Abstract
Background
This is Part 2 of the first consensus guidelines for optimal care of patients undergoing emergency laparotomy (EL) using an Enhanced Recovery After Surgery (ERAS) approach. This paper addresses intra- and postoperative aspects of care.
Methods
Experts in aspects of management of high-risk and emergency general surgical patients were invited to contribute by the International ERAS® Society. PubMed, Cochrane, Embase, and Medline database searches were performed for ERAS elements and relevant specific topics. Studies on each item were selected with particular attention to randomized clinical trials, systematic reviews, meta-analyses, and large cohort studies and reviewed and graded using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system. Recommendations were made on the best level of evidence, or extrapolation from studies on elective patients when appropriate. A modified Delphi method was used to validate final recommendations. Some ERAS® components covered in other guideline papers are outlined only briefly, with the bulk of the text focusing on key areas pertaining specifically to EL.
Results
Twenty-three components of intraoperative and postoperative care were defined. Consensus was reached after three rounds of a modified Delphi Process.
Conclusions
These guidelines are based on best available evidence for an ERAS® approach to patients undergoing EL. These guidelines are not exhaustive but pull together evidence on important components of care for this high-risk patient population. As much of the evidence is extrapolated from elective surgery or emergency general surgery (not specifically laparotomy), many of the components need further evaluation in future studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced Recovery After Surgery (ERAS) is a multidisciplinary structured approach providing standardized evidence-based components of care to patients undergoing specific types of surgery. To date, ERAS has largely been applied to elective surgery, but there is now evidence that high-risk surgical patients, such as those undergoing emergency laparotomy (EL), can also benefit from an ERAS approach [1,2,3,4,5,6,7,8,9,10,11]. The term “laparotomy” in the emergency situation encompasses a surgical exploration of the acute abdomen for a number of underlying pathologies [12,13,14,15,16,17]. Common indications include intestinal obstruction, perforation, or ischemia [13,14,15, 17]. For these ERAS® Society Guidelines, the term “emergency” is applied to all patients with non-elective, potentially life-threatening intra-abdominal conditions requiring surgery, excluding procedures for trauma, vascular conditions, appendicitis, and cholecystitis.
This is Part 2 of a three-part guideline. Part 1[18] dealt with background and preoperative care including diagnosis, rapid assessment, and optimization. This section includes intraoperative and postoperative care, and Part 3 covers organizational aspects of management. We suggest these ERAS® Society Guidelines should be used to improve the management of patients undergoing EL and to audit processes and outcomes of care.
Materials and methods
This project was initiated by the ERAS® Society. Lead authors (MS and CP) were invited by the Society to establish a guideline development group (GDG) of health care professionals with diverse clinical or academic expertise in the management of patients undergoing EL. The GDG consisted of surgeons, anesthesiologists, a nurse, a geriatrician, and a PhD who supported the organization of the literature. Several of the authors were also accredited in intensive care and the group was selected to ensure international representation. There was equal author representation from the USA and the UK (lead authors MS and CP have worked in both the USA and the UK), with more surgical representatives from the US, and more anesthetic representatives from the UK reflecting National Emergency Laparotomy (NELA) audit involvement. There were five European authors and two from the rest of the world. We recognize with regret in retrospect that Asia and Africa were not included and will correct this on the next iteration of these guidelines. A list of topics was generated, and groups of physicians with different backgrounds and from different countries were assigned to each topic, based on their expertise, to perform a literature review of English language publications and then to generate recommendations using the GRADE structure [19] and a modified Delphi process. Once the topic groups had drafted recommendations, these were collated and sent to the whole group for feedback. There was then significant review, editing, and response to comments, as well as extensive discussion of appropriate inclusion or modification of the recommendation list. The paper and recommendation list were then circulated again using a modified Delphi approach to rank the strength of the recommendation and seek further comment. A final Delphi was then undertaken highlighting areas where, prior to modification, there had been less than 80% agreement, and on this final round more than 80% consensus was reached. The time period searched was from 2005 until September 2021, with greater emphasis on recent publications, randomized clinical trials (RCTs), systematic reviews, meta-analyses, and large cohort studies. With delays in reconvening the group due to the COVID-19 pandemic, an updated search was performed in the Spring of 2022. Retrospective studies were considered where no other higher level of evidence was available, and with particular relevance to EL. The final guidelines were then circulated to all authors for review and identification of further relevant papers. All authors had access to papers reviewed using a reference library. The guideline development process used to reach consensus was based on that published by the ERAS® Society [20, 21]. Twenty-three key components of perioperative care were agreed on and assessed with three circulations of the paper. A reviewer from the International ERAS® Society (OL) was appointed to provide internal review of the guideline as it developed, on his suggestion and the need for ERAS recommendations to be measurable for compliance and actionable, the paper was re-ordered prior to the final Delhi round to place all clinical components into this paper (Part 2), and other components perhaps less amenable to change by clinicians, such as delivery system structure, into a second paper (Part 3 Peden et al. unpublished 2023). Discussion of implementation and delivery of these guidelines are done in Part 3. The components of these guidelines will be placed into the ERAS Society Interactive Audit System (EAIS) and will be tested to measure compliance and outcome.
Definitions
In these guidelines, EL is defined in line with criteria used by large cohort studies [16, 22] and definitions of high-risk emergency general surgical procedures [23], therefore, trauma laparotomies, appendectomy and cholecystectomy are excluded. Most vascular conditions are excluded, such as laparotomy for vascular pathology including ruptured aortic aneurysm and return to the operating room with complications following a vascular procedure. Conditions relating to bowel ischemia such as mesenteric vascular insufficiency are included [16, 22]. The definition of emergency can also vary, from classification of the case by the surgeon and anesthesiologist as an emergency [14] to a definition used in a major US epidemiology study of emergency surgery [24] as non-elective surgery within 48 h of admission. The UK National Emergency Laparotomy Audit (NELA) defines emergencies as patients having a non-elective admission with a potentially life-threatening condition [22]. In these guidelines the term “emergency” is applied to all patients with a non-elective, potentially life-threatening intra-abdominal condition requiring surgery.
Commentary
The components of a standard ERAS elective colorectal pathway were reviewed in relation to the patient undergoing EL [25]. However, EL is required to treat a range of upper and lower gastrointestinal conditions in patients who also require management of acute physiological derangement before, during and after surgery. This warrants a specific EL pathway. In particular, a high level of intraoperative and postoperative monitoring is needed to ensure desired physiological parameters are attained and maintained. Many of the elements of the pathway are contiguous across pre-, intra-, and postoperative phases of the pathway.
Results
Evidence and recommendations
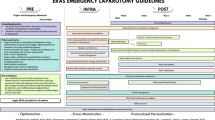
A summary of the 23 ERAS elements for intra- and postoperative care and grading of recommendations with their respective level of evidence is depicted in Table 1.
Preoperative phase
For preoperative pathway components, please see Part 1 of this guideline [18] which includes discussion on the timing of surgery. Since publication of Part 1, our recommendations on the management of sepsis and source control have been further endorsed [26].
Intraoperative phase
The aim of intraoperative management of an ERAS protocol for EL is to identify and rectify the primary surgical pathology and correct physiological derangement due to the pathology and associated surgery, such as blood loss, fluid shifts, and sepsis. Management of physiological derangement should occur alongside surgical intervention. Surgery, while essential, also drives further inflammation and pain both of which are additional physiological stressors for the patient, as is sub-optimal analgesia and anesthesia. The following evidence-based components should be incorporated into an intraoperative pathway of care for each patient undergoing EL.
Intraoperative surgical considerations
The choice of surgical technique should be based upon a judgment of factors related to the patient, the surgical pathology, preoperative imaging findings, surgeon’s preference and experience, and a risk/benefit assessment. Recent guidelines have been published addressing management of different types of colorectal surgical emergencies [27].
We have made no formal recommendations with regard to surgical technique and approach as each case must be considered based on the factors listed above, and the skill and resources of the surgical team.
Surgical approach
The use of initial diagnostic laparoscopy has increased during the past decade due to increased experience and training [28]. An initial diagnostic laparoscopy can always be converted to an open laparotomy technique. In a recent systematic review and meta-analysis of laparoscopic versus open emergency colorectal surgery comprising 7865 laparoscopic and 55, 862 open surgery procedures, a laparoscopic approach was associated with lower mortality, less overall morbidity, wound infection, wound dehiscence, ileus, pulmonary and cardiac complications, and shorter length of stay than an open approach [29]. However, all but one of the studies included were non-randomized retrospective cohorts, thus raising the strong possibility of confounding by indication. In an analysis of data from NELA, 11, 753 patients undergoing attempted emergency laparoscopic surgery were matched with 23, 506 patients undergoing emergency open laparotomy surgery (1:2 matching). The commonest laparoscopically performed procedures were colectomy, adhesiolysis, washout, and repair of perforated peptic ulcers. Laparoscopically attempted surgery was associated with lower mortality, blood loss and length of hospital stay [30]. Some studies have reported increased risk with conversion from laparoscopic to open surgery [31].
In summary, laparoscopic surgery should be considered when appropriate, considering underlying pathology, available resources, and surgeon experience.
Gastrointestinal/colorectal anastomoses in emergency laparotomy
A postoperative bowel anastomotic leak is a life-threatening major complication and even when survived is often associated with emergency re-operations, extended stay, prolonged recovery, and shorter disease-free cancer survival [32]. The risk factors for anastomotic leak have been well documented and include emergency surgery, American Society of Anesthesiology (ASA) physical status, advanced age, low serum albumin concentration, intraoperative blood loss and hypotension, extra peritoneal anastomosis, long operations [32,33,34,35,36], and vasopressor support [37]. All these risk factors are relevant to patients undergoing EL. Risk scoring systems for anastomotic leak exist [38] but are not yet in widespread clinical use.
Unlike elective surgery, there is much less opportunity for risk modification before EL and for many years, in the emergency setting, the risk of anastomotic leak was considered so high that standard treatment was to avoid an anastomosis and raise a surgical stoma when bowel resection was required. However, it is now clear that in many cases a primary anastomosis can be safely performed and emergency surgery per se is not an absolute contraindication to an anastomosis. There is evidence that subspecialist management of colorectal conditions is associated with low overall and operative mortality, while safely achieving high rates of primary anastomosis [39]. Large-scale EL audits show that only a minority of patients undergoing an emergency left-sided colonic resection have a primary anastomosis and there is wide inter-hospital variation in restorative resection rates [40]. When considering an anastomosis during EL, an individualized patient assessment should be conducted to determine the likely benefit and magnitude of risk, the likely consequences of an anastomotic leak and the suitability of any alternative operative strategy, noting that an anastomosis constructed in a patient requiring pressor support to treat shock is at high risk of failure.
