:
-
Minimally invasive surgery versus open surgery: High
-
Recommendation grade:
-
Minimally invasive surgery versus open surgery: Strong
16. Drainage of the peritoneal cavity and pelvis
The use of a drain in the pelvic cavity after rectal surgery or the peritoneal cavity after rectal or colonic surgery has historically been advocated to evacuate or prevent blood or serous collections and to prevent or detect anastomotic leakage.
In 2004, a Cochrane systematic review compared the safety and effectiveness of routine drainage after elective colorectal surgery. The primary outcome was clinically anastomotic leakage [193] and included 6 RCTs enrolling 1140 patients, but only 2 RCTs (191 patients) separated low rectal anastomoses. The authors could not find a significant difference in outcomes. In 2005, a meta-analysis of pelvic drains in rectal surgery [194] including three RCTs reported no effect on anastomotic leakage rate or overall outcome. A more recent systematic review and meta-analysis of 11 RCTs with 1803 patients concluded that pelvic and peritoneal drains did not decrease anastomotic leakage (clinical or radiological), mortality, wound infection, nor reoperation rates [195]. Lastly, a recently published RCT [196], including 469 patients, showed that the use of a pelvic drain after rectal surgery for rectal cancer conferred no benefit to the patient.
-
Summary and recommendation:
-
Pelvic and peritoneal drains show no effect on clinical outcome and should not be used routinely.
-
Evidence level: High
-
Recommendation grade: Strong
Postoperative items
See Fig. 4.
17. Nasogastric Intubation
Nasogastric tubes have been in use with the aim of reducing postoperative discomfort from gastric distention and vomiting. However, all recent data show that the routine use of a NG tube has no positive, but rather a series of negative effects.
A recent meta-analysis of RCTs including 1416 patients undergoing colorectal surgery showed that pharyngolaryngitis and respiratory infections occurred less frequently if postoperative nasogastric decompression was avoided but that vomiting was more common [197]. A Cochrane meta-analysis of 33 trials with > 5000 patients undergoing abdominal surgery confirmed significant differences by an earlier return of bowel function and a decrease in pulmonary complications if a nasogastric tube was avoided [198]. A Dutch study with > 2000 patients found that the use of nasogastric decompression after elective colonic surgery declined from 88 to 10% without increases in patient morbidity or mortality [199]. In an RCT, patients not receiving nasogastric tubes tolerated oral intake earlier suggesting that routine nasogastric decompression may unnecessarily delay important nutrition in the postoperative period [200, 201]. A meta-analysis comprising seven recent RCTs (587 patients) comparing the outcomes following early oral feeding versus traditional oral feeding with gastric decompression by tube found that early oral feeding reduced hospital LOS and total of postoperative complications significantly; there were no significant differences in anastomotic dehiscence, pneumonia, wound infections, rate of nasogastric tube reinsertion, vomiting or mortality [202].
The routine insertion of a nasogastric tube during elective colorectal surgery should be avoided except for evacuating air that may have entered the stomach during ventilation by the facial mask prior to endotracheal intubation. An orogastric tube will suffice for this purpose and is recommended in laparoscopic cases to prevent inadvertent gastric injury. If placed during surgery, nasogastric tubes should be removed before the reversal of anaesthesia. There is still a roll for inserting an NG tube in patients with postoperative ileus refractory to conservative management to decompress the stomach and reduce the risk of aspiration.
-
Summary and recommendation:
-
Postoperative nasogastric tubes should not be used routinely; if inserted during surgery, they should be removed before reversal of anaesthesia.
-
Quality of evidence: High
-
Recommendation grade: Strong
18. Postoperative analgesia
Postoperative analgesia resulting in adequate pain control is essential in enhanced recovery pathways in colorectal surgery [9, 10, 200, 203, 204]. Although colon and rectal surgery (open and laparoscopic) differ considerably regarding technique, surgical trauma and early outcome, opioid avoiding or sparing techniques in both types of surgery are associated with early mobilisation, fast return of bowel function, fewer complications and a reduction in LOS [9, 10, 200, 203, 204]. Therefore, the key is to avoid opioids and apply multimodal analgesia in combination with epidural analgesia (in open surgery) when indicated. In fact, this multimodal strategy should ideally be included in the intraoperative period already and be a continuum postoperatively [9, 10, 200, 204].
Multimodal analgesia
The benefit of using a multimodal approach to pain management is based on the concept that several multiple pain reducing mechanisms will improve pain control while avoiding the side effects of each drug. Paracetamol is a basic part of this strategy and can be administered easily [203]. NSAIDS are also vital and key opioid-sparing component in multimodal analgesia. However, there is still debate whether NSAIDs are associated with an increased incidence of anastomotic leakage, but literature shows inconclusive evidence to avoid NSAIDs in colorectal surgery patients other than the regular contraindications [4, 205]. COX 2 drugs that do not effect platelet aggregation can be used if surgeons are concerned for bleeding. Several studies investigated opioid-sparing techniques with systemic additives like lidocaine infusions, α2-agonists like dexmedetomidine, ketamine, magnesium sulphate, high dose steroids or gabapentinoids [9, 10, 200, 206,207,208,209,210]. Lidocaine and dexmedetomidine infusions do appear to reduce postoperative pain in colorectal surgery compared with placebo [207, 209, 210]. However, there are limited studies that have systematically assessed the combination of these systemic additives on adverse events, outcomes and the analgesic effects compared with other techniques or in combination with epidural analgesia and TAP blocks (discussed item 18d) [200]. In both colon and rectal surgery, the use of other additives seems to have promising pain relieving potential, but needs to be investigated more extensively regarding efficacy and safety. However, multimodal analgesia is the backbone to reduce opioids in both open and laparoscopic colorectal surgery. Surgical site infiltration or more specific port-site local infiltration with local anaesthetics does appear to reduce postoperative pain compared with placebo, but limited data are available [211].
-
Summary and recommendation: Avoid opioids and apply multimodal analgesia in combination with spinal/epidural analgesia or TAP blocks when indicated
-
Quality of evidence: Moderate
-
Recommendation grade: multimodal opioid-sparing analgesia: Strong
18 a Epidural blockade
Metabolic effects
It is well established that epidural blockade with local anaesthetics, initiated before and continued during and after surgery, is a successful modality to minimise the neuro-endocrine and catabolic response to surgery [212]. As one result, insulin resistance, an expression of surgical stress, is attenuated [213]. Epidural blockade has also shown to minimise postoperative protein breakdown [214]. This effect is particularly useful when patients are fed in the immediate postoperative period as postoperative nitrogen balance is normalised and protein synthesis facilitated [215, 216]. Current data on metabolic effects have been mainly shown for open surgery and data for laparoscopic surgery are yet to be found.
Analgesic outcomes
Thoracic epidural analgesia (TEA) (T7-T10) remains the gold standard in patients undergoing open colorectal surgery. Several RCTs and meta-analysis have demonstrated superior analgesia compared with patients receiving systemic opioids [217, 218]. Supplementary analgesia is required in patients undergoing abdominal perineal resection, in which perineal pain (S1–S3 dermatomes) is not controlled by TEA. Lumbar epidural blockade is discouraged because of insufficient upper sensory block covering the surgical incision, lack of blockade of sympathetic fibres and risk of lower limb motor block and urinary retention [212].
The same analgesic benefits have not been demonstrated in patients undergoing laparoscopic colorectal surgery [219] and epidurals may even increase LOS in patients undergoing minimally invasive surgery. In fact, alternative co-analgesic techniques, such as intravenous lidocaine [210, 220,221,222], spinal analgesia [223,224,225,226,227], abdominal trunk blocks (ultrasound guided or under direct laparoscopic guidance), intraperitoneal local anaesthetic [228, 229] or continuous wound infusion of local anaesthetics [230,231,232] have shown to provide adequate analgesia, similar to those obtained with TEA [233], but superior to those provided by systemic opioids alone. TEA might still be valuable in patients with chronic pain or in patients in whom the risk of conversion to laparotomy is high. The results of an RCT comparing TEA with intravenous lidocaine in patients undergoing laparoscopic colorectal surgery demonstrated that TEA might still be advantageous in the first 48 h after rectal surgery, as rectal extraction and anastomosis was performed through a 8–10 cm Pfannenstiel incision [234]. Awareness of the type of laparoscopic approach used can assist physicians to decide whether TEA can still be valuable in patients undergoing laparoscopic colorectal surgery.
A continuous epidural infusion of a mixture of local anaesthetic and lipophilic opioids provides better analgesia than local anaesthetic or opioids alone [217, 218, 235]. The addition of adjuvants such as clonidine [236, 237] or epinephrine (1.5–5 µg/ml) [238, 239] can also be added to improve segmental analgesia and reduce certain opioids side effects. A mixture containing local anaesthetic with epidural morphine instead of lipophilic opioids can provide better analgesia in patients with long midline incision. Because of its pre-emptive analgesic effect [240], TEA should be initiated before surgery and continued in the intraoperative and postoperative period, for 48–72 h. A disadvantage of the use of TEA is the primary epidural failure rates that continue to remain high in some reports (ranging between 22 and 32%). Additional methods to correctly identify the epidural space (i.e. epidural stimulation or wave form analysis) and increase the success rate of epidural blocks can be employed [241, 242]. Appropriate postoperative support such as a pain team is also important to troubleshoot analgesia issues related to TEA to improve efficacy.
Postoperative non-analgesic outcomes
Despite the results of the largest multicenter RCT assessing the impact of combining TEA with general anaesthesia on 30-day morbidity or mortality in high-risk patients after major open gastrointestinal surgery did not show any benefit [237], several subsequent meta-analyses have shown that TEA accelerates the recovery of bowel function after colorectal surgery [243,244,245] and reduces the risk of respiratory [245, 246] and cardiovascular complications [245]. There is, however, a higher risk of postoperative arterial hypotension and urinary retention [245]. It must be also acknowledged that the positive impact of TEA on postoperative morbidity originates from studies in open surgery with no context of an ERAS program. A recent meta-analysis including 5 RCTs of patients undergoing laparoscopic colorectal surgery and all treated with an ERAS programme does not demonstrate the same benefits [247]. Some recent evidence also demonstrates that TEA has no impact [248] or even delays [219, 247, 249] hospital discharge in patients undergoing laparoscopic colorectal surgery. This delay might be due to a higher incidence of hypotension, urinary retention or motor blockade requiring additional postoperative care [219, 250]. The impact of TEA on colorectal cancer recurrence and metastasis [251, 252] remains to be investigated further, especially in the context of an ERAS program.
-
Summary and recommendation:
-
TEA using low dose of local anaesthetic and opioids is recommended in open colorectal surgery to minimise the metabolic stress response and provide analgesia postoperatively. In patients undergoing laparoscopic surgery, TEA can be used, but cannot be recommended over several alternative choices.
-
To attenuate the neuro-endocrinal stress response:
-
Quality of Evidence: Laparotomy: High
-
Recommendation: Strong
-
Quality of Evidence: Laparoscopy: Low
-
Recommendation: weak
-
To provide optimal analgesia
-
Quality of Evidence: Laparotomy: High
-
Recommendation: strong
-
Quality of Evidence: Laparoscopy: Moderate, for not using it
-
Recommendation: strong for not using it.
-
Low-dose local anaesthetic and opioids:
-
Quality of Evidence: Moderate
-
Recommendation: Strong
-
To improve postoperative non-analgesic outcomes
-
Quality of Evidence: Recovery of bowel function: High, for using it
-
Morbidity and mortality: moderate, for using it
-
Length of hospital stay: high, for not using it (laparoscopy, within an ERAS program)
-
Recommendations: Strong
18 b Spinal Anaesthesia/Analgesia (as an adjunct for general anaesthesia) for laparoscopic Colorectal Surgery
Spinal anaesthesia has a high efficacy and relatively low complication profile [253]. It has been used to facilitate ultra-rapid recovery after laparoscopic colorectal surgery by minimising opioid consumption within an ERAS protocol [226]. As compared with epidural anaesthesia, the patient can be mobilised sooner and is at less risk of hypotension and fluid overload that is a risk due to the sympathetic block induced by continuous thoracic epidural analgesia [219]. A combination of local anaesthetic such as bupivacaine 0.5% and long-acting opioid (such as diamorphine or morphine) is usually used with the total volume dosing in the range of < 2.0 ml to avoid high spinal block. In addition to the local anaesthetic effect, spinals have been shown to reduce the endocrine-metabolic stress response but only for the duration of action of the local anaesthetic where after it returns to levels of controls [223]. The addition of a long-acting opioid has the benefit of reducing morphine requirements postoperatively by up to sixfold with the ability to mobilise patients very soon after surgery once the motor block has worn off [225]. In another study, although early recovery was superior there was no benefit on LOS compared with intravenous morphine alone [227]. The main concern of using intrathecal opioids is that of delayed respiratory depression. Commonly used doses are at the lower end of clinical practice: 300–500 mcg of diamorphine or 100–150 µg of preservative free morphine. Similar monitoring should be used as if the patient was using a patient-controlled analgesia pump.