Surgical Rescue: timing and damage control surgery
Damage control surgery (DCS) is a surgical strategy to control hemorrhage and/or sources of sepsis in critically ill patients not expected to survive prolonged initial definitive surgery [41,42,43,44,45]. Damage control in the septic abdomen is achieved by eliminating the source of infection and reducing bacterial contamination of the peritoneal cavity [46], while deferring anastomoses and temporarily closing the abdominal wall [45, 47]. After DCS, the patient should be transferred to the intensive care unit (ICU) to continue resuscitation efforts. Definitive surgical management, such as bowel anastomosis (if judged appropriate) and closure of the abdominal wall, should be performed at a subsequent operation. In a prospective observational multicenter study of 422 patients with severe sepsis or septic shock, inadequate source control was associated with a significantly higher 28-day mortality [48]. Clinical studies in DCS are limited and DCS exposes patients to multiple surgical interventions, prolonged ICU stay, open abdomen management, and related complications [49]. DCS usually results in planned re-laparotomy and in a RCT of patients undergoing laparotomy for severe secondary peritonitis re-laparotomy on demand and was associated with fewer negative laparotomies, fewer operations overall, shorter critical care stay, and shorter total hospital stay with no difference in mortality, than patients having a planned re-laparotomy [50]. Routine (indiscriminate) use of DCS in cases of severe secondary peritonitis was associated with an increased relative risk and odds ratio for death in one small RCT [46].
The decision for DCS and reoperation should be individualized, based on the patient’s condition during resuscitation and ongoing treatment. Multiple variables should be evaluated [49, 51, 52]. Patients with perforated hollow viscera can be managed using the principles of DCS to avoid an “ostomy” in the index surgery [53, 54]. A systematic review and meta-analysis of patients with non-trauma abdominal emergencies reported no difference regarding mortality between DCS or conventional management. However, the analysis used studies that compared observed and expected mortality, with a lower rate of observed mortality in patients with DCS [55].
Abdominal closure can be deferred, and negative pressure wound therapy (NPWT) initiated. The evidence is limited regarding temporary abdominal closure techniques between NPWT and non-NPWT [56]. However, studies of patients with abdominal sepsis treated with NPWT have reported benefits in mortality, complications, time to definitive abdominal closure, and reduced long-term costs [57, 58]. Evidence suggests that the risk of fascial closure complications and re-interventions is reduced when the patients have been managed in the postoperative period with NPWT [59, 60]. Combining NPWT with dynamic mesh mediated fascial traction can result in achieving successful delayed fascial closure in a high proportion of patients [61, 62] and has been associated with better outcomes than NPWT alone in some non-randomized studies [63]. Ongoing management should be individualized, with the aim of closing the abdomen as early and safely as possible. Some patients may benefit from delayed wound closure [49, 51, 52].
Intraoperative ERAS elements
Intra-abdominal surgical drains
Intra-abdominal drains have long been used to prevent and eliminate accumulation of infected or inflammatory peritoneal fluid. Nevertheless, their role as a prophylactic intervention after major elective abdominal surgery has been challenged [64]. In fact, in patients undergoing elective surgery, evidence for any of the proposed beneficial effects is lacking or weak; in general, patients with intra-abdominal drains have been found to have similar rates of mortality, morbidity, infections, anastomotic leaks, and re-interventions as patients without drains [65,66,67,68] and a meta-analysis of 4 RCTs in patients undergoing rectal surgery found no benefits from closed suction drains [69]. In a recent prospective international matched cohort study of 1805 patients undergoing elective colorectal surgery, drains were not associated with reduced rates or earlier detection of collections and were associated with delayed hospital discharge and an increased risk of surgical site infection [70].
In patients subjected to EL, there is a lack of high-quality studies, although a recent abstract from the EuroSurg Collaborative in emergency colorectal patients found no benefit for drain use [71]. In another study of trauma EL, the use of closed suction drains after acute laparotomies for hollow visceral injuries was associated with an increased rate of surgical site infections compared with those who did not receive an intra-abdominal drain [72], whereas the rate of deep surgical site infections, or re-interventions, in patients subjected to a laparotomy for solid organ injuries was unchanged [73]. “Routine drainage” after EL has also not shown any benefits over “no drain” with the same rates of surgical site infections measured [74]. Although appendectomy is not included in our definition of EL, a Cochrane review from 2018 investigating the role of drains in patients subjected to open appendectomies for complicated appendicitis could not find any benefits of drainage with regard to reduced surgical site infections [75]. The use of drains after appendectomies for perforated appendicitis, with or without the presence of an abscess or peritonitis, is discouraged by the World Society of Emergency Surgery [76]; their use provides no benefits with regard to preventing postoperative intra-abdominal abscess formation or surgical site infections and may lead to longer hospitalization [76, 77]. Finally, a prospective case–control study including patients who underwent laparotomies for a perforated peptic ulcer found that closure of the perforation with an omental patch technique was safe without prophylactic intra-abdominal drainage [78]. Furthermore, there was a high rate of drain-related morbidity (fever, wound infections, peritoneal fluid accumulation, and wound dehiscence) suggesting that drains should be avoided where possible [78].
Summary and recommendation
Routine, prophylactic use of intra-abdominal surgical drains is discouraged given a lack of evidence to their benefit in clean and clean/contaminated cases. The situation may differ in contaminated abdominal cases.
Level of evidence: Low
Recommendation grade: Weak
Prevention of infection: perioperative antibiotics, skin antisepsis, use of a fascial wound protector, irrigation, and glove change in abdominal closure
Surgical site infection is common and may account for 16% of all hospital acquired infections. The risk of infection is considerably higher when abdominal emergency operations take place and is estimated to affect 35% of all such patients [79]. The use of antibiotic prophylaxis before surgery has been shown to reduce surgical site infection [80]. Perioperative broad spectrum intravenous antibiotics should be administered within 60 min before skin incision if the patient has not already been commenced on them, some agents such as fluoroquinolones and vancomycin require administration over 1–2 h, and therefore, administration should begin, if possible within 120 min [26, 81]. Continuation of antibiotics should be decided according to the pathology and contamination found during surgery. The AHRQ “Technical Evidence Review for Emergency Major Abdominal Operation” [81] and the World Society of Emergency Surgery [82] provide more specific guidelines for antibiotics for a number of intra-abdominal emergency procedures. A systematic review found no specific evidence for skin antisepsis for major emergency general surgery but recommended preoperative skin antisepsis with alcohol-based solutions, or chlorhexidine for patients with an allergy to alcohol-based skin solutions [81].
The use of a fascial abdominal wound protector and new closure instruments after abdominal irrigation as well as a glove change is recommended by the American College of Surgeons (ACS) as part of the National Surgical Quality Improvement Program (NSQIP) bundle to reduce surgical site infections (SSI) and has been shown to be an effective way of reducing both superficial and deep SSI [83,84,85]. There is little specific evidence for wound protectors in EL, but a recent systematic review found some evidence of benefit for abdominal surgery in general, and no evidence of harm. A recent large cluster randomized trial in low- and middle-income countries, including a large proportion of emergency surgery patients and patients with intraoperative contamination, found that routine change of gloves and instruments before wound closure reduced surgical site infection by 13% [86]. Other key components to reduce SSI [87] include normothermia and control of blood glucose (both of which are discussed later in this document).
Summary and recommendations
Perioperative broad spectrum intravenous antibiotics should be administered within 60 min before skin incision unless the patient is already receiving appropriate antibiotic therapy, some agents such as fluoroquinolones and vancomycin require administration over 1–2 h, and therefore, administration should begin, if possible, within 120 min. Local and national guidelines should be followed for choice of antibiotic, dosing, and administration. Continuation of antibiotics should be based on pathology and contamination during surgery.
Level of evidence: High
Recommendation grade: Strong
Preoperative skin antisepsis with alcohol-based solutions, or chlorhexidine for patients with an allergy to alcohol-based skin solutions should be used. Chlorhexidine with alcohol is optimal.
Level of evidence: High
Recommendation grade: Strong
Routine use of a fascia abdominal wound protector, abdominal irrigation and new gloves and closure instruments are recommended to reduce SSI.
Evidence level: Moderate
Recommendation grade: Strong
Anesthesia and perioperative management
Overview
The goal of the anesthesiologist is to provide safe anesthesia while addressing the physiological disturbances caused by the pathological process and associated surgery, such as blood loss, sepsis, and significant fluid shifts. Patients who present for EL may be among the most complex and demanding that an anesthesiologist will meet in general service. Anesthesiologists should be suitably experienced and familiar with the considerations required to manage patients undergoing emergency general surgery (EGS) and specifically EL. Many aspects of management are not specific to EL but are common to routine anesthetic practice and form standards of care which must be adhered to in these vulnerable patients. Principles of sound anesthetic decision-making apply equally to emergency and non-emergency abdominal surgery.
Rapid sequence induction and intubation
Patients undergoing EL are at particularly high risk of regurgitation of gastric contents and subsequent aspiration into the lungs. The reasons for this include bowel and stomach obstruction and distension, sepsis, opioids, and the emergency nature of the surgery. For this reason, rapid sequence induction and intubation (RSII) has historically been seen as a standard part of anesthesia for patients undergoing EL. RSII was first described by Stept et al.[88] and incorporated the use of cricoid pressure [89] to protect the airway from contamination during the period between loss of consciousness and placement of a cuffed tracheal tube. This method of securing the airway has been widely practiced for many years but recently significant variation has taken place associated with the introduction of newer anesthetic agents and equipment, and lack of evidence of benefit for many parts of the original sequence. Newer induction drugs such as propofol have been used and non-depolarizing muscle relaxants such as rocuronium have been used as an alternative to succinylcholine [90]. A small RCT of 400 critically ill patients found no difference in intubating conditions or desaturation between rocuronium and succinylcholine[90] although a Cochrane review found less frequent excellent intubating conditions when a lower dose of rocuronium (0.6–0.7 mg/kg) was used [91]. The availability of sugammadex to reverse rocuronium rapidly may have encouraged the use of rocuronium in some settings when a selective relaxant binding agent (SRBA) is available, although the aspiration risk remains [92]. Recent guidelines from the European Society of Anaesthesiology and Intensive Care make a strong recommendation, based on a moderate level of evidence, for the use of a fast-acting muscle relaxant such as succinylcholine 1–2 mg kg −1 or rocuronium 0.9 to 1.2 mg kg −1 for RSII [93]. Some induction agents are likely to cause hypotension (propofol) or are relatively contraindicated in sepsis (etomidate) [92].
The use of cricoid pressure is under debate with some guidelines recommending its use, while others do not, citing lack of evidence for clinical efficacy and variation in appropriate application. A recent review article discussed the fact that practice in the use of cricoid pressure in an emergency operative induction varies internationally and that there is some evidence that cricoid pressure can make intubation more difficult but may not prevent aspiration of gastric contents [94]. If direct laryngoscopy is difficult, cricoid pressure should be released [95]. Although aspiration of gastric contents is rare, should it occur the risk of patient death or severe brain injury secondary to hypoxia are high, therefore in this high-risk EL patient population we recommend that the use of cricoid pressure should be in line with current standard of practice in the anesthesia practitioner’s respective country, e.g., for the UK [96] and the 2015 Difficult Airway Society Guidelines [95].
Recommendation
To minimize the risk of aspiration after induction of anesthesia rapid control of the airway with intubation using a fast-acting muscle relaxant such as succinylcholine 1–2 mg kg −1 or rocuronium 0.9 to 1.2 mg kg −1 for placement of an endotracheal tube should be used. We recommend the use of cricoid pressure according to the practitioner’s respective national guidelines. Drugs for induction of anesthesia should be selected and dosed appropriately to maintain hemodynamic stability.
Evidence level: Moderate
Recommendation grade: Strong
Maintenance anesthetic agent and depth of anesthesia monitoring
Inhaled anesthetic agents remain the drugs commonly used for maintenance of anesthesia in emergency surgery. Short acting agents such as sevoflurane or desflurane are easy to administer and monitor and allow rapid awakening at the end of surgery and return of protective reflexes. Intravenous anesthesia using target controlled propofol infusions reduces postoperative nausea and vomiting (PONV), and laboratory data and some retrospective studies suggest possible beneficial downstream effects on cancer outcomes [97]; however, this patient group is often hemodynamically challenged and the use of propofol can increase vasopressor requirements. There are no RCTs of total intravenous anesthesia (TIVA) versus inhalational anesthesia in this patient group. A Cochrane review did not show significant benefit of TIVA to reduce delirium in the elderly [98].
There is developing evidence that depth of anesthesia may be important in patients over 60 years of age, and avoiding volatile anesthetic overdose by close monitoring of age-adjusted minimum alveolar concentration (MAC) is critical to avoid side effects such as hypotension [99]. Variable evidence is available for older elective surgical patients, that titrating anesthesia using bispectral index (BIS) or another form of processed electroencephalography (EEG), and avoiding burst suppression of the EEG [99] reduces the risk of postoperative delirium [100]. A recent sub-study of a larger study targeting a lighter level of anesthesia depth with a BIS of 50 versus a deeper level with BIS 35 in major elective older surgical patients found a significant reduction in postoperative delirium in the lighter anesthesia group [101]. For the patient population undergoing EL, there is a high incidence of frailty and old age which increases the risk of postoperative delirium [102] as well as a higher incidence of accidental anesthetic awareness observed during emergency surgery [103]. While the cause of delirium is multifactorial, using depth of anesthesia monitoring to avoid extremely low BIS values may reduce this risk in older patients[99]. A recent review of the literature also concluded that processed EEG-guided anesthesia care may be appropriate if the goal is to facilitate rapid emergence and recovery [104].
Summary and recommendations
There is no evidence to recommend one anesthetic agent over another for maintenance of anesthesia.
Level of evidence: Low
Recommendation grade: Weak
Consider using depth of anesthesia monitoring in patients over 60 years of age at risk of postoperative delirium and anesthesia-induced hypotension.
Level of evidence: Moderate
Recommendation grade: Strong
Postoperative nausea and vomiting (PONV) reduction
PONV is a major cause of patient dissatisfaction and delays return to enteral intake in all surgery. All patients undergoing emergency laparotomy are at high risk of PONV due to physiological derangement and gastrointestinal insult. The use of intravenous opioids is also common in EL patients and is a risk factor for PONV. The use of opioids should be minimized using a multimodal approach (see section on analgesia). There are no RCTs of PONV prophylaxis in emergency general surgery but the international consensus guidelines for elective surgery recommend a multimodal approach to high-risk patients [105]. Other reviews support a multimodal approach of 2 or 3 agents used together [106]. There are several classes of antiemetic drugs including serotonin (5HT3) antagonists, dopamine (D2) antagonists, NK 1-antagonists, antihistamines, and corticosteroids. There is minimal harm to using most of these drugs apart from the increased risk of sedation or increasing the QTc interval [107]. Many patients in this group will be receiving steroids as part of the surviving sepsis guidelines [108] which also have antiemetic properties. Dexamethasone does not appear to increase the risk of wound infection [109]. Beers’ criteria should be followed to avoid high-risk drugs in the elderly population [110].
Recommendations
A multimodal approach to reducing PONV should be utilized, minimizing triggers and opioids.
Level of evidence: High.
Recommendation grade: Strong.
Temperature management
Patients are at risk of hypothermia due to exposure to the surroundings, the effects of anesthesia, and cold intravenous fluids. Hypothermia can impair drug metabolism, adversely affect coagulation, and increase bleeding, wound infection, and cardiac morbidity [111]. To avoid hypothermia forced air warming or underbody warming mattresses should be utilized [112]. Intravenous fluids and blood products should be administered using fluid warmers.
Recommendations
Measurement of core temperature, using a reliable method to monitor the efficacy of warming measures, should be routine.
Level of evidence: High
Recommendation grade: Strong
Active warming devices and warming of intravenous fluids should be used to maintain normothermia.
Level of evidence: High
Recommendation grade: Strong
Lung ventilation strategy
A multinational consensus developed questions and then produced evidence based statements using a modified Delphi method to recommend that, for intraoperative ventilation of surgical patients, a tidal volume of 6–8 ml/kg of predicted body weight and positive end-expiratory pressure (PEEP) 5 cm H2O should be used initially and then individualized thereafter using flow-volume loops [113]. The use of 8 mls/kg allows the use of Stroke Volume Variability (SVV) and Pulse Pressure Variability (PPV) to optimize preload if an arterial line and cardiac output monitoring is used. Recruitment maneuvers should use the lowest effective pressure for the shortest effective time [113]. An observational study in patients undergoing EL showed that high intraoperative peak inspiratory pressures were associated with development of a postoperative pulmonary complication (PPC) [114].
Recommendation
Routine use of low tidal volume (6–8 ml/kg predicted body weight) and positive end-expiratory PEEP ≥ 5 cm H2O with titration according to flow-volume loops and clinical evaluation is recommended.
Level of evidence: Moderate
Recommendation grade: Strong
Monitoring and reversal of neuromuscular block (NMB)
EL is a significant risk factor for PPCs [115]. While adequate reversal of NMB is always important, it is even more so after EL to prevent aspiration and associated PPCs. The metabolism and degradation of muscle relaxants may be unpredictable in patients undergoing EL, making them more likely to have residual muscle paralysis [116, 117]. It is imperative that there is adequate neuromuscular recovery before extubation [118, 119]. Formal monitoring is required, and recent guidelines recommend ulnar nerve stimulation with quantitative train of four (TOF) assessment, with the most reliable site of monitoring being the abductor pollicis muscle [93]. Nerve stimulators using acceleromyography are more accurate to monitor depth of NMB and ensure full reversal [93, 119]. Selective relaxant binding agents (SRBA) such as sugammadex have been shown to more predictably reverse NMB compared with neostigmine or glycopyrronium and so reduce the risk of bulbar dysfunction and aspiration. In an RCT of 200 older patients undergoing elective prolonged surgery, sugammadex was associated with a 40% reduction in residual NMB, a 10% reduction in 30-day readmission, but no reduction in postoperative pulmonary complications [120]. A recent meta-analysis showed that compared with neostigmine, SRBA use was associated with a lower risk of PPCs, mainly due to a lower incidence of postoperative respiratory failure [121].
Recent guidelines recommend SRBA to antagonize deep, moderate, and shallow NMB induced by aminosteroidal agents [93]. If neostigmine is used for reversal a spontaneous TOF ratio of > 0.2 should occur before administration, and a TOF ratio of more than 0.9 should be obtained before extubation [93].
Recommendations
Neuromuscular blockade should be monitored using a quantitative peripheral nerve monitor to ensure adequate reversal before endotracheal extubation, with the most reliable site of monitoring being the abductor pollicis muscle.
Level of evidence: High
Recommendation: Strong
Reversal of NMB using a selective relaxant binding agent as compared with neostigmine is recommended.
Level of evidence: Moderate
Recommendation grade: Strong
Intravenous fluid and electrolyte management and goal directed hemodynamic therapy
Resuscitation prior to, during, and after surgery is critical to the management of patients undergoing EL. Volume overload can lead to perioperative complications such as organ dysfunction, ventilator dependence, gut edema, and poor wound healing [122, 123], and too little fluid risks poor organ perfusion and associated consequences such as renal failure. Volume assessment is particularly challenging in emergency general surgery patients given their inflammatory response and physiologic derangement on presentation [124]. Many anesthesiologists routinely use some form of advanced hemodynamic monitoring. However, there are only a few small studies [125, 126] and few prospective trials for individual methods of advanced hemodynamic monitoring in EL [125, 126] although others are underway (https://floela.org/about). Arterial lines provide useful real-time blood pressure measurement in this patient group and allow frequent arterial blood gas sampling to guide therapy. Multi-lumen central venous catheters (CVCs) are mandated in many hospitals to deliver drugs such as vasopressors and inotropes required in many patients undergoing EL.
Fluid balance should be carefully recorded throughout and following surgery, and intraoperative volume therapy should be titrated by bolus, based on objective measures of hypovolemia [111]. A recent EL study targeted a postoperative fluid balance in the range of 0–2 L [126], which is in line with elective colorectal ERAS guidance [25].
Intravenous fluid and electrolyte replacement
Evidence for the type of fluid to use for laparotomy patients must be inferred from trials in elective surgery, the ICU literature and from mixed groups of other patients. An early study showed that patients who received 0.9% saline compared with lactated Ringer’s (LR) in hemorrhagic shock experienced a higher incidence of hyperchloremic metabolic acidosis, electrolyte derangements, dilutional coagulopathy, and higher overall volume requirements for adequate resuscitation [127]. Saline-induced disturbances in acid–base balance can have a negative impact on perioperative electrolyte management, end-organ function, and survival [128]. There is also evidence indicating a negative impact of solutions with high chloride content on renal function, resulting in decreased kidney perfusion and urine output, increased extravascular fluid accumulation, increased vasopressor requirements and acute kidney injury (AKI) [129,130,131,132].
The Isotonic Solutions and Major Adverse Renal Events Trial (SMART) in 15, 802 patients [133] was a pragmatic, unblinded, cluster-randomized, multiple-crossover study in ICU patients; approximately 21% of patients were admitted from the operating room. Patients who received 0.9% saline had a significantly higher incidence of a composite outcome of a major adverse kidney event within 30 days compared with patients in the balanced crystalloid group (15.4% vs 14.3%). There are some methodological limitations of the SMART trial, including that it was conducted at one facility and was not adequately powered to detect the different components of the composite outcome [134]. The Isotonic Solution Administration Logistical Testing (SALT) trial evaluated 974 adults admitted to the ICU mostly from the emergency department with a predominant diagnosis of sepsis, who received either a crystalloid solution or 0.9% saline. Patients who received crystalloid had lower 30 days in hospital mortality, and lower incidence of renal replacement therapy or renal dysfunction [135].