-
Summary and recommendation:
-
Spinal anaesthesia with low-dose opioids gives good analgesic effects, has a transient stress-reducing effect, and allows postoperative opiate sparing and is recommended as an adjunct option to general anaesthesia in laparoscopic surgery.
-
Quality of evidence: moderate
-
Recommendation: strong
18 c Lidocaine Infusions
The use of lidocaine infusions to reduce opioid use and nausea in colorectal surgery is now well established [210]. Published dosing ranges from 1.5 to 3 mg/kg/h depending on the bolus given (0 to 1.5 mg/kg) [254, 255]. Plasma lidocaine concentrations achieved are similar to those when running an epidural infusion (approximately 1 μM). Toxicity is related to the plasma concentration and appears to be rare, but monitoring in the postoperative period is important [256]. Continuous ECG monitoring is advised and nurses should be aware of symptoms of local anaesthetic toxicity such as tinnitus, blurred vision, dizziness, tongue paraesthesia and perioral tingling.
The analgesia benefit is in both open surgery and laparoscopic colorectal surgery. This beneficial effect appears to last longer than the infusion itself. Studies have been inconsistent in the duration of use of the infusion with some stopping at the end of surgery while others continue between 12 and 24 h postoperatively [254,255,256].
The incidence of postoperative ileus, which is a major cause of delayed hospital discharge in colorectal patients, is reduced in some studies. It is currently unclear whether this is due to the reduction in opiates or if there are other direct beneficial actions on the bowel or an anti-inflammatory effect [254].
-
Summary and recommendation:
-
Lidocaine infusions can reduce opiate consumption after surgery, whether the treatment reduces the risk of postoperative ileus remains unclear.
-
Quality of evidence: Use of lidocaine infusions to reduce opiate consumption after surgery: High
-
Recommendation: Strong
18 d Abdominal Wall Blocks
The role of epidural analgesia within the setting of an enhanced recovery programme has been questioned, especially with regard to laparoscopic operations [219, 257]. Interest in local anaesthetic abdominal walls blocks, as a component of multimodal analgesia, has thus increased.
Transversus abdominis plane (TAP) blocks are the most widely studied. Since the initial description, in 2001 [258], as the classic landmark-based technique, multiple variations have been described, including 2-point, 4-point, ultrasound-guided and laparoscopic-visualised blocks. TAP blocks provide analgesic coverage to the anterior abdominal wall from T10 to L1 [259] and have been demonstrated to provide an opioid-sparing approach in colorectal surgery [260]. As TAP blocks only provide analgesia reliably below the umbilicus, subcostal and rectus blocks are adjuncts, which can cover the upper abdomen.
An early Cochrane review of transversus abdominis plane (TAP) blocks found 5 heterogeneous studies, no comparisons with other methods of analgesia, and limited evidence of reduced opioid use [261]. A review of studies up to early 2016 of peripheral nerve blocks again demonstrated a lack of data [211]. There have been more recent RCTs indicating the benefits of TAP blocks in abdominal surgery in multiple specialties including gynaecologic, general, bariatric and transplant surgery [262,263,264,265,266] and also specifically in colorectal surgery with less opioid use, faster resumption of GI tract function and recovery [267, 268] although others have not shown benefits [269]. One major weakness with abdominal blocks is short duration. Conventional bupivacaine and ropivacaine used in traditional TAP blocks have a short half-life (usually 8–10 h) [270]. Various methods have been used to increase the duration of abdominal wall blocks including mixing standard local anaesthetics with dexamethasone [271], dextran [272] and use of infusion catheters [266]. Liposomal bupivacaine, initially approved for infiltration and not for nerve blocks, is currently approved for TAP blocks as the target is an anatomical musculofascial plane between the internal oblique and transversus abdominis muscles not a specific nerve [273].
-
Summary and recommendation: Small RCTs in laparoscopic colorectal and other surgeries show that TAP blocks reduce opioid consumption and improve recovery. Optimal pain relief appears to depend on the extent of spread within the fascial plane, which in turn is dependent on the type, volume, duration of action of injectate and the accuracy with which the correct plane is identified. Both ultrasound-guided and laparoscopic approaches have been described.
-
Quality of evidence: Moderate
-
Recommendation grade: TAP blocks in minimally invasive surgery: Strong
19. Thromboprophylaxis
Older data in traditional perioperative care showed that without thromboprophylaxis there was a 30% incidence of asymptomatic deep vein thrombosis (DVT) after colorectal surgery [274]. Recent reviews of risk factors (high-risk patients) include ulcerative colitis, advanced malignancy (Stage III + IV), hypercoaguable state, steroid use, advanced age and obesity [275].
All patients benefit from mechanical thromboprophylaxis achieved with compression stockings and/or intermittent pneumatic compression (ICP) during hospitalisation or until mobilised as proven measure to reduce the incidence of DVT after general surgery even in the absence of pharmacological treatment [OR 0.27 (0.20–0.38)] [276,277,278].
Pharmacological thromboprophylaxis with low molecular weight heparin (LMWH) or unfractionated heparin has been shown to reduce the incidence of symptomatic venous thromboembolism and also overall mortality with a very low risk of bleeding complications. A single administration of LMWH per day was as effective as twice-daily administration [279, 280]. A combination of ICP together with pharmacological prophylaxis decreased the incidence of pulmonary embolism (PE) and DVT when compared with a single modality at the expense of higher risk for bleeding complications when comparing to ICP alone [277].
The usefulness of extended thromboprophylaxis (ETP) for 28 days after colorectal surgery relies on data from traditional perioperative care. Based on a Cochrane meta-analysis of four RCTs [279], previous ERAS recommendations and other guidelines (NICE, NHMRC) recommended ETP (28 days) for patients having major cancer surgery in the abdomen or pelvis. However, with changes in surgical techniques from open to minimally invasive and advances towards less stress with modern anaesthesia, the extended prophylaxis regime has been questioned. A recent report indicates that only 8–27% of colorectal surgeons followed these traditional recommendations [281]. Furthermore, incidence of symptomatic VTE, DVT and PE after discharge is reported to be very low at 0.60–0.73%, 0.29–0.48% and 0.26–0.40%, respectively, in the three largest and recent cohort studies including 236,066 patients [275, 281]. Also, in non-cancer surgery, such as after hip replacement surgery, 90-day risk for VTE was indifferent for short thromboprophylaxis (1–6 days) as compared with standard (> 7 days) and extended (> 28 days) regimens [282]. So far, RCTs investigating the benefit or risks of ETP versus shorter prophylaxis show no reduction in symptomatic DVT, symptomatic pulmonary emboli or VTE-related death [281]. However, these studies are heavily underpowered and cannot answer this clinical question. The clinical value in that the same studies showed that subclinical thrombosis were several times more frequent with in-house short-term prophylaxis compared to 4 weeks is uncertain [281]. Given the reduction in stress using minimally invasive techniques and several other stress-reducing ERAS elements combined with the almost immediate mobilisation of the patients the relevance of older studies can be questioned and needs to be revisited. Lastly, no specific data are available supporting the use of ETP in low-risk ERAS patients.
However, given the seriousness of the complications, the risk factor spectrum for thrombosis among colorectal surgery patients and the lack of data showing no risk or a benefit from shorter or no prophylaxis, the recommendation needs to continue to rely on current old but available evidence.
Summary and recommendation:
Patients undergoing major colorectal surgery should have (I) mechanical thromboprophylaxis by well-fitting compression stockings and/or intermittent pneumatic compression until discharge and (II) receive pharmacological prophylaxis with LMWH once daily for 28 days after surgery.
-
Quality of evidence:
-
Postoperative mechanical thromboprophylaxis: High
-
In-hospital or until mobilised: Moderate
-
-
Postoperative LMWH: High
-
In-hospital or 7 days postop: Low
-
28 days after surgery: Low
-
-
Recommendation grade:
-
Mechanical thromboprophylaxis until discharge: Strong
-
LMWH In-hospital or 7 days postop: Weak
-
LMWH until 28 days postop: Strong
20. Postoperative fluid and electrolyte therapy
Intravenous fluid therapy is usually not necessary after the day of operation for most patients undergoing colorectal surgery. Patients should be encouraged to drink when they are awake and free of nausea after the operation and an oral diet can usually be started within 4 h after surgery [202, 283]. If oral fluid intake is tolerated, intravenous fluid administration should be discontinued as soon as feasible, preferably at least by day 1 POD and should be restarted only if clinical indications exist. In such situations and in the absence of surgical losses, physiological maintenance fluids should be given, when indicated, at a rate of 25–30 ml/kg per day with no more than 70–100 mmol sodium/day, along with potassium supplements (up to 1 mmol/kg/day) [284]. As long as this volume is not exceeded, hyponatraemia is very unlikely to occur despite the provision of hypotonic solutions [285, 286]. Any ongoing losses (e.g. vomiting or high stoma losses) should be replaced on a like for like basis for what is being lost in addition to the maintenance requirement. After ensuring the patient is normovolaemic, hypotensive patients receiving epidural analgesia should be treated with vasopressors rather than indiscriminate fluid boluses [287, 288]. It is important that patients are maintained in as near a state to zero fluid balance as possible in the perioperative period, as both fluid deficits and overload (of as little as 2.5 L [289]) can cause adverse effects in the form of increased postoperative complications, prolonged hospital stay and higher costs due to increased resource utilisation [6, 290, 291].
Balanced crystalloids versus 0.9% saline:
There is considerable evidence from physiological studies that large volumes of intravenous 0.9% saline cause a hyperchloraemic acidosis, interstitial fluid overload, impairment of renal haemodynamics and a reduction in urinary water and sodium excretion as a result of a reduction in renal blood flow and glomerular filtration rate [292,293,294,295,296].
Large observational, propensity-matched studies have suggested 0.9% saline, because of the high chloride content, may cause harm, especially to the kidney in patients undergoing surgery [297], critically ill patients [298] and those with the systemic inflammatory syndrome [299]. Another propensity-matched study has suggested that up to 22% of patients develop acute hyperchloraemia (> 110 mmol/L) in the postoperative period and that this is associated with an increased risk of 30-day mortality and longer LOS than those who do not develop hyperchloraemia [300]. However, as there are no large-scale RCTs yet comparing 0.9% saline with balanced crystalloids in a surgical population, the current evidence cannot be regarded as high. In addition, a recent meta-analysis that was limited by imprecision and studies of a small sample size has shown that for unselected critically ill or perioperative adult patients there was no benefit evidence of low versus high chloride solutions [301].
Management of Oliguria
Oliguria in the adult is usually defined as urine output < 0.5 ml/kg/h, or < 500 ml in 24 h, in an adult. However, urine output and oliguria alone are not reliable indicators of hypovolaemia in the first 48 h after surgery as the postoperative metabolic response to stress leads to renal vasoconstriction and physiological salt and water retention [302]. Oliguria should be assessed carefully (fluid balance chart), and a thorough clinical examination performed before commencing intravenous fluid resuscitation for dehydration or hypovolaemia, as excess fluid administration has been associated with acute kidney injury [303, 304]. If the patient does not demonstrate clinical signs of hypovolaemia (e.g. tachycardia, hypotension, sweating, confusion and decreased capillary return), it is useful to average urine output out over 4 h. A conservative fluid regimen does not appear to increase the risk of postoperative oliguria or acute kidney injury [305,306,307,308]. In addition, supplemental intravenous fluids or diuretics do not improve renal function or protect against acute kidney injury [304,305,306, 309]. Indeed, allowing a lower urine output in the perioperative phase appears safe and results in significantly reduced administration of intravenous fluid [308].
-
Summary and recommendation: Net “near-zero” fluid and electrolyte balance should be maintained. To cover pure maintenance needs, hypotonic crystalloids should be used (rather than isotonic crystalloids, which contain high concentrations of sodium and cations). For replacement of losses, saline 0.9% and saline-based solutions should be avoided, with balanced solutions being preferred. In patients receiving epidural analgesia, arterial hypotension should be treated with vasopressors after ensuring the patient is normovolaemic.
-
Quality of evidence:
-
Neutral fluid balance: High
-
Hypotonic crystalloids for maintenance needs: Low
-
Balanced salt solutions instead of 0.9% saline: Low
-
Recommendation grade:
-
“Near-zero” fluid balance: Strong
-
Hypotonic crystalloids for maintenance needs: Strong
-
0.9% saline should be avoided: Strong (only in hyperchloraemic and acidotic patients).