Overall, the existing evidence suggests that balanced crystalloids may result in improved patient outcomes and reduce morbidity and mortality. The use of 0.9% saline use should be limited, especially in higher-risk patients with existing electrolyte derangements such as acidosis or hyperchloremia and those who might require a significant amount of fluid resuscitation. The use of hydroxyethyl starch (HES) solutions is not recommended due to the increased risk of kidney failure and mortality and lack of benefit demonstrated in the FLASH study [136] and in a systematic review [137].
Patients undergoing EL are likely to experience electrolyte abnormalities. Critically-ill patients (which can include patients undergoing EL) are especially susceptible to electrolyte disturbances, including hypo- and hypernatremia [138, 139], hypo- and hyperkalemia [140, 141], hypophosphatemia [142], hypocalcemia [143], and hypomagnesemia [144]. Electrolyte disturbances can lead to a variety of adverse events in the intraoperative and postoperative setting, including cardiac dysrhythmias, particularly atrial fibrillation [145]. Correcting electrolyte disturbances is important to maintain body homeostasis. Existing guidelines and institutional protocols should be used to guide treatment. Patients should be appropriately monitored when significant electrolyte abnormalities are suspected.
Recommendation
Patients should have ongoing treatment to correct electrolyte disturbances throughout the perioperative period.
Level of evidence: Moderate
Recommendation grade: Strong
Recommendation
Balanced crystalloids should be used in preference to 0.9% normal saline for resuscitation and to maintain intravascular volume.
Level of evidence: Low
Recommendation grade: Weak
Goal directed hemodynamic therapy, cardiovascular monitoring, maintenance of blood pressure, and vasopressor use
Goal-directed hemodynamic therapy (GDHT) is the process of using cardiac output monitoring to guide the administration of fluid and vasopressors. The key components involve optimizing flow by maintaining a patient’s stroke volume while avoiding the deleterious effects of hypotension. Intraoperative GDHT improves outcomes in elective surgery in some studies, with most benefits observed in high-risk patients [146,147,148], while others have demonstrated little benefit [149,150,151,152]. In the emergency setting, all patients can be considered to be at high risk and some small observational studies involving GDHT as part of perioperative management protocols in this group have demonstrated improved outcomes and a mortality benefit [1, 153] although others have shown no benefit [126].
There is no single GDHT protocol that has shown clear benefit over others in the emergency general surgery setting. Regarding the physiologic goals of GDHT plans, a broad assessment is difficult given the heterogeneous clinical trial protocols and populations studied [146]. Paired with clinical judgment, the use of stroke volume as a guide to resuscitation and vasopressor use is likely to reduce unnecessary fluid overload and improve outcomes. A recent two-arm multicenter study in 312 patients did not show a benefit in the flow directed group compared with control [126]. Maximizing stroke volume may not be the correct approach, but diligence in avoiding hypovolemia and hypotension and ensuring adequate perfusion is key. The importance of avoiding hypotension in elective surgery is now recognized [154], but it is important to optimize flow prior to the commencement of vasopressors [155, 156].
Transthoracic echocardiography (TTE) and ultrasound are increasingly utilized to assess patients who are hemodynamically unstable and septic in the emergency department, operating room, and critical care unit [157]. The increased availability of cheaper quality bedside ultrasound machines and increased training has driven this trend. Bedside TTE can assess left ventricular and right ventricular contractility and structural/valvular abnormalities and guide the use of inotropes and vasopressors once optimal intravascular volume has been achieved [157]. Minimally invasive cardiac output devices can utilize the arterial waveform to calculate values for stroke volume, stroke volume variation (SVV), pulse pressure variation (PPV), and cardiac index [158]. Arterial lines facilitate earlier detection of hypotension and allow regular arterial blood gas analysis.
Norepinephrine (noradrenaline) infusions are now viewed as the first vasopressor of choice to maintain mean arterial pressure (MAP) ≥ 65 mm Hg where sepsis is present or suspected, as it has both vasoconstrictor effects but also some beta-agonism to support cardiac contractility. Epinephrine (adrenaline) can be added as an additional agent to maintain adequate blood pressure or increase cardiac output and vasopressin (0.03 U/min) can be added to norepinephrine to raise mean arterial pressure [159]. MAP target of ≥ 65 mmHg during elective surgery has been shown to be effective in reducing end-organ injury such as AKI and myocardial injury after non-cardiac surgery [154]. Lower MAPs occurring over longer periods are associated with a greater degree of injury. However, a recent study in the UK in 2463 patients over the age of 65 years which aimed to minimize vasopressor exposure in vasodilatory shock and allow a MAP to go down to 60 mmHg showed that 90-day mortality was no different from a targeted MAP of 65 mmHg [160]. A pooled analysis in patients with septic shock showed that higher doses of vasopressors to target higher MAPs may reduce the risk of AKI but increased the risk of mortality [161]. Starting norepinephrine via a large peripheral vein in a shocked patient is safe until central access is established [162].
Summary and recommendations
Use of arterial lines and/or central venous pressure catheters should be considered at an early stage to aid in physiological assessment and to deliver and titrate vasopressors and fluid therapy.
Level of evidence: Moderate
Recommendation grade: Strong
GDHT should be considered during surgery in high-risk patients to optimize cardiac index. A MAP of 60–65 mmHg and Cardiac Index > 2.2 L/min/m2 individualized to the patient, should be maintained during surgery using appropriate vasopressors and inotropes as needed.
Level of evidence: Moderate
Recommendation grade: Strong
Management of blood glucose
Hyperglycemia (blood glucose > 10 mmol/l or 180 mg/dl) is a risk factor for many complications after surgery for patients with and without diabetes mellitus [163, 164]. The risk of complications and mortality is dependent on many factors but notably long-term glycemic control and blood glucose concentration at admission [164]. Hyperglycemia can impair neutrophil function and cause overproduction of reactive oxygen species, inflammatory mediators, and free fatty acids [165]. These changes can contribute to direct cellular damage as well as vascular and immune dysfunction. Hyperglycemia is driven by the physiological stress response causing insulin resistance, and so is also an indirect marker of tissue injury. Treatment with insulin to reduce hyperglycemia can reduce complications [166]. Given that EL patients frequently have fluid shifts and acidosis, use of a variable rate insulin infusion is likely to be most appropriate intraoperatively and can be continued postoperatively until the patient is more stable [167]. Infusion regimens and blood glucose targets vary depending on guidelines and patient circumstances, but a perioperative range of 7.7–10 mmol/l (140–180 mg/dl) has been suggested [167]. Point of care intraoperative measurement of blood glucose should be performed at a minimum every hour while on the infusion and until serum glucose levels are stable.
Recommendation
Patients should have their glucose closely monitored and controlled in the range of 7.7–10 mmol/l, preferably with the use of a variable rate insulin infusion.
Level of evidence: Moderate.
Recommendation grade: Strong.
Blood product management
Transfusion of packed red blood cells occurs in up to 30% of the emergency surgical population even where primary blood loss is not the cause for admission [168, 169]. Blood transfusion can be associated with increased risk of mortality and complications. Based on evidence review and consensus opinion “Blood Management Guidelines” from the American Society of Anesthesiologists suggest that red cell transfusion should be restrictive to maintain an Hb of 60–100 g/l based on potential or actual ongoing bleeding, intravascular volume status, signs of organ ischemia, and adequacy of cardiopulmonary reserve [170]. Similar guidelines give a range of 70–90 g/l from the European Society of Anaesthesiology and Intensive Care [171], and much of the evidence in both these guidelines is extrapolated from critical care. In an analysis of 470 407 patients in the ACS NSQIP database, high-risk patients did not have significantly increased risk, but low-risk patients had an 8–tenfold excess risk of adverse outcomes if they received an equivalent blood transfusion [172]. It is imperative to maintain cardiac output, mean arterial pressure, and hematocrit to reduce the incidence of organ dysfunction. A meta-analysis of practice recommendations from 14 trials identified that the following intraoperative issues were used to guide transfusion in guidelines—blood loss, signs of end-organ ischemia, and hemodynamic instability—although only one of these studies included general surgery patients [173].
Recommendations
Transfusion of red blood cells should be restrictive (trigger Hb 70 -90 g/l), with exceptions based on individualized clinical status and comorbidities.
Level of evidence: Moderate.
Recommendation grade: Strong.
Multimodal systemic analgesia
Multimodal pain management strategies, utilizing primarily non-opioid analgesics and techniques, should be used when possible to reduce the perioperative consumption of opioids which may delay patient recovery [174, 175]. Compared with traditional ERAS pathways for elective surgery, an ERAS pathway for EL may differ; for instance, placement of an epidural catheter may not be appropriate in a patient who is coagulopathic or has known or suspected bacteremia. Nevertheless, the analgesic principles for both elective and emergency ERAS protocols remain the same—provision of superior analgesia and decrease in reliance on perioperative opioids to facilitate recovery [176, 177].
Regular dosing of acetaminophen (paracetamol) up to 15 mg/kg every 6 h (with a maximum of 4 g per 24 h) is a good analgesic base in all patients except those with liver dysfunction. It is available in intravenous and rectal preparations, so it can be administered even when patients are unable to have enteral intake.
The use of non-steroidal anti-inflammatory drugs (NSAIDs) in the perioperative period should be used with caution due to the risk of platelet dysfunction with subsequent bleeding and effect on renal blood flow as this patient group has a high risk of acute kidney injury (AKI). Intravenous NSAIDs are available and can be introduced postoperatively once renal function is not impaired, and the risk of bleeding has passed. There is little evidence for the use of non-opioid analgesics in the EL setting; however, elective surgical data support the use of these agents if there are no contraindications [25, 81, 177]. Although there has been discussion around NSAIDs increasing anastomotic leak, a recent systematic review in colorectal cancer surgery did not support this suggestion [178]. There is increasing evidence that gabapentinoids can be potentially harmful and should not be used as part of a multimodal regimen in older patients for major surgery [179].
The elective colorectal guidelines for ERAS and the accompanying anesthesia guidelines for colorectal surgery suggest involvement of an acute pain team if available that recommendation should also apply to EL patients who are likely to benefit from an acute pain team consult in the postoperative period [25, 111].
Nerve blocks, catheters, and local anesthetic infiltration
The common types of nerve blocks/catheters used for laparotomy include neuraxial (epidural/spinal) analgesia and non-neuraxial (transversus abdominis or rectus sheath) blocks, or local infiltration techniques.
Neuraxial blocks
A meta-analysis of 58 RCTs (5904 patients) comparing epidural vs. systemic analgesia in patients undergoing abdominal and thoracic surgery found that epidural analgesia was associated with a significant decrease in odds of pneumonia, the need for prolonged ventilation or reintubation, and improved lung function and blood oxygenation, but increased the risk of hypotension, urinary retention and pruritus [180]. Another meta-analysis (128 RCTs, 8754 patients) in patients undergoing abdominal surgery demonstrated that epidural local anesthetics (compared with opioid-based regimens) significantly accelerated the return of gastrointestinal transit and decreased postoperative pain without a difference in the incidence of anastomotic leak [181]. Epidural analgesia provides superior analgesia compared with patient-controlled analgesia (PCA) with opioids in patients undergoing intra-abdominal surgery [182]. Single-shot spinal anesthesia with opioids in addition to the local anesthetic mix reduces the postoperative need for opioids. However, in emergency general surgery where hemodynamic instability is common it may be preferable to use an opioid alone and omit the local anesthetic to avoid the sympathetic blockade and subsequent vasodilatory hypotension. Neuraxial blocks and catheters should be placed with caution in any patient on concurrent anticoagulation therapy or systemic sepsis.
Local abdominal blocks and catheters
Several meta-analyses indicate that use of a transversus abdominis plane (TAP) block in patients undergoing abdominal surgery is associated with a decrease in pain scores (both at rest and with activity) and reduction in opioid consumption, although there may not be a decrease in postoperative nausea and vomiting [183,184,185]. One small RCT in EL found that TAP blocks lowered pain scores compared with placebo [186]. The duration of analgesia for TAP blocks may potentially be extended with use of TAP catheters [187]. A systematic review noted that wound infiltration with local anesthetics (vs. placebo) during abdominal surgery (cesarean delivery) was associated with a decrease in morphine consumption at 24 h [188]. TAP blocks and local anesthetic wound infiltration in lower abdominal surgical procedures provide comparable short-term postoperative analgesia, but TAP blocks provide a long-lasting analgesic effect [189]. A meta-analysis of continuous wound infusion of local anesthetics agents following colorectal surgery did not provide conclusive evidence of analgesic benefit [190]. Finally, intraperitoneal instillation of local anesthetics for patients undergoing laparoscopic abdominal procedures may significantly decrease pain for up to 6 h after laparoscopy [191]. Caution should be exercised whenever multiple sources of local anesthetics are used, and doses should be reduced accordingly, to minimize risk of systemic toxicity.
Some meta-analyses indicate that perioperative IV lidocaine (lignocaine) infusions in patients undergoing elective abdominal surgical procedures may decrease postoperative pain, reduce opioid consumption, and possibly decrease length of hospital stay in part from the earlier return of gastrointestinal function [192,193,194]. One meta-analysis of a heterogeneous group of RCTs found that use of local anesthetic wound infiltration was associated with pain scores comparable to those obtained with epidural analgesia [195]. The optimum dose, timing, and duration of lidocaine infusions in patients undergoing abdominal surgical procedures are uncertain [194, 196]. There are safety concerns, and appropriate monitoring for toxic effects should be performed, clear guidelines for indications, dosing, and the use of ideal body weight for dose calculation are given in an international consensus statement [197].
Subanesthetic infusions of ketamine have been increasingly used for postoperative analgesia to reduce opioid needs and can reduce opioid requirements, with some theoretical influence on chronic pain mechanisms. Doses of 0.1–0.5 mg/kg/h are frequently reported with higher infusion rates increasing the risk of side effects such as hallucinations and delirium which if they occur should prompt cessation for 1–2 h and then recommencing at a lower dose [198].
Summary
Minimization of perioperative opioid use improves both respiratory function and return of gastrointestinal motility. Optimal analgesic management will reduce the stress response and facilitate postoperative management in line with ERAS principles.
Recommendations
Each patient should be assessed for the optimal perioperative analgesic regimen, considering the presence of sepsis and coagulation abnormalities. Multimodal management should include acetaminophen and non-steroidal anti-inflammatory drugs if there are no contraindications.
Level of Evidence: Low
Recommendation: Strong
The use of wound catheters and/or local abdominal wall blocks and catheters should be considered to reduce postoperative opioid demand but may have variable efficacy.
Level of evidence: Low
Recommendation grade: Weak
Thoracic epidural analgesia and spinal anesthesia should be used only after assessment for sepsis and abnormal coagulation. Hypotension necessitates appropriate monitoring, volume, and vasopressor therapy.
Level of evidence: Low
Recommendation grade: Weak
End of surgery, evaluation and endotracheal extubation
Patients undergoing EL are at high risk for Postoperative Pulmonary Complications (PPCs). A study of a cohort of 30, 000 patients who underwent in-patient general anesthesia for major surgery found that of those patients who required reintubation within the first 3 days after primary extubation, the highest odds ratios of requiring reintubation were ASA physical status (PS) III or more (OR 5.23), emergency surgery (OR 4.21) or high-risk surgery including abdominal surgery[199]. In this data, reintubation was associated with a 72-fold increase in in-hospital death [199]. A secondary analysis of 6063 patients in a prospective study from 146 centers worldwide identified 13 perioperative risk factors for occurrence of a postoperative pulmonary complication including older age and higher ASA PS score, urgent or emergency surgery, and surgery lasting ≥ 1 h [200]. In another large multicenter prospective observational study [201] of 1200 patients ASA PS III or more undergoing prolonged non-cardiothoracic surgery, variables identified with the greatest significant association with one or more PPCs were emergency surgery (OR, 4.47) and abdominal/pelvic surgery (OR, 2.54). Occurrence of even a single PPC increases the risk of postoperative mortality, requirement for ICU, and length of stay [201].
The high risk of a postoperative pulmonary complication in patients undergoing EL and the impact of surgery, sepsis, and ongoing metabolic derangement may mean that extubation at the end of surgery is not appropriate. The anesthesiologist and surgical team should assess the patient carefully, considering the conditions listed above, as well as blood loss and intraoperative ventilatory requirements. Further evaluation with a risk scoring system may guide management. The concept of an “end of surgery” bundle was suggested in the Higher Risk Surgical patient guidelines [202] including risk scoring, assessment of an arterial blood gas sample to assess lactate and acid–base status, assessment of the ratio of arterial oxygen concentration to the fraction of inspired oxygen (P/F ratio), review of fluids given and ongoing fluid requirements, and check and documentation of temperature.
Recommendation
A multidisciplinary discussion at the end of surgery should be used to assess suitability for endotracheal extubation as the risk of postoperative pulmonary complications and reintubation is high.
Level of evidence: Moderate
Recommendation grade: Strong
Postoperative care
Prevention of postoperative pulmonary complications
Patients who have undergone EL are at high risk of postoperative hypoxemia and PPCs related to atelectasis, diaphragmatic dysfunction, retained secretions, pain, and aspiration [203, 204]. Guidelines from the European Society for Anaesthesiology and Intensive Care and European Society of Intensive Care Medicine (ESA/ESICM) [205] suggest using noninvasive positive pressure ventilation (NIPPV) or continuous positive airway pressure (CPAP), rather than conventional oxygen therapy, immediately post-extubation for patients who are hypoxemic and at risk of developing acute respiratory failure after abdominal surgery. The level of evidence for this recommendation was graded as moderate quality (1B) with a strong recommendation, based on two RCTs, one of which provided early helmet CPAP to elective intra-abdominal surgical patients who were hypoxemic [206] and the other gave therapeutic noninvasive ventilation (NIV) to patients in acute respiratory failure [207]. Almost 50% of patients in the latter study, which used therapeutic NIV delivered via facemask, had undergone emergency intra-abdominal surgery and no adverse events were reported [207]. Patients who received NIV were less likely to require reintubation or mechanical ventilation and had fewer episodes of health care-associated infection. An earlier Cochrane review reported very low strength of evidence that prophylactic CPAP in the postoperative period reduced atelectasis, pneumonia, and reintubation after major abdominal surgery, in comparison with standard management [208]. A large multicenter study (PRISM) evaluating the prophylactic use of noninvasive respiratory support in patients who underwent elective intra-abdominal surgery demonstrated that CPAP was safe, but showed no benefit for prophylactic use of CPAP to reduce incidence of pneumonia, endotracheal re-intubation, or death after major elective abdominal surgery [209].
The ESA/ESCIM guidelines [205] recommend that patients receiving NIPPV or CPAP do so in a clinical area where staff are competent in the management of these therapies, and where clinical examination and continuous physiological monitoring plus frequent arterial blood gas sampling can occur.
A systematic review and meta-analysis of perioperative interventions to prevent PPCs found low-to-moderate quality of evidence to reduce incidence [210]. Interventions relevant to EL patients in the postoperative period included use of enhanced recovery pathways, prophylactic mucolytics (evidence low and limited evidence on adverse effects and safety), prophylactic CPAP (level of 8 cm H20 for at least 8–12 h following extubation or admission to PACU), and respiratory physiotherapy—although the largest RCT [211] influencing the findings was for pre- and postoperative physiotherapy in elective major abdominal surgery. There is evidence that even one session of physiotherapy preoperatively may be helpful if time and patient condition permits [211]. A major study is underway to provide more evidence for benefit, timing, and intensity of physiotherapy after emergency abdominal surgery [212], and pilot findings are promising [213].
Goal-directed hemodynamic therapy and epidural analgesia also had moderate evidence of benefit in the prevention of PPCs. No evidence was found for incentive spirometry [210]. HiFlo oxygen is becoming increasingly utilized since the COVID-19 pandemic, and it increases oxygenation with less risk of aspiration, but no studies have been performed in Emergency Laparotomy as yet [214].
Summary and recommendations
Patients who have undergone EL and show evidence of hypoxemia should receive CPAP pressure or NIPPV (technique based on local expertise) rather than standard oxygen therapy, if the risk of pulmonary aspiration is considered to be low. This should occur in an environment where staff are skilled in these techniques, continuous physiological monitoring is available, and arterial blood gases can be sampled.
Level of evidence: High
Recommendation: Strong
Respiratory physiotherapy involving the training and supervision of patients’ sputum clearance, developing inspiratory muscle strength, and deep breathing exercises should be used in EL patients in the postoperative period.
Level of evidence: Moderate
Recommendation: Strong
Admission to the intensive care unit (ICU) or higher level of care postoperatively
Many EL patients have ongoing requirements for active management and monitoring until the physiological insult from the underlying condition and associated surgery have resolved. These needs may be best met in an intensive care unit (ICU) [124, 215].