21. Urinary drainage
Urinary drainage during and after colorectal surgery is used traditionally for two main reasons: prevention of urinary retention and monitoring of urine output. The duration of catheterisation is directly related to a risk of urinary tract infection (UTI) and may hinder postoperative mobilisation and should therefore be limited. In a RCT of catheter removal after major abdominal and thoracic surgery on day 1 (n = 105) versus day 4 (n = 110), the risk of UTI was markedly reduced with early removal (2 vs 14%) and the risk of retention was low in both groups (8 vs 2% single in–out catheterisation; 3 vs 0% 24-h catheterisation) [310]. A large observational study (n = 513) confirms low retention rates (14%) in patients undergoing colorectal surgery within an established ERAS protocol that included early catheter removal [311]. This study highlighted male gender and postoperative epidural analgesia as important independent predictors of retention. Thus, tailored removal of the bladder catheter can be guided by such risk factors.
The importance of closely monitoring the perioperative urine output to avoid oliguria has recently been challenged. In renal medicine, oliguria is defined as a daily urine output < 400 ml, or approximately < 0.2 ml/kg/h in average weight adults [312]. In the perioperative setting, oliguria is traditionally defined as a urine output < 0.5 ml/kg/h, and additional fluid is administered to reach above this target. There are no data to support this practice. A recent RCT demonstrated that fluid therapy guided by the lower target of 0.2 ml/kg/h during and after colorectal surgery is not only safe but also spares a significant volume of intravenous fluids compared with the standard target of 0.5 ml/kg/h [308]. An hourly measurement of urinary output is therefore by itself no longer an indication for bladder catheterisation.
Special considerations for pelvic surgery
Patients undergoing pelvic surgery appear to be at particular risk of postoperative urinary retention. A RCT performed in the 1990s found retention rates of 25% versus 10% when the transurethral catheter was removed on day 1 versus day 5 after proctectomy [313]. A more recent RCT of transurethral catheterisation for 1, 3 or 5 days after pelvic surgery (n = 118) supported a higher risk of retention with early removal (15, 5 and 10%, respectively) [314]. The 15–25% urinary retention rate associated with catheter removal on the first day after pelvic surgery suggests that delaying catheter removal in this group to 2 or 3 days is justified in this group.
Extended bladder catheterisation may be required in selected cases undergoing complex pelvic reconstructive surgery. A recent meta-analysis has confirmed that when the duration of postoperative catheterisation exceeds 5 days, a suprapubic tube or clean intermittent catheterisation are safer alternatives to the standard transurethral catheter [315].
-
Summary and recommendation:
-
Routine transurethral catheterisation is recommended for 1–3 days after colorectal surgery. The duration should be individualised based on known risk factors for retention: male gender, epidural analgesia and pelvic surgery. Patients at low risk should have routine removal of catheter on the first day after surgery, while patients with moderate or high risk require catheterisation for up to 3 days.
-
Quality of evidence level: High
-
Recommendation grade: Strong
22. Prevention of postoperative ileus
Prolonged postoperative ileus is a major contributor to patient discomfort, delayed discharge and increased cost; hence, its prevention is a key objective of enhanced recovery protocols. Many of the core elements of these protocols, such as (1) limiting opioid administration through application of multimodal analgesia techniques (including use of mid-thoracic epidurals and peripheral nerve blocks), (2) use of minimally invasive surgery, (3) eliminating routine nasogastric tube placement, and (4) maintaining fluid balance including goal-directed fluid therapy, can limit the duration of postoperative ileus [9]. These elements are supported by high-quality evidence and are discussed elsewhere in these guidelines. This section focuses on additional interventions and pharmacological agents that specifically target ileus.
Peripherally acting µ-opioid receptor (PAM-OR) antagonists with limited ability to cross the blood–brain barrier include alvimopan, methylnaltrexone, naloxone and naloxegol. These agents can ameliorate opioid-induced bowel dysfunction without reversing analgesia through central µ-opioid receptor antagonism. Of these agents, alvimopan is the best studied in the context of limiting duration of postoperative ileus. This drug is currently approved by the US Food and Drug Administration (FDA) but not universally available, for the indication of accelerating upper and lower gastrointestinal recovery following partial large or small bowel resection with primary anastomosis. However, in a recently published systematic review of eight randomised, placebo-controlled, clinical trials evaluating of the efficacy of alvimopan in reducing duration of postoperative ileus following major abdominal surgery, six of these studies found a reduction with alvimopan administration, whereas two found no difference among study groups [316]. Each of these studies were graded as moderate or low in quality and tended to focus on patients undergoing open surgery. In addition, two randomised, placebo-controlled, clinical trial found no difference between methylnaltrexone and placebo in decreasing duration of postoperative ileus following segmental colectomy [317]. Conflicting data on efficacy, costs and concerns over cardiovascular complications, limit recommendation for routine use of these agents, particularly in the context of increasingly wide-spread application of opioid-sparing anaesthesia and analgesia techniques and of minimally invasive surgery.
Numerous RCTs have evaluated the efficacy of postoperative gum chewing in reducing duration of postoperative ileus. A Cochrane review of this topic concluded that, while gum chewing may be associated with mild reductions in ileus duration, the evidence on this topic is largely limited to small, poor quality studies [318]; in particular, most studies lack appropriate blinding of patients and investigators [319]. Further, the benefits of gum chewing in the context of ERAS pathways have been unclear. Recently, a well-designed, large-scale multicentre RCT evaluating the effects of postoperative gum chewing in patients undergoing abdominal surgery and on ERAS pathways was reported [320]. Gum chewing had no impact on time to first postoperative flatus or bowel movement, on postoperative length of stay, or on incidence of postoperative complications. Thus, while gum chewing is associated with little, if any, harm in postoperative patients, currently available evidence does not support the efficacy of gum chewing in reducing duration of ileus in patients undergoing abdominal surgery on ERAS pathways. Accordingly, its routine inclusion as a component of ERAS care is not recommended.
Various other agents that have been tested for efficacy in reducing duration of postoperative ileus, including laxatives and coffee. In prospective controlled trials, reductions in various indices of postoperative ileus have been observed to occur with oral bisacodyl administration in patients undergoing colorectal surgery [321], with oral magnesium oxide administration in patients undergoing hysterectomy [322], with oral daikenchuto (a traditional Japanese herbal medicine) administration in patients undergoing gastrectomy [323], and with oral coffee administration in patients undergoing colorectal surgery [324]. Interestingly, another RCT revealed greater reductions in indices of postoperative ileus with de-caffeinated coffee administration than with caffeinated coffee in patients undergoing left-sided laparoscopic colectomy [325]. These studies have methodological limitations, and confirmatory studies are needed before routine application is recommended. Nevertheless, we recommend against withholding coffee from postoperative patients who tolerate oral liquids.
-
Summary and recommendation: A multimodal approach to minimise the development of postoperative ileus include: limit opioid administration through use of multimodal anaesthesia and analgesia techniques, use minimally invasive surgical techniques (when feasible), eliminate routine placement of nasogastric tubes and use goal-directed fluid therapy. Peripherally acting µ-opioid receptor antagonists, chewing gum, bisacodyl, magnesium oxide, daikenchuto and coffee have all some indications of affecting an established ileus.
-
Quality of evidence:
-
Multimodal prevention of ileus: High.
-
Peripherally acting µ-opioid receptor antagonists (e.g. alvimopan): Moderate.
-
Bisacodyl, magnesium oxide, daikenchuto and coffee: Low
-
Recommendation grade: Multimodal prevention of ileus: Strong. Peripherally acting µ-opioid receptor antagonists (e.g. alvimopan): Weak. Bisacodyl, magnesium oxide, daikenchuto, and coffee: Weak
23. Postoperative glycaemic control
A hallmark of the physiological response to surgical trauma is insulin resistance, or so-called pseudodiabetes of injury, which persists for several weeks after elective surgery [326]. This leads to an osmotic shift of fluid into the vascular space and an increased availability of glucose for glucose-dependent tissues such as white blood cells and the brain. Although hyperglycaemia after surgery was reported in 1934, it was not until 2001 that negative consequences of perioperative hyperglycaemia were fully recognised, with the publication of a large RCT comparing permissive hyperglycaemia with strict glycaemic control by intensive insulin therapy [327]. Morbidity and mortality were decreased in the intervention group.
No further trials of strict glycaemic control in surgical patients have been reported, although a subgroup analysis of trauma patients in a multi-centre trial shows similar results [328]. Intensive insulin therapy can therefore not be recommended in routine colorectal surgery, but these trials do highlight the clinical risks posed by perioperative hyperglycaemia.
In elective surgery, there are opportunities to prevent insulin resistance from developing in the first place. Several interventions that blunt insulin resistance are part of the ERAS care pathway, including oral preoperative carbohydrate treatment, laparoscopic surgery and thoracic epidural analgesia. A recent large RCT showed that preoperative carbohydrates moderated postoperative glucose concentrations and reduced the need for insulin [124]. Two trials have shown that surgery within ERAS is associated with partial or complete attenuation of key stress responses. In the first, unchanged postoperative nitrogen losses, neutral nitrogen balance, minimal insulin resistance and preserved normoglycaemia during feeding were found after major open colorectal surgery [329]. A recent four-way randomised study of laparoscopic versus open surgery and ERAS versus traditional care assessed the independent effects of laparoscopic surgery and ERAS [191, 330]. ERAS was associated with a blunted stress mediator response, measured by growth hormone concentration changes. Nevertheless, observational studies have revealed that hyperglycaemia remains prevalent during the postoperative period, in particular in patients with an increased preoperative haemoglobin A1c [331]. The association to postoperative adverse outcomes appears to be the strongest in subjects without a diagnosis of diabetes [332].
-
Summary and recommendation:
-
Hyperglycaemia is a risk factor for complications and should therefore be avoided. Several interventions in the ERAS protocol prevent insulin resistance, thereby improving glycaemic control with no risk of causing hypoglycaemia. For in patients, insulin should be used judiciously to maintain blood glucose as low as feasible with the available resources.
-
Quality of evidence:
-
Using stress-reducing elements of ERAS to minimise hyperglycaemia: Moderate (study quality, extrapolations).
-
Insulin treatment in the ICU: Moderate (inconsistency, uncertain target concentration of glucose).
-
Glycaemic control (using insulin) in the ward setting: Low (inconsistency, extrapolations)
-
Recommendation grade:
-
Using stress-reducing elements of ERAS to minimise hyperglycaemia: Strong
-
Insulin treatment in the ICU (severe hyperglycaemia): Strong
-
Insulin treatment in the ICU (mild hyperglycaemia): Weak (uncertain target concentration of glucose)
-
Insulin treatment in the ward setting: Weak (risk of hypoglycaemia, evidence level)
24. Postoperative nutritional care
Postoperative resumption of oral intake.
It has been well established that any delay in the resumption of normal oral diet after major surgery is associated with increased rates of infectious complications and delayed recovery [333]. Early oral diet has been shown to be safe 4 h after surgery [3] in patients with a new non-diverted colorectal anastomosis. Some report that low residue diet, rather than clear liquid diet, after colorectal surgery is associated with less nausea, faster return of bowel function, and a shorter hospital stay without increasing postoperative morbidity when administered in association with prevention of postoperative ileus [334]. Spontaneous food intake rarely exceeds 1200–1500 kcal/day [331]. To reach energy and protein requirements, additional oral nutritional supplements have been shown to be useful [335].
Immunonutrition
Surgical stress can cause an acute depletion of arginine, which both impairs T cell function and wound healing [336]. This acute nutritional deficiency is potentially modifiable and has been the target of nutritional optimisation around the time of surgery. Therefore, supplementation of enteral feeds with immunomodulators such as l-arginine, l-glutamine, ω-3 fatty acids and nucleotides (immunonutrition) is thought to modify immune and inflammatory responses favourably and result in reduced postoperative infective complications and shorter LOS [337, 338]. The recent ESPEN guideline on perioperative nutrition presented an extensive review of multiple RCTs and meta-analysis and concluded that peri-or at least postoperative immunonutrition (arginine, omega 3 fatty acids and ribonucleotides) should be given to malnourished patients undergoing major cancer surgery [53]. A reduction in infectious complications was reported in favour of immunonutrition over standard ONS in two recent prospective RCTs within an ERAS protocol [339, 340].
Summary and recommendation:
Most patients can and should be offered food and ONS from the day of surgery. Perioperative immunonutrition in malnourished patients is beneficial in colorectal cancer surgery.
Quality of evidence:
Postoperative resumption of oral intake: Moderate
Immunonutrition: Low
Recommendation grade:
Postoperative resumption of oral intake: Strong
Immunonutrition: Strong (no harm)
25. Early Mobilisation
Early mobilisation after abdominal surgery is widely regarded as an important component of perioperative care for enhanced recovery. Prolonged bed rest is associated with risk for developing pulmonary complications, decreased skeletal muscle strength, thromboembolic complications and insulin resistance [341,342,343]. Early mobilisation has therefore been an integral component of enhanced recovery after surgery protocols. While there is strong evidence regarding the harmful effects of immobilisation, evidence is more limited regarding the benefit of dedicated interventions specifically designed to increase early mobilisation after surgery.