The incidence of postoperative morbidity and mortality following EL is higher than for major elective procedures where direct admission to ICU may be routine [216,217,218]. Failure to detect deterioration and facilitate rapid intervention, including a return to the OR is associated with worse outcomes [17]. Many patients undergoing EL die in the first 72 h after surgery [219], and morbidity and mortality remain high until at least 30 days [220]. Observational studies suggest that failure to admit to higher levels of care immediately after surgery contributes to poor outcomes and death [217, 221, 222]. Older people and those living with frailty tolerate complications less well [220, 223, 224]. Proactive admission of high-risk EGS patients directly to ICU after surgery reduced mortality in observational studies [1, 6] and resulted in shorter length of stay (LOS) and lower short- and long-term mortality rates [225]. Hospitals with a higher ratio of intensive care beds to ward/floor beds have reduced mortality for EGS patients [226]. Consensus guidelines for standards of care for emergency surgery consistently recommend that protocols should be in place for admission to critical care postoperatively, based on objective risk-assessment, procedure specific risk, hemodynamic instability, ongoing unstable physiology, and clinical judgment of the anesthesia and surgical team [202, 227, 228]. Patient destination for immediate postoperative care should be based on a validated preoperative risk score, patient age, comorbidities and frailty, the impact of the surgical procedure, ongoing physiological instability, and continuing supportive and therapeutic requirements. Admission to an ICU is likely to be appropriate for many patients.
Recommendation
Health systems should establish protocols for determining the appropriate location for postoperative care based on a validated preoperative risk score, impact of the surgical procedure, ongoing physiological instability, and continuing supportive and therapeutic requirements.
Level of evidence: Moderate
Recommendation Grade: Strong
Postoperative delirium screening and prevention
Delirium and perioperative neurocognitive disorders (PND) were covered in Part 1 of these guidelines [18] but are repeated here as patients remain at high risk postoperatively. To summarize, key points are that patients ≥ 65 years of age who undergo emergency surgery are at particular risk for delirium and perioperative neurocognitive disorders [229,230,231]. Patients ≥ 65 years of age and those with preexisting cognitive impairment should have regular postoperative screening for delirium with a simple validated tool such as the 4AT test, the Confusion Assessment Method (CAM) or short 3 min CAM (3D-CAM) [232, 233]. Screening after surgery should begin in the recovery room and continue ideally twice a day until day 5 or discharge [99, 232, 234,235,236,237]. The ACS and the American Geriatric Society (AGS) have joint guidelines on how to prevent, diagnose, and care for delirium in the surgical patient [235]. Delirium is preventable in about 40% of cases with simple steps [235, 238, 239] and avoidance of drugs that fall under AGS Beers criteria drugs, such as benzodiazepines and anticholinergics [110, 238]. Incorporation of a “hospital elder life program” (HELP) with simple measures such as mouth care and regular orienting communication for patients undergoing major elective intra-abdominal surgery demonstrated a significant reduction in the incidence of delirium [239, 240]. Since publication of Part 1 of these guidelines [18], more guidelines and evidence on best practice for delirium avoidance and management have emerged [232, 237] including one specifically for older patients undergoing EL[241]. There is mounting evidence on the costs of delirium [242] and the efficacy of a HELP-type approach in prevention [243].
Summary and recommendations
Patients over 65 years of age should receive regular postoperative delirium screening.
At-risk patients should be managed with non-pharmaceutical interventions such as regular orientation, sleep hygiene approaches and cognitive stimulation to prevent delirium, and medication triggers minimized.
Level of Evidence: High
Recommendation Grade: Strong
Continuation of venous thromboembolism risk assessment and treatment
Compared with elective surgical patients, emergency patients undergoing comparable intra-abdominal procedures are at increased risk of venous thromboembolism (VTE) [244,245,246,247]. Part 1 of these guidelines [18] discussed in more detail that patients should be risk assessed with a validated tool at admission, and VTE prophylaxis (mechanical and/or pharmacologic) should be initiated as soon as possible [244, 247,248,249]. Pharmacologic prophylaxis is preferred but must be balanced against risk of bleeding, if mechanical prophylaxis is used intermittent compression devices are preferred over graduated stockings [250]. Combined pharmacological and mechanical prophylaxis should be considered for very high-risk patients [250].
Postoperatively patients should be reassessed at regular intervals [244]. VTE risk can remain elevated for up to 12 weeks after surgery, especially for patients with an underlying malignancy [244,245,246, 251, 252]. Guidelines recommend extended prophylaxis (4 weeks with low molecular weight heparins) for high-risk patients undergoing abdominal and pelvic surgery, such as those with malignancy or inflammatory bowel disease [81, 244, 253]. Travel requirements after surgery may also increase risk. Around one third of VTEs in EGS patients occur after discharge and about 70% require readmission, suggesting that extended prophylaxis should be considered in high-risk EGS patients [81, 254].
Summary and recommendations
Patients should be assessed with a validated tool for VTE risk on admission and throughout their hospital stay. If pharmacological prophylaxis is not possible, mechanical prophylaxis should be administered. For very high-risk patients (many EL patients will fall into this category), pharmacological combined with mechanical prophylaxis should be given. Reassessment should occur daily postoperatively.
Level of evidence: High
Recommendation Grade: Strong
The duration of prophylaxis, including after discharge, should be determined by patient risk factors and underlying conditions.
Level of evidence: Moderate
Recommendation grade: Strong
Urinary catheter removal
Urinary catheters are routinely placed for patients undergoing major abdominal surgery for fluid balance, bladder decompression, and to prevent urinary retention. Enhanced recovery protocols in the elective setting advocate for their early removal postoperatively to encourage mobility and improve patient comfort [25, 255] and to reduce the incidence of catheter-associated urinary tract infection (CAUTI), which increases with duration of catheterization [256,257,258]. In older adults, presence of a urinary catheter is associated with a significantly increased risk of delirium [229, 230]. Early removal in major abdominal surgery encourages mobility, speeds recovery, reduces LOS, and decreases CAUTI [258,259,260]. However, in the emergency surgical setting strict monitoring of urine output and fluid balance may be necessary in patients with sepsis or acute physiological derangement [261, 262]. While source control may have been achieved with surgery, ongoing resuscitation and a urinary catheter may be required past the first postoperative day in EL patients. A catheter may also continue to be needed in cases of pelvic surgery, patient immobility, sedation, or epidural analgesia [257, 261]. These conditions aside, when the patient no longer requires strict fluid management, the urinary catheter should be removed as early as possible, and mobilization encouraged.
Recommendation
Urinary catheter use should be evaluated daily, and the catheter should be removed as early as possible.
Level of evidence: Moderate (extrapolated from elective studies)
Recommendation grade: Strong
Peri- and postoperative nasogastric tube use
Prophylactic nasogastric intubation (NGI) following abdominal operations has been used to decrease postoperative complications, such as nausea, vomiting, gastric distention, and anastomotic leakage. However, a Cochrane review of 33 RCTs including both elective and emergency abdominal surgery (although numbers of included EGS patients is unclear) showed no reduction in complications with NGI [263]. Another meta-analysis of mainly elective abdominal surgery found no benefit of prophylactic NGI on gastrointestinal or pulmonary complications but an increase in undesired outcomes, such as discomfort and delayed return to liquid or regular diet [264]. Patients not subjected to NGI had earlier passage of feces and return to fluid intake [264]. Reduction of time from surgery to the first passage of flatus was also detected in 862 patients from eight RCTs when routine NGI was avoided [265]. A trend toward shorter hospital LOS was detected in patients not subjected to NGI in several RCTs, but statistical significance was not reached [263,264,265]. Therapeutic NGI is indicated in patients presenting with ileus or those with gross intestinal edema at the end of the procedure. Otherwise, no convincing data is available to support prophylactic postoperative nasogastric decompression [263]. Most of the reviewed RCTs and meta-analyses are not in patients undergoing EL, although two small studies in EGS patients also suggest no evidence for routine use [266, 267].
Summary and recommendation
The evidence for the use of gastric decompression and evidence is inconclusive and the authors feel that a strategy of selective or therapeutic use of postoperative NGI is more appropriate than routine or prophylactic use, even in this population of emergency surgical patients. Postoperatively once gastric aspirate volumes are controlled/minimized, nasogastric tubes should be removed to encourage oral enteral intake.
Recommendation
Nasogastric tube use should be considered on an individual basis taking into account the risk of gastric stasis and aspiration related to gut dysfunction. Daily revaluation of the need for NGI should occur and it should be removed as early as possible.
Level of Evidence: Moderate
Recommendation Grade: Strong
Postoperative nutrition
While it is not possible to optimize patients nutritionally before undergoing EL, postoperative diet and nutrition should be managed proactively in line with ERAS principles [268, 269] and assessed on a case by case basis dependent on underlying pathology. Even in the emergency situation early feeding may be of benefit. An RCT [270] of EGS patients showed no increase in complications with feeding within 24 h of surgery compared with a traditional approach of starting with a liquid diet after passage of flatus or stool. There were no differences between the two groups in complications rates nor postoperative ileus or LOS. In this study, early feeding appeared safe after emergency abdominal surgery although it was associated with more vomiting (treated easily and without patient discomfort) and less hunger than with a traditional approach. A retrospective propensity-matched study of patients fed early after EGS compared with delayed feeding showed the early group had lower mortality and complications, although it is likely that lower risk patients may have been selected as suitable to be fed early [271].
Some patients who have undergone EL will not tolerate oral nutrition postoperatively. Enteral nutrition has been proposed as a viable alternative when oral nutrition is not feasible but may take up to 5 postoperative days to achieve desired protein and calorie intake in patients undergoing major elective abdominal surgery [272]. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines on perioperative nutrition state, “If the energy and nutrient requirements cannot be met by oral and enteral intake alone (< 50% of caloric requirement) for more than 7 days, a combination of enteral and parenteral nutrition is recommended” [273]. However, enteral feeding may be contraindicated in patients who have intestinal obstruction or ileus, sepsis, intestinal ischemia, high output fistulae, and severe gastrointestinal intestinal hemorrhage [268, 273]. For these patients, early parenteral nutrition is indicated to mitigate the period of inadequate oral/enteral intake. Enteral or oral nutrition may be reinitiated as gastrointestinal function recovers, and/or contraindications end and replace parenteral nutrition when caloric needs can be safely met through the oral/enteral route [268]. Although postoperative immune modulating nutrition may be beneficial in patients undergoing elective abdominal surgery, there is no evidence of benefit in patients undergoing EL [274].
Regular reassessment of nutritional status should be performed during hospital stay. Nutrition therapy and dietary counseling after discharge is advised for patients in whom energy and protein requirements are not met by the oral route [275].
Recommendations
Early tube feeding (within 24 h) should be initiated in patients in whom early oral nutrition cannot be started, and in whom oral intake will be inadequate (< 50% of caloric requirement) for more than 7 days.
Level of evidence: Moderate
Recommendation grade: Strong
If enteral feeding is contraindicated, early parenteral nutrition is indicated to mitigate the period of inadequate oral/enteral intake. Enteral or oral nutrition may be reinitiated as gastrointestinal function recovers and/or contraindications end and replace parenteral nutrition when caloric needs can be safely met through oral/enteral routes.