A recent systematic review of the effect of early mobilisation protocols on postoperative outcomes following abdominal and thoracic surgery evaluated a total of 8 studies [344] (including 3 RCTs and 1 prospective observational study) in abdominal surgery and 4 (including 3 RCTs and 1 retrospective observational study) in thoracic surgery. The specific outcomes of interest included postoperative complications, LOS, gastrointestinal function recovery, performance-based outcomes and patient-reported outcomes. While not all studies evaluated all outcomes, for each outcome, only one study could demonstrate a benefit for the intervention group.
The impact of early mobilisation in critically ill patients was recently demonstrated in an international multicentre randomised trial of goal-directed mobilisation versus usual care in intensive care unit patients [345]. The intervention arm included basic manoeuvres such as sitting and standing or stepping in place at the bedside. Compared with usual care, goal-directed early mobilisation was associated with a short duration of surgical intensive care unit stay and better functional mobility at discharge. Moreover, lack of early mobilisation after abdominal surgery has been associated with an up to 3.0 (95% confidence interval 1.2–8.0) fold increased in likelihood of developing a pulmonary complication [346]. Yet the applicability of these findings to patients who have few limitations for mobility following elective surgery is uncertain.
Early mobilisation is an essential component of multimodality strategies for enhanced recovery after surgery. A multivariate linear regression analysis of data collected during the LAFA trial supported the notion that mobilisation on postoperative days 1, 2 and 3 is a factor significantly associated with a successful outcome of ERAS [191]. However, despite the demonstrated effectiveness of the ERAS pathways, there remains considerable variation in the extent to which the different ERAS pathway interventions are implemented, including with respect to the implementation of early mobilisation [347, 348]. Although the degree of compliance to ERAS principles including early mobilisation has been associated with improved outcomes [203], a recently reported RCT comparing facilitated mobilisation during postoperative days 0–3 to a standard enhanced recovery programme increased out-of-bed activities but did not improve outcomes [349].
Finally, another important consideration is that failure of early mobilisation may be due to a variety of factors such as inadequate control of pain, continued intravenous intake of fluids, prolonged indwelling urinary catheter, patient motivation, and pre-existing comorbidities which are likely themselves associated with poorer outcomes, leading to question of whether the observed outcomes are associated with early mobilisation or are due to the underlying factors that lead to the inability to mobilise.
Taken together, the studies suggest that bedrest should be discouraged in favour of early mobilisation, but the allocation of additional resources to implement structured early mobilisation beyond integration into multimodal enhanced recovery protocols has not shown to be of benefit.
Summary and recommendation:
Early mobilisation through patient education and encouragement is an important component of enhanced recovery after surgery programmes; prolonged immobilisation is associated with a variety of adverse effects and patients should therefore be mobilised.
Quality of evidence: Moderate
Recommendation grade: Strong
Audit
Audit forms the basis for insights to practice and outcomes. Countries in Northern Europe and the UK have had a tradition of national audits with yearly reports on basic surgical data and crude outcomes such as major complications and mortality. Over the years, these have become more or less mandatory for most surgical procedures. In the USA, the American College of Surgeons runs the ACS National Surgical Quality Improvement program (www.facs.org/quality-programs/acs-nsqip) involving several hundred hospitals collecting sample outcome data throughout the surgical spectrum. In many countries, however, there are no systems available to study or compare outcomes. The ERAS®Society has taken on the mission to spread the use of audit and to develop systems not only for annual reports, but for daily use to implement changes and improvements and to sustain high-level care (www.erassociety.org).
Surgical patients undergoing major operations are most often treated by a large number of more or less specialised healthcare professionals delivering a long list of different care elements. Each caregiver is focused on his/her specific target with their treatment during that specific period he/she cares for the patient. The complexity of the patient’s journey makes it very hard for each and everyone involved in the care to know what their role is in the bigger picture, nor how their choices of treatments will affect the patient’s journey later on. A poor choice in treatment early in the patient journey will affect the possibilities to deliver other care elements later on. For instance, if the patient is overhydrated during the operation, the chances of feeding the patient orally postoperatively are diminished [285]. For this reason, it is important to document and feed back to all involved in the patients care pathway which care is actually given to the patient throughout and relate that to the outcomes that the unit delivers. To know this seemingly basic information there is a need to collect relevant data, analyse them and feed back in a structured way. A Cochrane analysis reported that audit and feedback have a significant effect on healthcare professionals adherence to a given protocol [350]. Audit and feedback has its best effects when done repeatedly (monthly), delivered by colleagues and given both in writing and verbally, with specific targets for change and for multifaceted interventions.
For ERAS implementation, an early report showed that a protocol alone was not enough to achieve good outcomes [351], and with more experience assembled, a recent Delphi study suggested the use of standardised audit and feedback as an important part of an implementation programme [352].
There are several reports from different countries showing that better compliance with ERAS® Society guidelines associates with better 30 day outcomes in terms of complications and time to discharge and recovery [204] and even long-term survival [353], which is contrasted by a recent report from 12 hospitals in central western Europe showing low adherence and hospitals stays of almost 2 weeks while having had no structured ERAS implementation or continuous use of audit [348]. While there is convincing evidence that audit and feedback is important in implementation of ERAS, there are fewer insights to the effect of audit and feedback on sustainability of ERAS. There is, however, one report from the Netherlands where a successful implementation programme was followed up several years later when audit had been dropped after the completion of the programme. The authors found that compliance had fallen back in 7 of the 10 units investigated, and despite the introduction of minimally invasive surgery to a large extent, LOS was increased [354].
There are different ways to collect data and to review them, and some use homemade systems using daily used software. The ERAS® Society has developed the ERAS® Interactive Audit System, which is used in the ERAS® Implementation Programmes worldwide and that is tailored for use when making changes, sustaining improvements and for research [204]. It also allows comparisons and benchmarking.
Summary and recommendation:
Collection of key outcome and process data used for repeated audit and feedback is essential to drive change for improvements and to know and control practice. Outcomes (complications and mortality 30 days) and processes should be audited and feed back to all healthcare providers on a regular basis when driving change or implementing ERAS programmes, as well as for sustaining improvements.
Quality of evidence: High
Recommendation grade: Strong
Implications of ERAS for nursing practice
Implementation of an ERAS programme can be challenging for clinical staff. Nurses can often find certain elements of the process difficult as they are often expected to alter their practice based on current evidence. ERAS education is essential to inform and update all members of the clinical team about all the ERAS interventions and this should begin at nursing colleges and universities. Regular multi-disciplinary conversations need to occur so that the evidence-based recommendations can be implemented effectively. Some research has been conducted on the impact of ERAS on nursing workload [355, 356] but more qualitative research would be beneficial to better understand ERAS from a nursing perspective.
Documentation is also a crucial factor in ERAS implementation—the documents need to be concise and agreed locally so that the nurse can progress the patient along the pathway autonomously and meet ERAS targets more effectively.
Setting discharge criteria and daily goals is important. Highlighting these goals to the patient before surgery is a key to avoid unrealistic expectations and keep both the patient and relatives informed that short LOS is to be expected. Patient follow-up is integral to an ERAS programme—patient’s should be given discharge advice and should be contacted by the ERAS team, particularly if the patient is discharged within 2–4 days of surgery. This provides an added ‘safety net’ for patients so that they and their clinicians know that they are being reviewed following discharge.
Comment
The current guidelines from the ERAS society for clinical perioperative care of patients undergoing elective colorectal surgery are the fourth in order published since the ERAS study group was formed in 2001. A continually growing evidence base in perioperative medicine necessitates frequent updates in the knowledge base for continuous training and development in practise for those involved in the treatment of surgical patients. The current evidence-based recommendations were evaluated according to the GRADE system and the quality of evidence for each item were crosschecked by several authors in the author list.
Even though the guidelines are based on formal criteria on how to evaluate the evidence base behind perioperative treatment, it cannot be ignored that grading of evidence is demanding and also difficult. That the evidence base is low in certain research areas can have many reasons and does not obviously mean that an effect is missing or that the outcome of one item is worse than another item. Thus, a strong recommendation together with low evidence may seem conflicting. However, current review of the evidence must be put into the perspective of the level of evidence in general for common medical practices and treatments and that the evidence for components in the ERAS protocol is at a level that is commonly in use throughout medicine today.
The quality of evidence and recommendations in these guidelines are intended to be used by experienced clinicians either as a tool to implement an ERAS protocol or to upgrade a protocol that already has been implemented. However, in clinical practice one has to remember that many of the healthcare professionals who are involved in perioperative care may have limited knowledge in ERAS care pathways and therefore need an overview of the topic more quickly. In the current guidelines, we have renewed the layout so that the reader is able to obtain an efficient overview with the graphs and still find more details on different items in the text. We hope that the way the ERAS items are listed in this document will make the guidelines easier to read as they follow the natural perioperative journey.
Previous versions of these guidelines have been extensively tested in different parts of the world and shown to be efficacious [203]. Continuous issues when discussing ERAS programmes are which elements are the most important for outcome from surgery, as some may argue that only a few are needed. These questions have no evidence-based simple answer. Some units may use certain aspects of perioperative care and then as other evidence elements are added, they will improve their outcomes. Other units may have a completely different starting point. What has been shown, however, is that with increased compliance to the items within the whole ERAS protocol, short-term outcomes are improved [3, 203], and may also have impact on improving long-term survival [353]. Therefore, all elements that may have an impact on outcome, greater or smaller, have been included in the guidelines.
The lack of updated evidence is a potential weakness for some of the recommendations as well as the fact that many studies were not performed under optimal ERAS conditions. While it would be ideal to test all elements in optimal perioperative conditions, this will not reflect real-life perioperative care of today. By reviewing national databases, large registries or cohort studies it is obvious that key outcome data such as LOS and complications differ significantly between centres in different countries. This difference also applies to centres practising the ERAS protocol. In addition, traditions and recommendations in one country may vary from another. This may be especially true when the evidence base is weak. The intention of this update is thus to provide a comprehensive overview of the optimal perioperative care of patients undergoing major colorectal surgery as found in the current up to date medical literature.
References
Basse L, Raskov HH, Hjort Jakobsen D et al (2002) Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg 89:446–453
Basse L, Werner M, Kehlet H (2000) Is urinary drainage necessary during continuous epidural analgesia after colonic resection? Reg Anesth Pain Med 25:498–501
Gustafsson UO, Hausel J, Thorell A et al (2011) Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 146:571–577
Khoo CK, Vickery CJ, Forsyth N et al (2007) A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 245:867–872
Serclova Z, Dytrych P, Marvan J et al (2009) Fast-track in open intestinal surgery: prospective randomized study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr 28:618–624
Varadhan KK, Lobo DN (2010) A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 69:488–498
Wind J, Polle SW, Fung Kon Jin PH et al (2006) Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 93:800–809
Fearon KC, Ljungqvist O, Von Meyenfeldt M et al (2005) Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 24:466–477
Gustafsson UO, Scott MJ, Schwenk W et al (2013) Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 37:259–284. https://doi.org/10.1007/s00268-012-1772-0
Nygren J, Thacker J, Carli F et al (2013) Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 37:285–305. https://doi.org/10.1007/s00268-012-1787-6
Verhagen AP, de Vet HC, de Bie RA et al (1998) The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51:1235–1241
Guyatt GH, Oxman AD, Kunz R et al (2008) Going from evidence to recommendations. BMJ 336:1049–1051
Guyatt GH, Oxman AD, Kunz R et al (2008) What is “quality of evidence” and why is it important to clinicians? BMJ 336:995–998
Guyatt GH, Oxman AD, Vist GE et al (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Ayyadhah Alanazi A (2014) Reducing anxiety in preoperative patients: a systematic review. Br J Nurs 23:387–393
Gan TJ, Habib AS, Miller TE et al (2014) Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 30:149–160
Hounsome J, Lee A, Greenhalgh J et al (2017) A systematic review of information format and timing before scheduled adult surgery for peri-operative anxiety. Anaesthesia 72:1265–1272
Wilson CJ, Mitchelson AJ, Tzeng TH et al (2016) Caring for the surgically anxious patient: a review of the interventions and a guide to optimizing surgical outcomes. Am J Surg 212:151–159
Ziehm S, Rosendahl J, Barth J et al (2017) Psychological interventions for acute pain after open heart surgery. Cochrane Database Syst Rev 7:CD009984
Granziera E, Guglieri I, Del Bianco P et al (2013) A multidisciplinary approach to improve preoperative understanding and reduce anxiety: a randomised study. Eur J Anaesthesiol 30:734–742
Sjoling M, Nordahl G, Olofsson N et al (2003) The impact of preoperative information on state anxiety, postoperative pain and satisfaction with pain management. Patient Educ Couns 51:169–176
Wongkietkachorn A, Wongkietkachorn N, Rhunsiri P (2017) Preoperative needs-based education to reduce anxiety, increase satisfaction, and decrease time spent in day surgery: a randomized controlled trial. World J Surg. https://doi.org/10.1007/s00268-017-4207-0
Forsmo HM, Pfeffer F, Rasdal A et al (2016) Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis 18:603–611
Powell R, Scott NW, Manyande A et al (2016) Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008646.pub2
Bekelis K, Calnan D, Simmons N et al (2017) Effect of an immersive preoperative virtual reality experience on patient reported outcomes: a randomized controlled trial. Ann Surg 265:1068–1073
Aasa A, Hovback M, Bertero CM (2013) The importance of preoperative information for patient participation in colorectal surgery care. J Clin Nurs 22:1604–1612
Cohen ME, Liu Y, Ko CY et al (2017) An examination of american college of surgeons NSQIP surgical risk calculator accuracy. J Am Coll Surg 224(787–795):e781
Jie B, Jiang ZM, Nolan MT et al (2012) Impact of preoperative nutritional support on clinical outcome in abdominal surgical patients at nutritional risk. Nutrition 28:1022–1027
Kristensen SD, Knuuti J, Saraste A et al (2014) 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 31:517–573
Rockwood K, Song X, MacKnight C et al (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ 173:489–495
Bluman LG, Mosca L, Newman N et al (1998) Preoperative smoking habits and postoperative pulmonary complications. Chest 113:883–889
Mills E, Eyawo O, Lockhart I et al (2011) Smoking cessation reduces postoperative complications: a systematic review and meta-analysis. Am J Med 124(144–154):e148
Thomsen T, Villebro N, Moller AM (2014) Interventions for preoperative smoking cessation. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002294.pub4
Wong J, Lam DP, Abrishami A et al (2012) Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth 59:268–279
Kork F, Neumann T, Spies C (2010) Perioperative management of patients with alcohol, tobacco and drug dependency. Curr Opin Anaesthesiol 23:384–390
Tonnesen H (2003) Alcohol abuse and postoperative morbidity. Dan Med Bull 50:139–160
Shabanzadeh DM, Sorensen LT (2015) Alcohol consumption increases post-operative infection but not mortality: a systematic review and meta-analysis. Surg Infect (Larchmt) 16:657–668
Oppedal K, Moller AM, Pedersen B et al (2012) Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD008343.pub2
Wilson RJ, Davies S, Yates D et al (2010) Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth 105:297–303
Carli F, Zavorsky GS (2005) Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care 8:23–32
Silver JK, Baima J (2013) Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 92:715–727
Carli F, Charlebois P, Stein B et al (2010) Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 97:1187–1197
Gillis C, Li C, Lee L et al (2014) Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 121:937–947
Barberan-Garcia A, Ubre M, Roca J et al (2018) Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 267:50–56
Correia MI, Waitzberg DL (2003) The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 22:235–239
Norman K, Pichard C, Lochs H et al (2008) Prognostic impact of disease-related malnutrition. Clin Nutr 27:5–15
Pacelli F, Bossola M, Rosa F et al (2008) Is malnutrition still a risk factor of postoperative complications in gastric cancer surgery? Clin Nutr 27:398–407
Pressoir M, Desne S, Berchery D et al (2010) Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br J Cancer 102:966–971
Gustafsson UO, Ljungqvist O (2011) Perioperative nutritional management in digestive tract surgery. Curr Opin Clin Nutr Metab Care 14:504–509
Schwegler I, von Holzen A, Gutzwiller JP et al (2010) Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg 97:92–97
Gibbs J, Cull W, Henderson W et al (1999) Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg 134:36–42
Hennessey DB, Burke JP, Ni-Dhonochu T et al (2010) Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg 252:325–329
Weimann A, Braga M, Carli F et al (2017) ESPEN guideline: clinical nutrition in surgery. Clin Nutr 36:623–650
Abe Vicente M, Barao K, Silva TD et al (2013) What are the most effective methods for assessment of nutritional status in outpatients with gastric and colorectal cancer? Nutr Hosp 28:585–591
Boleo-Tome C, Monteiro-Grillo I, Camilo M et al (2012) Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br J Nutr 108:343–348
Detsky AS, Baker JP, O’Rourke K et al (1987) Predicting nutrition-associated complications for patients undergoing gastrointestinal surgery. JPEN J Parenter Enteral Nutr 11:440–446
Faramarzi E, Mahdavi R, Mohammad-Zadeh M et al (2013) Validation of nutritional risk index method against patient-generated subjective global assessment in screening malnutrition in colorectal cancer patients. Chin J Cancer Res 25:544–548
Hakonsen SJ, Pedersen PU, Bath-Hextall F et al (2015) Diagnostic test accuracy of nutritional tools used to identify undernutrition in patients with colorectal cancer: a systematic review. JBI Database System Rev Implement Rep 13:141–187
Bozzetti F, Gianotti L, Braga M et al (2007) Postoperative complications in gastrointestinal cancer patients: the joint role of the nutritional status and the nutritional support. Clin Nutr 26:698–709
Waitzberg DL, Saito H, Plank LD et al (2006) Postsurgical infections are reduced with specialized nutrition support. World J Surg 30:1592–1604. https://doi.org/10.1007/s00268-005-0657-x
Butcher A, Richards T, Stanworth SJ et al (2017) Diagnostic criteria for pre-operative anaemia-time to end sex discrimination. Anaesthesia 72:811–814
Munoz M, Gomez-Ramirez S, Martin-Montanez E et al (2014) Perioperative anemia management in colorectal cancer patients: a pragmatic approach. World J Gastroenterol 20:1972–1985
Munoz M, Acheson AG, Auerbach M et al (2017) International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 72:233–247
Baron DM, Hochrieser H, Posch M et al (2014) Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 113:416–423
Smilowitz NR, Oberweis BS, Nukala S et al (2016) Association between anemia, bleeding, and transfusion with long-term mortality following noncardiac surgery. Am J Med 129(315–323):e312
Acheson AG, Brookes MJ, Spahn DR (2012) Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 256:235–244
Mazzeffi M, Tanaka K, Galvagno S (2017) Red blood cell transfusion and surgical site infection after colon resection surgery: a cohort study. Anesth Analg 125:1316–1321
Bennett S, Baker LK, Martel G et al (2017) The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford) 19:321–330
Schiergens TS, Rentsch M, Kasparek MS et al (2015) Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum 58:74–82
American Society of Anesthesiologists Task Force on Perioperative Blood M (2015) Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 122:241–275
Chertow GM, Mason PD, Vaage-Nilsen O et al (2006) Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant 21:378–382
Rampton D, Folkersen J, Fishbane S et al (2014) Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 99:1671–1676
Froessler B, Palm P, Weber I et al (2016) The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg 264:41–46
Gan TJ, Diemunsch P, Habib AS et al (2014) Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 118:85–113
Hill RP, Lubarsky DA, Phillips-Bute B et al (2000) Cost-effectiveness of prophylactic antiemetic therapy with ondansetron, droperidol, or placebo. Anesthesiology 92:958–967
Sarin P, Urman RD, Ohno-Machado L (2012) An improved model for predicting postoperative nausea and vomiting in ambulatory surgery patients using physician-modifiable risk factors. J Am Med Inform Assoc 19:995–1002
Apfel CC, Laara E, Koivuranta M et al (1999) A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 91:693–700
Apfel CC, Philip BK, Cakmakkaya OS et al (2012) Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology 117:475–486
Singh BN, Dahiya D, Bagaria D et al (2015) Effects of preoperative carbohydrates drinks on immediate postoperative outcome after day care laparoscopic cholecystectomy. Surg Endosc 29:3267–3272
Apfel CC, Kranke P, Eberhart LH et al (2002) Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth 88:234–240
Kappen TH, Moons KG, van Wolfswinkel L et al (2014) Impact of risk assessments on prophylactic antiemetic prescription and the incidence of postoperative nausea and vomiting: a cluster-randomized trial. Anesthesiology 120:343–354
Kappen TH, Vergouwe Y, van Wolfswinkel L et al (2015) Impact of adding therapeutic recommendations to risk assessments from a prediction model for postoperative nausea and vomiting. Br J Anaesth 114:252–260
Kranke P, Eberhart LH (2011) Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol 28:758–765
Apfel CC, Bacher A, Biedler A et al (2005) A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. Anaesthesist 54:201–209
Eberhart LH, Mauch M, Morin AM et al (2002) Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia 57:1022–1027
De Oliveira GS Jr, Castro-Alves LJ, Ahmad S et al (2013) Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 116:58–74
Collaborators DT, West Midlands Research C (2017) Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial). BMJ 357:j1455
Grant MC, Betz M, Hulse M et al (2016) The effect of preoperative pregabalin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg 123:1100–1107
Grant MC, Lee H, Page AJ et al (2016) The effect of preoperative gabapentin on postoperative nausea and vomiting: a meta-analysis. Anesth Analg 122:976–985
Liu M, Zhang H, Du BX et al (2015) Neurokinin-1 receptor antagonists in preventing postoperative nausea and vomiting: a systematic review and meta-analysis. Medicine (Baltimore) 94:e762
Apfel CC, Turan A, Souza K et al (2013) Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain 154:677–689
Stoicea N, Gan TJ, Joseph N et al (2015) Alternative therapies for the prevention of postoperative nausea and vomiting. Front Med (Lausanne) 2:87
Hovaguimian F, Lysakowski C, Elia N et al (2013) Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 119:303–316
Orhan-Sungur M, Kranke P, Sessler D et al (2008) Does supplemental oxygen reduce postoperative nausea and vomiting? A meta-analysis of randomized controlled trials. Anesth Analg 106:1733–1738
Ip HY, Abrishami A, Peng PW et al (2009) Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology 111:657–677
Mavros MN, Athanasiou S, Gkegkes ID et al (2011) Do psychological variables affect early surgical recovery? PLoS ONE 6:e20306
Kassie GM, Nguyen TA, Kalisch Ellett LM et al (2017) Preoperative medication use and postoperative delirium: a systematic review. BMC Geriatr 17:298
By the American Geriatrics Society Beers Criteria Update Expert P (2015) American Geriatrics Society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 63:2227–2246
Mijderwijk H, Vanb S, Duivenvoorden HJ et al (2016) Effectiveness of benzodiazepine premedication on recovery in day-case surgery: a systematic review with meta-analysis. Minerva Anestesiol 82:438–464
Walker KJ, Smith AF (2009) Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002192.pub2