Level of evidence: Moderate
Recommendation grade: Weak
Postoperative ileus minimization
Postoperative ileus is one of the most frequent complications after abdominal surgery [276, 277]. Pathophysiology is multifactorial and the incidence and duration depends on multiple parameters related to the patient, the procedure, and perioperative care [276, 278,279,280,281]. A major aim of ERAS protocols is prevention of postoperative ileus (POI); the most important items are minimally invasive surgery, optimized fluid management and opioid-sparing analgesia. Other measures, which may or may not be possible for the EL patient, are early mobilization, early postoperative food intake, laxatives, and omission of postoperative nasogastric tubes [25, 276, 277, 279]. In the emergency setting [5], where modulation of metabolic stress response and optimization of perioperative care are even more important, fluid optimization must be balanced between adequate resuscitation and avoidance of fluid overload. Efforts should be taken to correct fluid status early, aiming to have weight gain limited to < 3 kg at postoperative day three [25, 280,281,282]. In patients who present with ileus or those with gross intestinal edema, early oral intake should be encouraged to maintain intestinal function, and small portions should be offered initially, especially after right-sided resections and small-bowel anastomosis [279, 283]. There is some low-level evidence to support other approaches including laxatives such as bisacodyl and magnesium oxide [25]. Chewing gum has been used to prevent POI, but currently available evidence does not support its use in elective ERAS pathways [25]. Some evidence exists for the use of water-soluble contrast agents and neostigmine to treat POI [278, 284, 285].
Recommendations
A multifaceted approach to minimizing postoperative ileus, including minimally invasive surgery, optimized fluid management, opioid-sparing analgesia, early mobilization, early postoperative food intake, laxative administration, and omission/early removal of nasogastric intubation, should be used.
Level of evidence: Moderate
Recommendation grade: Strong
Early mobilization
Early mobilization is an important component of enhanced recovery protocols. Prolonged bed rest increases pulmonary complications, thromboembolism, insulin resistance and decreases muscle strength [286]. The benefits of early mobilization may be even greater in the emergency population with higher prevalence of older patients with preexisting sarcopenia [287, 288], and patients with sepsis at increased risk of muscle catabolism. Loss of functional independence is a significant risk for older patients living with frailty undergoing emergency surgery [224, 289]. Promotion of patient-oriented rehabilitation as part of an older patient care pathway resulted in a significant reduction in mortality, length of stay, and discharge to a higher level of care [290]. A systematic review of early mobilization protocols following mainly elective abdominal and thoracic surgery found little hard evidence of benefit [291], and a RCT in elective colorectal patients found no benefit of staff-facilitated early mobilization [292]. In contrast, a multicenter study showed benefit of early mobilization in surgical ICU patients, length of stay was shortened and patients’ functional ability at discharge was increased [293].
Recommendations
Patients should be assisted to mobilize as soon as possible after surgery.
Level of evidence: Weak
Recommendation grade: Strong
Conclusion
Significant components of the intra- and postoperative management of patients undergoing EL have been identified, with recommendations given based on levels of evidence, safety, and relevance to management of an EL ERAS pathway. Many components are based on evidence extrapolated from the elective setting or from emergency general surgery studies, and not specifically laparotomy. Therefore, many components may require further prospective validation. We suggest the components described in these guidelines are combined into a comprehensive pathway for the management of the EL patient. Aspects of organizational management and approaches to implementation needed to provide an EL ERAS pathway are provided in part 3 of these guidelines.
These guidelines are based on best available current evidence and will be revised when new evidence that changes the recommendations becomes available. While every effort has been made to list correct drug dosages, readers should check drug formulations and dosages in their National/Hospital formularies before prescribing.
Abbreviations
- ACS:
-
American College of Surgeons
- AGS:
-
American Geriatric Society
- AKI:
-
Acute kidney injury
- ASA:
-
American Society of Anesthesiology
- BIS:
-
Bispectral index
- CAM:
-
Confusion assessment method
- CAUTI:
-
Catheter-associated urinary tract infection
- CPAP:
-
Continuous positive airways pressure
- CS:
-
Damage control surgery
- CVC:
-
Central venous catheter
- EEG:
-
Electroencephalography
- EGS:
-
Emergency general surgery
- EL:
-
Emergency laparotomy
- ESA/ESICM:
-
European Society for Anesthesiology and European Society of Intensive Care Medicine
- ESPEN:
-
European Society for Clinical Nutrition and Metabolism
- ERAS:
-
Enhanced Recovery After Surgery
- GDHT:
-
Goal-directed hemodynamic therapy
- HELP:
-
Hospital end of life pathway
- ICU:
-
Intensive care unit
- IV:
-
Intravenous
- LOS:
-
Length of stay
- MAC:
-
Minimum alveolar concentration
- MAP:
-
Mean arterial pressure
- NELA:
-
National Emergency Laparotomy Audit
- NGI:
-
Nasogastric intubation
- NIPPV:
-
Noninvasive positive pressure ventilation
- NIV:
-
Noninvasive ventilation
- NMB:
-
Neuromuscular blockade
- NPWT:
-
Negative pressure wound therapy
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- NSQIP:
-
National Surgical Quality Improvement Program
- OR:
-
Odds ratio
- PACU:
-
Post-anesthesia care unit
- PCA:
-
Patient-controlled analgesia
- PEEP:
-
Positive end-expiratory pressure
- PND:
-
Perioperative neurocognitive disorder
- POI:
-
Postoperative ileus
- PONV:
-
Postoperative nausea and vomiting
- PPC:
-
Postoperative pulmonary complications
- PPV:
-
Pulse pressure variation
- RCT:
-
Randomized clinical trial
- RSI:
-
Rapid sequence induction
- SSI:
-
Surgical site infection
- SVV:
-
Stroke volume variation
- TAP:
-
Transversus abdominis plane
- TIVA:
-
Total intravenous anesthesia
- TTE:
-
Transthoracic echography
- VTE:
-
Venous thromboembolism
References
Huddart S, Peden CJ, Swart M et al (2015) Use of a pathway quality improvement care bundle to reduce mortality after emergency laparotomy. Br J Surg 102:57–66
Lohsiriwat V (2014) Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol 20:13950–13955
Møller MH, Adamsen S, Thomsen RW et al (2011) Multicentre trial of a perioperative protocol to reduce mortality in patients with peptic ulcer perforation. Br J Surg 98:802–810
Wisely JC, Barclay KL (2016) Effects of an enhanced recovery after surgery programme on emergency surgical patients. ANZ J Surg 86:883–888
Roulin D, Blanc C, Muradbegovic M et al (2014) Enhanced recovery pathway for urgent colectomy. World J Surg 38:2153–2159
Aggarwal G, Peden CJ, Mohammed MA et al (2019) Evaluation of the collaborative use of an evidence-based care bundle in emergency laparotomy. JAMA Surg 154:e190145
Paduraru M, Ponchietti L, Casas IM et al (2017) Enhanced recovery after surgery (ERAS) - the evidence in geriatric emergency surgery: a systematic review. Chirurgia 112:546–557
Gonenc M, Dural AC, Celik F et al (2014) Enhanced postoperative recovery pathways in emergency surgery: a randomised controlled clinical trial. Am J Surg 207:807–814
Mohsina S, Shanmugam D, Sureshkumar S et al (2018) Adapted ERAS pathway vs. standard care in patients with perforated duodenal ulcer—a randomized controlled trial. J Gastrointest Surg 22:107–116
Shida D, Tagawa K, Inada K et al (2017) Modified enhanced recovery after surgery (ERAS) protocols for patients with obstructive colorectal cancer. BMC Surg 17:18
Shang Y, Guo C, Zhang D (2018) Modified enhanced recovery after surgery protocols are beneficial for postoperative recovery for patients undergoing emergency surgery for obstructive colorectal cancer. Medicine 97:e12348
Symons NRA, Moorthy K, Almoudaris AM et al (2013) Mortality in high-risk emergency general surgical admissions. Br J Surg 100:1318–1325
Shafi S, Aboutanos MB, Agarwal S Jr et al (2013) Emergency general surgery: definition and estimated burden of disease. J Trauma Acute Care Surg 74:1092–1097
Al-Temimi MH, Griffee M, Enniss TM et al (2012) When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg 215:503–511
Lee KC, Sturgeon D, Lipsitz S et al (2020) Mortality and health care utilization among medicare patients undergoing emergency general surgery vs those with acute medical conditions. JAMA Surg 155:216–223
American College of Surgeons (2018) American College of Surgeons (2019) User Guide for the 2018 Participant Use Data File (PUF) https://www.facs.org/media/xunbqzy5/nsqip_puf_userguide_2018.pdf
NELA Project Team (2019) Fifth Patient Report of the National Emergency Laparotomy Audit https://www.nela.org.uk/downloads/The%20Fifth%20Patient%20Report%20of%20the%20NELA%202019%20-%20Full%20Patient%20Report.pdf
Peden CJ, Aggarwal G, Aitken RJ et al (2021) Guidelines for perioperative care for emergency laparotomy enhanced recovery after surgery (ERAS) Society recommendations: Part 1-preoperative: Diagnosis, rapid assessment and optimization. World J Surg 45:1272–1290
Guyatt GH, Oxman AD, Kunz R et al (2008) Going from evidence to recommendations. BMJ 336:1049–1051
Brown BB (1968) Delphi process: a methodology used for the elicitation of opinions of experts. RAND Corporation, Santa Monica
Brindle M, Nelson G, Lobo DN et al (2020) Recommendations from the ERAS® Society for standards for the development of enhanced recovery after surgery guidelines. BJS Open 4:157–163
NELA Project Team (2017) Audit Inclusion and Exclusion Criteria: NELA Inclusion Criteria. In: National Emergency Laparotomy Audit. https://www.nela.org.uk/downloads/NELA%20Inclusion-Exclusion%20Criteria%20-%20Updated%2024-02-17.pdf Accessed March 21st 2023
Feeney T, Castillo-Angeles M, Scott JW et al (2018) The independent effect of emergency general surgery on outcomes varies depending on case type: a NSQIP outcomes study. Am J Surg 216:856–862
Scott JW, Olufajo OA, Brat GA et al (2016) Use of national burden to define operative emergency general surgery. JAMA Surg 151:e160480
Gustafsson UO, Scott MJ, Hubner M, Nygren J (2019) Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J Surg 43:659–695
Statement on the initial antimicrobial treatment of sepsis V2.0 (2022) In: Academy of Medical Royal Colleges. https://www.aomrc.org.uk/reports-guidance/statement-on-the-initial-antimicrobial-treatment-of-sepsis-v2-0/. Accessed 4 Nov 2022
Miller AS, Boyce K, Box B et al (2021) The association of coloproctology of Great Britain and Ireland consensus guidelines in emergency colorectal surgery. Colorectal Dis 23:476–547