Hansen MV, Halladin NL, Rosenberg J et al (2015) Melatonin for pre- and postoperative anxiety in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009861.pub2
Hurley RW, Cohen SP, Williams KA et al (2006) The analgesic effects of perioperative gabapentin on postoperative pain: a meta-analysis. Reg Anesth Pain Med 31:237–247
Mishriky BM, Waldron NH, Habib AS (2015) Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth 114:10–31
Nelson RL, Gladman E, Barbateskovic M (2014) Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001181.pub4
Weber WP, Mujagic E, Zwahlen M et al (2017) Timing of surgical antimicrobial prophylaxis: a phase 3 randomised controlled trial. Lancet Infect Dis 17:605–614
Chen M, Song X, Chen LZ et al (2016) Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum 59:70–78
Kiran RP, Murray AC, Chiuzan C et al (2015) Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 262:416–425 (discussion 423–415)
Darouiche RO, Wall MJ Jr, Itani KM et al (2010) Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26
Zhang D, Wang XC, Yang ZX et al (2017) Preoperative chlorhexidine versus povidone-iodine antisepsis for preventing surgical site infection: a meta-analysis and trial sequential analysis of randomized controlled trials. Int J Surg 44:176–184
Ploegmakers IB, Olde Damink SW, Breukink SO (2017) Alternatives to antibiotics for prevention of surgical infection. Br J Surg 104:e24–e33
Webster J, Osborne S (2015) Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004985.pub5
Tanner J, Norrie P, Melen K (2011) Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004122.pub4
Sanders G, Mercer SJ, Saeb-Parsey K et al (2001) Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. Br J Surg 88:1363–1365
Rollins KE, Javanmard-Emamghissi H, Lobo DN (2018) Impact of mechanical bowel preparation in elective colorectal surgery: a meta-analysis. World J Gastroenterol 24:519–536
Toneva GD, Deierhoi RJ, Morris M et al (2013) Oral antibiotic bowel preparation reduces length of stay and readmissions after colorectal surgery. J Am Coll Surg 216:756–762 (discussion 762-753)
Kim EK, Sheetz KH, Bonn J et al (2014) A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg 259:310–314
Koullouros M, Khan N, Aly EH (2017) The role of oral antibiotics prophylaxis in prevention of surgical site infection in colorectal surgery. Int J Colorectal Dis 32:1–18
Garfinkle R, Abou-Khalil J, Morin N et al (2017) Is there a role for oral antibiotic preparation alone before colorectal surgery? ACS-NSQIP analysis by coarsened exact matching. Dis Colon Rectum 60:729–737
Sanders G, Arthur CH, Hosie KB et al (2007) Is patient outcome affected by the administration of intravenous fluid during bowel preparation for colonic surgery? Ann R Coll Surg Engl 89:487–489
(2017) Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Task Force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology 126:376–393
Brady M, Kinn S, Ness V et al (2009) Preoperative fasting for preventing perioperative complications in children. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005285.pub2
Brady M, Kinn S, Stuart P (2003) Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004423
Nygren J (2006) The metabolic effects of fasting and surgery. Best Pract Res Clin Anaesthesiol 20:429–438
Gianotti L, Biffi R, Sandini M et al (2018) Preoperative oral carbohydrate load versus placebo in major elective abdominal surgery (PROCY): a randomized, placebo-controlled, multicenter, phase III trial. Ann Surg 267:623–630
Lee B, Soh S, Shim JK et al (2017) Evaluation of preoperative oral carbohydrate administration on insulin resistance in off-pump coronary artery bypass patients: a randomised trial. Eur J Anaesthesiol 34:740–747
Smith MD, McCall J, Plank L et al (2014) Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009161.pub2
Maltby JR, Pytka S, Watson NC et al (2004) Drinking 300 mL of clear fluid two hours before surgery has no effect on gastric fluid volume and pH in fasting and non-fasting obese patients. Can J Anaesth 51:111–115
Gustafsson UO, Nygren J, Thorell A et al (2008) Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand 52:946–951
Hellstrom PM, Samuelsson B, Al-Ani AN et al (2017) Normal gastric emptying time of a carbohydrate-rich drink in elderly patients with acute hip fracture: a pilot study. BMC Anesthesiol 17:23
Azagury DE, Ris F, Pichard C et al (2015) Does perioperative nutrition and oral carbohydrate load sustainably preserve muscle mass after bariatric surgery? A randomized control trial. Surg Obes Relat Dis 11:920–926
Wigmore TJ, Mohammed K, Jhanji S (2016) Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 124:69–79
Martin DS, Grocott MP (2015) Oxygen therapy and anaesthesia: too much of a good thing? Anaesthesia 70:522–527
Myles PS, Leslie K, Chan MT et al (2014) The safety of addition of nitrous oxide to general anaesthesia in at-risk patients having major non-cardiac surgery (ENIGMA-II): a randomised, single-blind trial. Lancet 384:1446–1454
Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N (2014) Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003843.pub3
Chan MT, Cheng BC, Lee TM et al (2013) BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 25:33–42
Demyttenaere S, Feldman LS, Fried GM (2007) Effect of pneumoperitoneum on renal perfusion and function: a systematic review. Surg Endosc 21:152–160
Bruintjes MH, van Helden EV, Braat AE et al (2017) Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: a systematic review and meta-analysis. Br J Anaesth 118:834–842
Sodha S, Nazarian S, Adshead JM et al (2016) Effect of pneumoperitoneum on renal function and physiology in patients undergoing robotic renal surgery. Curr Urol 9:1–4
McLean DJ, Diaz-Gil D, Farhan HN et al (2015) Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology 122:1201–1213
Brull SJ, Prielipp RC (2017) Neuromuscular monitoring and the cost of antagonism: when will we learn? Anaesthesia 72:1557–1558
Abad-Gurumeta A, Ripolles-Melchor J, Casans-Frances R et al (2015) A systematic review of sugammadex vs neostigmine for reversal of neuromuscular blockade. Anaesthesia 70:1441–1452
Thiele RH, Raghunathan K, Brudney CS et al (2016) American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on perioperative fluid management within an enhanced recovery pathway for colorectal surgery. Perioper Med (Lond) 5:24
Gan TJ, Soppitt A, Maroof M et al (2002) Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 97:820–826
Noblett SE, Snowden CP, Shenton BK et al (2006) Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 93:1069–1076
Feldheiser A, Aziz O, Baldini G et al (2016) Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 60:289–334
Brandstrup B, Svendsen PE, Rasmussen M et al (2012) Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth 109:191–199
Gomez-Izquierdo JC, Trainito A, Mirzakandov D et al (2017) Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Anesthesiology 127:36–49
Srinivasa S, Taylor MH, Singh PP et al (2013) Randomized clinical trial of goal-directed fluid therapy within an enhanced recovery protocol for elective colectomy. Br J Surg 100:66–74
Rollins KE, Lobo DN (2016) Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg 263:465–476
Bijker JB, van Klei WA, Vergouwe Y et al (2009) Intraoperative hypotension and 1-year mortality after noncardiac surgery. Anesthesiology 111:1217–1226
Feldheiser A, Conroy P, Bonomo T et al (2012) Development and feasibility study of an algorithm for intraoperative goal directed haemodynamic management in noncardiac surgery. J Int Med Res 40:1227–1241
Billeter AT, Hohmann SF, Druen D et al (2014) Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery 156:1245–1252
Sessler DI (2016) Perioperative thermoregulation and heat balance. Lancet 387:2655–2664
Rajagopalan S, Mascha E, Na J et al (2008) The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 108:71–77
(2008) Hypothermia: prevention and management in adults having surgery. National Institute for Health and Care Excellence, London
(2017) Bair Hugger for measuring core temperature during perioperative care. National Institute for Health and Care Excellence, London
Dean M, Ramsay R, Heriot A et al (2017) Warmed, humidified CO2 insufflation benefits intraoperative core temperature during laparoscopic surgery: a meta-analysis. Asian J Endosc Surg 10:128–136
Birch DW, Dang JT, Switzer NJ et al (2016) Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev 10:CD007821
Connelly L, Cramer E, DeMott Q et al (2017) The optimal time and method for surgical prewarming: a comprehensive review of the literature. J Perianesth Nurs 32:199–209
Roberson MC, Dieckmann LS, Rodriguez RE et al (2013) A review of the evidence for active preoperative warming of adults undergoing general anesthesia. AANA J 81:351–356
de Neree Tot Babberich MPM, van Groningen JT, Dekker E et al (2018) Laparoscopic conversion in colorectal cancer surgery; is there any improvement over time at a population level? Surg Endosc 32:3234–3246
Coleman MG, Hanna GB, Kennedy R et al (2011) The national training programme for laparoscopic colorectal surgery in England: a new training paradigm. Colorectal Dis 13:614–616
Bonjer HJ, Deijen CL, Abis GA et al (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372:1324–1332
Green BL, Marshall HC, Collinson F et al (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Jeong SY, Park JW, Nam BH et al (2014) Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 15:767–774
Kang SB, Park JW, Jeong SY et al (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11:637–645
Lacy AM, Delgado S, Castells A et al (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248:1–7
Weeks JC, Nelson H, Gelber S et al (2002) Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 287:321–328
Gietelink L, Wouters MW, Bemelman WA et al (2016) Reduced 30-day mortality after laparoscopic colorectal cancer surgery: a population based study from the Dutch Surgical Colorectal Audit (DSCA). Ann Surg 264:135–140
Kuhry E, Schwenk WF, Gaupset R et al (2008) Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003432.pub2
Schwenk W, Haase O, Neudecker J et al (2005) Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003145.pub2
Vennix S, Pelzers L, Bouvy N et al (2014) Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD005200.pub3
Lim DR, Bae SU, Hur H et al (2017) Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg Endosc 31:1728–1737
Fleshman J, Branda M, Sargent DJ et al (2015) Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 Randomized Clinical Trial. JAMA 314:1346–1355
Stevenson AR, Solomon MJ, Lumley JW et al (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314:1356–1363
Tyler JA, Fox JP, Desai MM et al (2013) Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum 56:458–466
Gash KJ, Goede AC, Chambers W et al (2011) Laparoendoscopic single-site surgery is feasible in complex colorectal resections and could enable day case colectomy. Surg Endosc 25:835–840
Lacy AM, Tasende MM, Delgado S et al (2015) Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg 221:415–423
Trastulli S, Farinella E, Cirocchi R et al (2012) Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 14:e134–e156
Jayne D, Pigazzi A, Marshall H et al (2017) Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR Randomized Clinical Trial. JAMA 318:1569–1580
Atallah S, Martin-Perez B, Albert M et al (2014) Transanal minimally invasive surgery for total mesorectal excision (TAMIS-TME): results and experience with the first 20 patients undergoing curative-intent rectal cancer surgery at a single institution. Tech Coloproctol 18:473–480
Buchs NC, Nicholson GA, Ris F et al (2015) Transanal total mesorectal excision: a valid option for rectal cancer? World J Gastroenterol 21:11700–11708
Ma B, Gao P, Song Y et al (2016) Transanal total mesorectal excision (taTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer 16:380
Simillis C, Hompes R, Penna M et al (2016) A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis 18:19–36
Penninckx F, Kartheuser A, Van de Stadt J et al (2013) Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg 100:1368–1375
Deijen CL, Velthuis S, Tsai A et al (2016) COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 30:3210–3215
Watt DG, McSorley ST, Horgan PG et al (2015) Enhanced recovery after surgery: which components, if any, impact on the systemic inflammatory response following colorectal surgery?: a systematic review. Medicine (Baltimore) 94:e1286
Esteban F, Cerdan FJ, Garcia-Alonso M et al (2014) A multicentre comparison of a fast track or conventional postoperative protocol following laparoscopic or open elective surgery for colorectal cancer surgery. Colorectal Dis 16:134–140
Kennedy RH, Francis EA, Wharton R et al (2014) Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 32:1804–1811
Vlug MS, Wind J, Hollmann MW et al (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254:868–875
Currie AC, Malietzis G, Jenkins JT et al (2016) Network meta-analysis of protocol-driven care and laparoscopic surgery for colorectal cancer. Br J Surg 103:1783–1794
Jesus EC, Karliczek A, Matos D et al (2004) Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002100.pub2
Bretagnol F, Slim K, Faucheron JL (2005) Anterior resection with low colorectal anastomosis. To drain or not? Ann Chir 130:336–339
Zhang HY, Zhao CL, Xie J et al (2016) To drain or not to drain in colorectal anastomosis: a meta-analysis. Int J Colorectal Dis 31:951–960
Denost Q, Rouanet P, Faucheron JL et al (2017) To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: the GRECCAR 5 randomized trial. Ann Surg 265:474–480
Rao W, Zhang X, Zhang J et al (2011) The role of nasogastric tube in decompression after elective colon and rectum surgery: a meta-analysis. Int J Colorectal Dis 26:423–429
Nelson R, Edwards S, Tse B (2007) Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004929.pub3
Jottard K, Hoff C, Maessen J et al (2009) Life and death of the nasogastric tube in elective colonic surgery in the Netherlands. Clin Nutr 28:26–28
Carmichael JC, Keller DS, Baldini G et al (2017) Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 60:761–784
Li K, Zhou Z, Chen Z et al (2011) “Fast Track” nasogastric decompression of rectal cancer surgery. Front Med 5:306–309
Zhuang CL, Ye XZ, Zhang CJ et al (2013) Early versus traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg 30:225–232
Group EC (2015) The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 261:1153–1159
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced recovery after surgery: a review. JAMA Surg 152:292–298
Kverneng Hultberg D, Angenete E, Lydrup ML et al (2017) Nonsteroidal anti-inflammatory drugs and the risk of anastomotic leakage after anterior resection for rectal cancer. Eur J Surg Oncol 43:1908–1914
Bell RF, Dahl JB, Moore RA et al (2006) Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004603.pub2
Cheung CW, Qiu Q, Ying AC et al (2014) The effects of intra-operative dexmedetomidine on postoperative pain, side-effects and recovery in colorectal surgery. Anaesthesia 69:1214–1221
Eipe N, Penning J, Yazdi F et al (2015) Perioperative use of pregabalin for acute pain-a systematic review and meta-analysis. Pain 156:1284–1300
Ge DJ, Qi B, Tang G et al (2016) Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep 6:21514
Kranke P, Jokinen J, Pace NL et al (2015) Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD009642.pub2
Hamilton TW, Athanassoglou V, Mellon S et al (2017) Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev 2:CD011419
Carli F, Kehlet H, Baldini G et al (2011) Evidence basis for regional anesthesia in multidisciplinary fast-track surgical care pathways. Reg Anesth Pain Med 36:63–72
Uchida I, Asoh T, Shirasaka C et al (1988) Effect of epidural analgesia on postoperative insulin resistance as evaluated by insulin clamp technique. Br J Surg 75:557–562
Carli F, Halliday D (1997) Continuous epidural blockade arrests the postoperative decrease in muscle protein fractional synthetic rate in surgical patients. Anesthesiology 86:1033–1040
Schricker T, Meterissian S, Wykes L et al (2004) Postoperative protein sparing with epidural analgesia and hypocaloric dextrose. Ann Surg 240:916–921
Soop M, Carlson GL, Hopkinson J et al (2004) Randomized clinical trial of the effects of immediate enteral nutrition on metabolic responses to major colorectal surgery in an enhanced recovery protocol. Br J Surg 91:1138–1145
Block BM, Liu SS, Rowlingson AJ et al (2003) Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 290:2455–2463
Werawatganon T, Charuluxanun S (2005) Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004088.pub2
Hubner M, Blanc C, Roulin D et al (2015) Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg 261:648–653
McCarthy GC, Megalla SA, Habib AS (2010) Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 70:1149–1163
Tikuisis R, Miliauskas P, Samalavicius NE et al (2014) Intravenous lidocaine for post-operative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctol 18:373–380
Ventham NT, Kennedy ED, Brady RR et al (2015) Efficacy of intravenous lidocaine for postoperative analgesia following laparoscopic surgery: a meta-analysis. World J Surg 39:2220–2234. https://doi.org/10.1007/s00268-015-3105-6
Day AR, Smith RV, Scott MJ et al (2015) Randomized clinical trial investigating the stress response from two different methods of analgesia after laparoscopic colorectal surgery. Br J Surg 102:1473–1479
Hubner M, Lovely JK, Huebner M et al (2013) Intrathecal analgesia and restrictive perioperative fluid management within enhanced recovery pathway: hemodynamic implications. J Am Coll Surg 216:1124–1134
Levy BF, Scott MJ, Fawcett W et al (2011) Randomized clinical trial of epidural, spinal or patient-controlled analgesia for patients undergoing laparoscopic colorectal surgery. Br J Surg 98:1068–1078
Levy BF, Scott MJ, Fawcett WJ et al (2009) 23-hour-stay laparoscopic colectomy. Dis Colon Rectum 52:1239–1243
Wongyingsinn M, Baldini G, Stein B et al (2012) Spinal analgesia for laparoscopic colonic resection using an enhanced recovery after surgery programme: better analgesia, but no benefits on postoperative recovery: a randomized controlled trial. Br J Anaesth 108:850–856
Fares KM, Mohamed SA, Abd El-Rahman AM et al (2015) Efficacy and safety of intraperitoneal dexmedetomidine with bupivacaine in laparoscopic colorectal cancer surgery, a randomized trial. Pain Med 16:1186–1194
Park YH, Kang H, Woo YC et al (2011) The effect of intraperitoneal ropivacaine on pain after laparoscopic colectomy: a prospective randomized controlled trial. J Surg Res 171:94–100
Fustran N, Dalmau A, Ferreres E et al (2015) Postoperative analgesia with continuous wound infusion of local anaesthesia vs saline: a double-blind randomized, controlled trial in colorectal surgery. Colorectal Dis 17:342–350
Krishnan S, Morris RG, Hewett PJ et al (2014) A randomized double-blind clinical trial of a continuous 96-hour levobupivacaine infiltration after open or laparoscopic colorectal surgery for postoperative pain management—including clinically important changes in protein binding. Ther Drug Monit 36:202–210
Ventham NT, O’Neill S, Johns N et al (2014) Evaluation of novel local anesthetic wound infiltration techniques for postoperative pain following colorectal resection surgery: a meta-analysis. Dis Colon Rectum 57:237–250
Joshi GP, Bonnet F, Kehlet H et al (2013) Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis 15:146–155
Wongyingsinn M, Baldini G, Charlebois P et al (2011) Intravenous lidocaine versus thoracic epidural analgesia: a randomized controlled trial in patients undergoing laparoscopic colorectal surgery using an enhanced recovery program. Reg Anesth Pain Med 36:241–248
Curatolo M, Petersen-Felix S, Scaramozzino P et al (1998) Epidural fentanyl, adrenaline and clonidine as adjuvants to local anaesthetics for surgical analgesia: meta-analyses of analgesia and side-effects. Acta Anaesthesiol Scand 42:910–920
Persec J, Persec Z, Bukovic D et al (2007) Effects of clonidine preemptive analgesia on acute postoperative pain in abdominal surgery. Coll Antropol 31:1071–1075
Wu CT, Jao SW, Borel CO et al (2004) The effect of epidural clonidine on perioperative cytokine response, postoperative pain, and bowel function in patients undergoing colorectal surgery. Anesth Analg 99:502–509 (table of contents)
Niemi G, Breivik H (2002) Epinephrine markedly improves thoracic epidural analgesia produced by a small-dose infusion of ropivacaine, fentanyl, and epinephrine after major thoracic or abdominal surgery: a randomized, double-blinded crossover study with and without epinephrine. Anesth Analg 94:1598–1605 (table of contents)
Niemi G, Breivik H (2003) The minimally effective concentration of adrenaline in a low-concentration thoracic epidural analgesic infusion of bupivacaine, fentanyl and adrenaline after major surgery. A randomized, double-blind, dose-finding study. Acta Anaesthesiol Scand 47:439–450
Ong CK, Lirk P, Seymour RA et al (2005) The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg 100:757–773 (table of contents)
Arnuntasupakul V, Van Zundert TC, Vijitpavan A et al (2016) A randomized comparison between conventional and waveform-confirmed loss of resistance for thoracic epidural blocks. Reg Anesth Pain Med 41:368–373
Tran DQ, Van Zundert TC, Aliste J et al (2016) Primary failure of thoracic epidural analgesia in training centers: the invisible elephant? Reg Anesth Pain Med 41:309–313
Guay J, Nishimori M, Kopp S (2016) Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev 7:CD001893
Khan SA, Khokhar HA, Nasr AR et al (2013) Effect of epidural analgesia on bowel function in laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc 27:2581–2591
Popping DM, Elia N, Van Aken HK et al (2014) Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 259:1056–1067
Popping DM, Elia N, Marret E et al (2008) Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 143:990–999 (discussion 1000)
Borzellino G, Francis NK, Chapuis O et al (2016) Role of epidural analgesia within an ERAS program after laparoscopic colorectal surgery: a review and meta-analysis of randomised controlled studies. Surg Res Pract 2016:7543684
Liu H, Hu X, Duan X et al (2014) Thoracic epidural analgesia (TEA) vs. patient controlled analgesia (PCA) in laparoscopic colectomy: a meta-analysis. Hepatogastroenterology 61:1213–1219
Halabi WJ, Kang CY, Nguyen VQ et al (2014) Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg 149:130–136
Hanna MH, Jafari MD, Jafari F et al (2017) Randomized clinical trial of epidural compared with conventional analgesia after minimally invasive colorectal surgery. J Am Coll Surg 225:622–630
Christopherson R, James KE, Tableman M et al (2008) Long-term survival after colon cancer surgery: a variation associated with choice of anesthesia. Anesth Analg 107:325–332
Day A, Smith R, Jourdan I et al (2012) Retrospective analysis of the effect of postoperative analgesia on survival in patients after laparoscopic resection of colorectal cancer. Br J Anaesth 109:185–190
Cook TM, Counsell D, Wildsmith JA et al (2009) Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 102:179–190
Dunn LK, Durieux ME (2017) Perioperative use of intravenous lidocaine. Anesthesiology 126:729–737
Sun Y, Li T, Wang N et al (2012) Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 55:1183–1194
Khan JS, Yousuf M, Victor JC et al (2016) An estimation for an appropriate end time for an intraoperative intravenous lidocaine infusion in bowel surgery: a comparative meta-analysis. J Clin Anesth 28:95–104
Turunen P, Carpelan-Holmstrom M, Kairaluoma P et al (2009) Epidural analgesia diminished pain but did not otherwise improve enhanced recovery after laparoscopic sigmoidectomy: a prospective randomized study. Surg Endosc 23:31–37
Rafi AN (2001) Abdominal field block: a new approach via the lumbar triangle. Anaesthesia 56:1024–1026
Tran TM, Ivanusic JJ, Hebbard P et al (2009) Determination of spread of injectate after ultrasound-guided transversus abdominis plane block: a cadaveric study. Br J Anaesth 102:123–127
Keller DS, Ermlich BO, Delaney CP (2014) Demonstrating the benefits of transversus abdominis plane blocks on patient outcomes in laparoscopic colorectal surgery: review of 200 consecutive cases. J Am Coll Surg 219:1143–1148
Charlton S, Cyna AM, Middleton P et al (2010) Perioperative transversus abdominis plane (TAP) blocks for analgesia after abdominal surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007705.pub2
Elamin G, Waters PS, Hamid H et al (2015) Efficacy of a laparoscopically delivered transversus abdominis plane block technique during elective laparoscopic cholecystectomy: a prospective, double-blind randomized trial. J Am Coll Surg 221:335–344
Fields AC, Gonzalez DO, Chin EH et al (2015) Laparoscopic-assisted transversus abdominis plane block for postoperative pain control in laparoscopic ventral hernia repair: a randomized controlled trial. J Am Coll Surg 221:462–469
Guner Can M, Goz R, Berber I et al (2015) Ultrasound/laparoscopic camera-guided transversus abdominis plane block for renal transplant donors: a randomized controlled trial. Ann Transplant 20:418–423
Sinha A, Jayaraman L, Punhani D (2013) Efficacy of ultrasound-guided transversus abdominis plane block after laparoscopic bariatric surgery: a double blind, randomized, controlled study. Obes Surg 23:548–553
Taylor R Jr, Pergolizzi JV, Sinclair A et al (2013) Transversus abdominis block: clinical uses, side effects, and future perspectives. Pain Pract 13:332–344
Tikuisis R, Miliauskas P, Lukoseviciene V et al (2016) Transversus abdominis plane block for postoperative pain relief after hand-assisted laparoscopic colon surgery: a randomized, placebo-controlled clinical trial. Tech Coloproctol 20:835–844
Walter CJ, Maxwell-Armstrong C, Pinkney TD et al (2013) A randomised controlled trial of the efficacy of ultrasound-guided transversus abdominis plane (TAP) block in laparoscopic colorectal surgery. Surg Endosc 27:2366–2372
Oh TK, Yim J, Kim J et al (2017) Effects of preoperative ultrasound-guided transversus abdominis plane block on pain after laparoscopic surgery for colorectal cancer: a double-blind randomized controlled trial. Surg Endosc 31:127–134
Stokes AL, Adhikary SD, Quintili A et al (2017) Liposomal bupivacaine use in transversus abdominis plane blocks reduces pain and postoperative intravenous opioid requirement after colorectal surgery. Dis Colon Rectum 60:170–177
Akkaya A, Yildiz I, Tekelioglu UY et al (2014) Dexamethasone added to levobupivacaine in ultrasound-guided tranversus abdominis plain block increased the duration of postoperative analgesia after caesarean section: a randomized, double blind, controlled trial. Eur Rev Med Pharmacol Sci 18:717–722
Hamada T, Tsuchiya M, Mizutani K et al (2016) Levobupivacaine-dextran mixture for transversus abdominis plane block and rectus sheath block in patients undergoing laparoscopic colectomy: a randomised controlled trial. Anaesthesia 71:411–416
Gadsden J, Ayad S, Gonzales JJ et al (2015) Evolution of transversus abdominis plane infiltration techniques for postsurgical analgesia following abdominal surgeries. Local Reg Anesth 8:113–117
McLeod RS, Geerts WH, Sniderman KW et al (2001) Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg 233:438–444
Iannuzzi JC, Aquina CT, Rickles AS et al (2016) Risk factors for postdischarge venothromboembolism after colorectal resection. Dis Colon Rectum 59:224–229
Hill J, Treasure T (2010) [Decreasing the venous thromboembolism risk Summary of the NICE guidelines]. Praxis (Bern 1994) 99:977–980
Kakkos SK, Caprini JA, Geroulakos G et al (2016) Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev 9:CD005258
Sachdeva A, Dalton M, Amaragiri SV et al (2014) Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD001484.pub3
Rasmussen MS, Jorgensen LN, Wille-Jorgensen P (2009) Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004318.pub2
van Dongen CJ, MacGillavry MR, Prins MH (2005) Once versus twice daily LMWH for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003074.pub2
Sammour T, Chandra R, Moore JW (2016) Extended venous thromboembolism prophylaxis after colorectal cancer surgery: the current state of the evidence. J Thromb Thrombolysis 42:27–32
Pedersen AB, Sorensen HT, Mehnert F et al (2015) Effectiveness and safety of different duration of thromboprophylaxis in 16,865 hip replacement patients—a real-word, prospective observational study. Thromb Res 135:322–328
Lassen K, Kjaeve J, Fetveit T et al (2008) Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 247:721–729
Padhi S, Bullock I, Li L et al (2013) Intravenous fluid therapy for adults in hospital: summary of NICE guidance. BMJ 347:f7073
Lobo DN, Bostock KA, Neal KR et al (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 359:1812–1818
Van Regenmortel N, De Weerdt T, Van Craenenbroeck AH et al (2017) Effect of isotonic versus hypotonic maintenance fluid therapy on urine output, fluid balance, and electrolyte homeostasis: a crossover study in fasting adult volunteers. Br J Anaesth 118:892–900
Gould TH, Grace K, Thorne G et al (2002) Effect of thoracic epidural anaesthesia on colonic blood flow. Br J Anaesth 89:446–451
Holte K, Foss NB, Svensen C et al (2004) Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology 100:281–286
Lobo DN (2009) Fluid overload and surgical outcome: another piece in the jigsaw. Ann Surg 249:186–188
Shin CH, Long DR, McLean D et al (2018) Effects of intraoperative fluid management on postoperative outcomes: a hospital registry study. Ann Surg 267:1084–1092
Thacker JK, Mountford WK, Ernst FR et al (2016) Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg 263:502–510
Hansen PB, Jensen BL, Skott O (1998) Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension 32:1066–1070
Lobo DN, Awad S (2014) Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury?: con. Kidney Int 86:1096–1105
Reid F, Lobo DN, Williams RN et al (2003) (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci (Lond) 104:17–24
Wilcox CS (1983) Regulation of renal blood flow by plasma chloride. J Clin Invest 71:726–735
Williams EL, Hildebrand KL, McCormick SA et al (1999) The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg 88:999–1003
Shaw AD, Bagshaw SM, Goldstein SL et al (2012) Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 255:821–829
Yunos NM, Bellomo R, Hegarty C et al (2012) Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 308:1566–1572
Shaw AD, Schermer CR, Lobo DN et al (2015) Impact of intravenous fluid composition on outcomes in patients with systemic inflammatory response syndrome. Crit Care 19:334
McCluskey SA, Karkouti K, Wijeysundera D et al (2013) Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 117:412–421
Kawano-Dourado L, Zampieri FG, Azevedo LCP et al (2018) Low- versus high-chloride content intravenous solutions for critically ill and perioperative adult patients: a systematic review and meta-analysis. Anesth Analg 126:513–521
Myles PS, Andrews S, Nicholson J et al (2017) Contemporary approaches to perioperative IV fluid therapy. World J Surg 41:2457–2463. https://doi.org/10.1007/s00268-017-4055-y
Prowle JR, Kirwan CJ, Bellomo R (2014) Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 10:37–47
Zacharias M, Mugawar M, Herbison GP et al (2013) Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003590.pub4
Egal M, de Geus HR, van Bommel J et al (2016) Targeting oliguria reversal in perioperative restrictive fluid management does not influence the occurrence of renal dysfunction: a systematic review and meta-analysis. Eur J Anaesthesiol 33:425–435
Matot I, Paskaleva R, Eid L et al (2012) Effect of the volume of fluids administered on intraoperative oliguria in laparoscopic bariatric surgery: a randomized controlled trial. Arch Surg 147:228–234
Neal JM, Wilcox RT, Allen HW et al (2003) Near-total esophagectomy: the influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med 28:328–334
Puckett JR, Pickering JW, Palmer SC et al (2017) Low versus standard urine output targets in patients undergoing major abdominal surgery: a randomized noninferiority trial. Ann Surg 265:874–881
Ho KM, Sheridan DJ (2006) Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 333:420
Zaouter C, Kaneva P, Carli F (2009) Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med 34:542–548
Grass F, Slieker J, Frauche P et al (2017) Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surg Res 207:70–76
Klahr S, Miller SB (1998) Acute oliguria. N Engl J Med 338:671–675
Benoist S, Panis Y, Denet C et al (1999) Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery 125:135–141
Zmora O, Madbouly K, Tulchinsky H et al (2010) Urinary bladder catheter drainage following pelvic surgery—is it necessary for that long? Dis Colon Rectum 53:321–326
Han CS, Kim S, Radadia KD et al (2017) Comparison of urinary tract infection rates associated with transurethral catheterization, suprapubic tube and clean intermittent catheterization in the postoperative setting: a network meta-analysis. J Urol 198:1353–1358
Schwenk ES, Grant AE, Torjman MC et al (2017) The efficacy of peripheral opioid antagonists in opioid-induced constipation and postoperative ileus: a systematic review of the literature. Reg Anesth Pain Med 42:767–777
Yu CS, Chun HK, Stambler N et al (2011) Safety and efficacy of methylnaltrexone in shortening the duration of postoperative ileus following segmental colectomy: results of two randomized, placebo-controlled phase 3 trials. Dis Colon Rectum 54:570–578
Short V, Herbert G, Perry R et al (2015) Chewing gum for postoperative recovery of gastrointestinal function. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006506.pub3
Matros E, Rocha F, Zinner M et al (2006) Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized, placebo-controlled trial. J Am Coll Surg 202:773–778
de Leede EM, van Leersum NJ, Kroon HM et al (2018) Multicentre randomized clinical trial of the effect of chewing gum after abdominal surgery. Br J Surg 105:820–828
Zingg U, Miskovic D, Pasternak I et al (2008) Effect of bisacodyl on postoperative bowel motility in elective colorectal surgery: a prospective, randomized trial. Int J Colorectal Dis 23:1175–1183
Hansen CT, Sorensen M, Moller C et al (2007) Effect of laxatives on gastrointestinal functional recovery in fast-track hysterectomy: a double-blind, placebo-controlled randomized study. Am J Obstet Gynecol 196(311):e311–e317
Yoshikawa K, Shimada M, Wakabayashi G et al (2015) Effect of daikenchuto, a traditional japanese herbal medicine, after total gastrectomy for gastric cancer: a multicenter, randomized, double-blind, placebo-controlled, phase II trial. J Am Coll Surg 221:571–578
Muller SA, Rahbari NN, Schneider F et al (2012) Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg 99:1530–1538
Dulskas A, Klimovskij M, Vitkauskiene M et al (2015) Effect of coffee on the length of postoperative ileus after elective laparoscopic left-sided colectomy: a randomized, prospective single-center study. Dis Colon Rectum 58:1064–1069
Thorell A, Efendic S, Gutniak M et al (1994) Insulin resistance after abdominal surgery. Br J Surg 81:59–63
van den Berghe G, Wouters P, Weekers F et al (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345:1359–1367
Australian N-SSIft, New Zealand Intensive Care Society Clinical Trials G, the Canadian Critical Care Trials G et al (2015) Intensive versus conventional glucose control in critically ill patients with traumatic brain injury: long-term follow-up of a subgroup of patients from the NICE-SUGAR study. Intensive Care Med 41:1037–1047
Soop M, Nygren J, Thorell A et al (2004) Preoperative oral carbohydrate treatment attenuates endogenous glucose release 3 days after surgery. Clin Nutr 23:733–741
Veenhof AA, Vlug MS, van der Pas MH et al (2012) Surgical stress response and postoperative immune function after laparoscopy or open surgery with fast track or standard perioperative care: a randomized trial. Ann Surg 255:216–221
Gustafsson UO, Thorell A, Soop M et al (2009) Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg 96:1358–1364
Kotagal M, Symons RG, Hirsch IB et al (2015) Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 261:97–103
Andersen HK, Lewis SJ, Thomas S (2006) Early enteral nutrition within 24 h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004080.pub2
Lau C, Phillips E, Bresee C et al (2014) Early use of low residue diet is superior to clear liquid diet after elective colorectal surgery: a randomized controlled trial. Ann Surg 260:641–647 (discussion 647–649)
Smedley F, Bowling T, James M et al (2004) Randomized clinical trial of the effects of preoperative and postoperative oral nutritional supplements on clinical course and cost of care. Br J Surg 91:983–990
Mauskopf JA, Candrilli SD, Chevrou-Severac H et al (2012) Immunonutrition for patients undergoing elective surgery for gastrointestinal cancer: impact on hospital costs. World J Surg Oncol 10:136
Cerantola Y, Hubner M, Grass F et al (2011) Immunonutrition in gastrointestinal surgery. Br J Surg 98:37–48
Marimuthu K, Varadhan KK, Ljungqvist O et al (2012) A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg 255:1060–1068
Moya P, Miranda E, Soriano-Irigaray L et al (2016) Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc 30:4946–4953
Moya P, Soriano-Irigaray L, Ramirez JM et al (2016) Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an Enhanced Recovery (ERAS) protocol: a multicenter randomized clinical trial (SONVI Study). Medicine (Baltimore) 95:e3704
(1958) BED REST, thrombosis, and embolism. Lancet 1:465–466
Brower RG (2009) Consequences of bed rest. Crit Care Med 37:S422–S428
Harper CM, Lyles YM (1988) Physiology and complications of bed rest. J Am Geriatr Soc 36:1047–1054
Castelino T, Fiore JF Jr, Niculiseanu P et al (2016) The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: a systematic review. Surgery 159:991–1003
Schaller SJ, Anstey M, Blobner M et al (2016) Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 388:1377–1388
Haines KJ, Skinner EH, Berney S et al (2013) Association of postoperative pulmonary complications with delayed mobilisation following major abdominal surgery: an observational cohort study. Physiotherapy 99:119–125
Smart NJ, White P, Allison AS et al (2012) Deviation and failure of enhanced recovery after surgery following laparoscopic colorectal surgery: early prediction model. Colorectal Dis 14:e727–e734
van Zelm R, Coeckelberghs E, Sermeus W et al (2017) Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Int J Colorectal Dis 32:1471–1478
Fiore JF Jr, Castelino T, Pecorelli N et al (2017) Ensuring early mobilization within an enhanced recovery program for colorectal surgery: a randomized controlled trial. Ann Surg 266:223–231
Ivers N, Jamtvedt G, Flottorp S et al (2012) Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000259.pub3
Maessen J, Dejong CH, Hausel J et al (2007) A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg 94:224–231
Francis NK, Walker T, Carter F et al (2018) Consensus on training and implementation of enhanced recovery after surgery: a Delphi study. World J Surg. https://doi.org/10.1007/s00268-017-4436-2
Gustafsson UO, Oppelstrup H, Thorell A et al (2016) Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg 40:1741–1747. https://doi.org/10.1007/s00268-016-3460-y
Gillissen F, Ament SM, Maessen JM et al (2015) Sustainability of an enhanced recovery after surgery program (ERAS) in colonic surgery. World J Surg 39:526–533. https://doi.org/10.1007/s00268-014-2744-3
Bernard H, Foss M (2014) The impact of the enhanced recovery after surgery (ERAS) programme on community nursing. Br J Community Nurs 19(184):186–188
Hubner M, Addor V, Slieker J et al (2015) The impact of an enhanced recovery pathway on nursing workload: a retrospective cohort study. Int J Surg 24:45–50
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gustafsson has nothing to disclose. Dr. Scott reports personal fees from Merck and personal fees from Edwards Lifescience, outside the submitted work. Dr. Hubner has nothing to disclose. Dr. Nygren has nothing to disclose. Dr. Demartines reports payments from In Court and payments from MDT pancreas cancer, outside the submitted work. Dr. Francis has nothing to disclose. Dr. Rockall has nothing to disclose. Dr. Young Fadok reports personal fees from Pacira, outside the submitted work; and is President of ERAS USA and organises an ERAS CME course at Mayo annually. Dr. Hill has nothing to disclose. Dr. Soop reports personal fees from IBD Congress News, Sweden Shire Pharmaceuticals Ltd, UK, outside the submitted work. Dr. de Boer is the Treasurer of the ERAS Society and reports no financial benefits. Dr. Urman reports personal fees from Mallinckrodt Pharmaceuticals, personal fees from 3 M, personal fees from Merck, grants from Merck, grants from Mallinckrodt, grants from Medtronic, outside the submitted work; and is Treasurer, ERAS Society—USA. Dr. Chang reports personal fees from J&J and MORE Health, outside the submitted work. Dr. Fichera has nothing to disclose. Dr. Kessler has nothing to disclose. Dr. Grass has nothing to disclose. Dr. Whang has nothing to disclose. Dr. Fawcett reports personal fees and non-financial support from MSD and Smiths-Medical and Grunethal, outside the submitted work; and is an Executive Committee Member of ERAS® Society. Dr. Carli has nothing to disclose. Dr. Lobo reports grants and personal fees from BBraun, grants and personal fees from Baxter Healthcare, personal fees from Fresenius Kabi, and personal fees from Shire, outside the submitted work. Dr. Rollins has nothing to disclose. Dr. Balfour has nothing to disclose. Dr. Baldini has nothing to disclose. Dr. Riedel reports personal fees from Edwards Lifesciences, outside the submitted work. Dr. Ljungqvist reports other from Encare AB (Sweden), personal fees and other from Nutricia (NL), outside the submitted work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gustafsson, U.O., Scott, M.J., Hubner, M. et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 43, 659–695 (2019). https://doi.org/10.1007/s00268-018-4844-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-018-4844-y