Heywood N, Parmar KL, Stott M et al (2021) The laparoscopy in emergency general surgery (LEGS) study: a questionnaire survey of UK practice. Ann R Coll Surg Engl 103:120–129
Warps A-LK, Zwanenburg ES, Dekker JWT et al (2021) Laparoscopic versus open colorectal surgery in the emergency setting: a systematic review and meta-analysis. Annals of Surgery Open 2:e097
Pucher PH, Mackenzie H, Tucker V, Mercer SJ (2021) A national propensity score-matched analysis of emergency laparoscopic versus open abdominal surgery. Br J Surg 108:934–940
Moghadamyeghaneh Z, Masoomi H, Mills SD et al (2014) Outcomes of conversion of laparoscopic colorectal surgery to open surgery. JSLS 18(e2014):00230
Boccola MA, Buettner PG, Rozen WM et al (2011) Risk factors and outcomes for anastomotic leakage in colorectal surgery: a single-institution analysis of 1576 patients. World J Surg 35:186–195
McDermott FD, Heeney A, Kelly ME et al (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102:462–479
2017 European Society of Coloproctology (ESCP) collaborating group (2018) Safety of primary anastomosis following emergency left sided colorectal resection: an international, multi-centre prospective audit. Colorectal Dis 20(Suppl 6):47–57
Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T (2017) Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis 19:288–298
Kang CY, Halabi WJ, Chaudhry OO et al (2013) Risk factors for anastomotic leakage after anterior resection for rectal cancer. JAMA Surg 148:65–71
Zakrison T, Nascimento BA Jr, Tremblay LN et al (2007) Perioperative vasopressors are associated with an increased risk of gastrointestinal anastomotic leakage. World J Surg 31:1627–1634
Dekker JWT, Liefers GJ, de Mol van Otterloo JCA et al (2011) Predicting the risk of anastomotic leakage in left-sided colorectal surgery using a colon leakage score. J Surg Res 166:e27-34
Boyce SA, Bartolo DCC, Paterson HM, Unit EC (2013) Subspecialist emergency management of diverticulitis is associated with reduced mortality and fewer stomas. Colorectal Dis 15:442–447
Burmas M, Aitken RJ, Broughton KJ (2018) Outcomes following emergency laparotomy in Australian public hospitals. ANZ J Surg 88:998–1002
Weber DG, Bendinelli C, Balogh ZJ (2014) Damage control surgery for abdominal emergencies. Br J Surg 101:e109–e118
Ball CG (2015) Damage control surgery. Curr Opin Crit Care 21:538–543
Rodrigues RR, Carmona MJC, Junior JOCA (2016) Bleeding and damage control surgery. Curr Opin Anaesthesiol 29:229–233
Girard E, Abba J, Boussat B et al (2018) Damage control surgery for non-traumatic abdominal emergencies. World J Surg 42:965–973
Ordoñez CA, Parra M, García A et al (2021) Damage control surgery may be a safe option for severe non-trauma peritonitis management: proposal of a new decision-making algorithm. World J Surg 45:1043–1052
Robledo FA, Luque-de-León E, Suárez R et al (2007) Open versus closed management of the abdomen in the surgical treatment of severe secondary peritonitis: a randomized clinical trial. Surg Infect 8:63–72
Sharrock AE, Midwinter M (2013) Damage control - trauma care in the first hour and beyond: a clinical review of relevant developments in the field of trauma care. Ann R Coll Surg Engl 95:177–183
Bloos F, Thomas-Rüddel D, Rüddel H et al (2014) Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care 18:R42
Waibel BH, Rotondo MF (2012) Damage control for intra-abdominal sepsis. Surg Clin North Am 92:243–257
van Ruler O, Boermeester MA (2017) Surgical treatment of secondary peritonitis. Chirurg 88:1–6
Boyd-Carson H, Gana T, Lockwood S et al (2020) A review of surgical and peri-operative factors to consider in emergency laparotomy care. Anaesthesia 75(Suppl 1):e75–e82
Benz D, Balogh ZJ (2017) Damage control surgery: current state and future directions. Curr Opin Crit Care 23:491–497
Ordóñez CA, Sánchez AI, Pineda JA et al (2010) Deferred primary anastomosis versus diversion in patients with severe secondary peritonitis managed with staged laparotomies. World J Surg 34:169–176
Ordoñez CA, Puyana JC (2006) Management of peritonitis in the critically ill patient. Surg Clin North Am 86:1323–1349
Haltmeier T, Falke M, Quaile O et al (2021) Damage control surgery in patients with non-traumatic abdominal emergencies: a systematic review and meta-analysis. J Trauma Acute Care Surg 92:1075–1085
Cirocchi R, Birindelli A, Biffl WL et al (2016) What is the effectiveness of the negative pressure wound therapy (NPWT) in patients treated with open abdomen technique? A systematic review and meta-analysis. J Trauma Acute Care Surg 81:575–584
Bleszynski MS, Chan T, Buczkowski AK (2016) Open abdomen with negative pressure device vs primary abdominal closure for the management of surgical abdominal sepsis: a retrospective review. Am J Surg 211:926–932
Betancourt AS, Milagros GC, Sibaja P et al (2020) Cost evaluation of temporary abdominal closure methods in abdominal sepsis patients successfully treated with an open abdomen. Should we take temporary abdominal closure methods at face value? Health Economic evaluation. Ann Med Surg (Lond) 56:11–16
Willms A, Schaaf S, Schwab R et al (2016) Abdominal wall integrity after open abdomen: long-term results of vacuum-assisted wound closure and mesh-mediated fascial traction (VAWCM). Hernia 20:849–858
Fortelny RH, Hofmann A, Gruber-Blum S et al (2014) Delayed closure of open abdomen in septic patients is facilitated by combined negative pressure wound therapy and dynamic fascial suture. Surg Endosc 28:735–740
Beltzer C, Eisenächer A, Badendieck S et al (2016) Retrospective analysis of a VACM (vacuum-assisted closure and mesh-mediated fascial traction) treatment manual for temporary abdominal wall closure - results of 58 consecutive patients. GMS Interdiscip Plast Reconstr Surg DGPW 5:Doc19
Tolonen M, Mentula P, Sallinen V et al (2017) Open abdomen with vacuum-assisted wound closure and mesh-mediated fascial traction in patients with complicated diffuse secondary peritonitis: a single-center 8-year experience. J Trauma Acute Care Surg 82:1100–1105
Salamone G, Licari L, Guercio G et al (2018) Vacuum-assisted wound closure with mesh-mediated fascial traction achieves better outcomes than vacuum-assisted wound closure alone: a comparative study. World J Surg 42:1679–1686
Schein M (2008) To drain or not to drain? The role of drainage in the contaminated and infected abdomen: an international and personal perspective. World J Surg 32:312–321
Jesus EC, Karliczek A, Matos D et al (2004) Prophylactic anastomotic drainage for colorectal surgery. Cochrane Datab Syst Rev 2004(4):002100
Hüttner FJ, Probst P, Knebel P et al (2017) Meta-analysis of prophylactic abdominal drainage in pancreatic surgery. Br J Surg 104:660–668
Petrowsky H, Demartines N, Rousson V, Clavien P-A (2004) Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg 240:1074–1084 (discussion 1084–5)
Urbach DR, Kennedy ED, Cohen MM (1999) Colon and rectal anastomoses do not require routine drainage: a systematic review and meta-analysis. Ann Surg 229:174–180
Guerra F, Giuliani G, Coletta D et al (2018) A meta-analysis of randomized controlled trials on the use of suction drains following rectal surgery. Dig Surg 35:482–490
Collaborative E (2022) Intraperitoneal drain placement and outcomes after elective colorectal surgery: international matched, prospective, cohort study. Br J Surg 109:520–529
Collaborative E (2022) 256 the effect of intraperitoneal drain placement on postoperative outcomes after emergency colorectal surgery: a propensity-score matched analysis. Br J Surg 109(znac268):026
Talving P, Mohseni S, Inaba K et al (2011) Closed suction drain after isolated hollow viscus injury: a friend or foe? J Trauma 70:1424–1428
Mohseni S, Talving P, Kobayashi L et al (2012) Closed-suction drain placement at laparotomy in isolated solid organ injury is not associated with decreased risk of deep surgical site infection. Am Surg 78:1187–1191
Manzoor B, Heywood N, Sharma A (2015) Review of subcutaneous wound drainage in reducing surgical site infections after laparotomy. Surg Res Pract 2015:715803
Li Z, Zhao L, Cheng Y et al (2018) Abdominal drainage to prevent intra-peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database Syst Rev 5:CD010168
Di Saverio S, Podda M, De Simone B et al (2020) Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg 15:27
Schlottmann F, Reino R, Sadava EE et al (2016) Could an abdominal drainage be avoided in complicated acute appendicitis? Lessons learned after 1300 laparoscopic appendectomies. Int J Surg 36:40–43
Ansari M, Akhtar A, Haleem S et al (2012) Is there a role of abdominal drainage in primarily repaired perforated peptic ulcers? J Exp Integr Med 2:47
Alkaaki A, Al-Radi OO, Khoja A et al (2019) Surgical site infection following abdominal surgery: a prospective cohort study. Can J Surg 62:111–117
Nelson RL, Gladman E (2024) Barbateskovic M (2014) Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 5:001181
Hu LQ, Grant MC, Hornor MA et al (2020) Technical evidence review for emergency major abdominal operation conducted for the AHRQ safety program for improving surgical care and recovery. J Am Coll Surg 231:743-764.e5
Sartelli M, Chichom-Mefire A, Labricciosa FM et al (2017) The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg 12:29
Keenan JE, Speicher PJ, Thacker JKM et al (2014) The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg 149:1045–1052
Zywot A, Lau CSM, Stephen Fletcher H, Paul S (2017) Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg 21:1915–1930
Reese SM, Knepper B, Amiot M et al (2020) Implementation of colon surgical site infection prevention bundle-The successes and challenges. Am J Infect Control 48:1287–1291
NIHR Global Research Health Unit on Global Surgery (2022) Routine sterile glove and instrument change at the time of abdominal wound closure to prevent surgical site infection (ChEETAh): a pragmatic, cluster-randomised trial in seven low-income and middle-income countries. Lancet 400:1767–1776
Leaper DJ, Edmiston CE (2017) World Health Organization: global guidelines for the prevention of surgical site infection. J Hosp Infect 95:135–136
Stept WJ, Safar P (1970) Rapid induction/intubation for prevention of gastric-content aspiration. Anesth Analg 49:633–636
Sellick BA (1961) Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet 2:404–406
Marsch SC, Steiner L, Bucher E et al (2011) Succinylcholine versus rocuronium for rapid sequence intubation in intensive care: a prospective, randomized controlled trial. Crit Care 15:R199
Tran DTT, Newton EK, Mount VAH et al (2017) Rocuronium vs. succinylcholine for rapid sequence intubation: a Cochrane systematic review. Anaesthesia 72:765–777
Pillay L, Hardcastle T (2017) Collective review of the status of rapid sequence intubation drugs of choice in trauma in low- and middle-income settings (prehospital, emergency department and operating room setting). World J Surg 41:1184–1192
Fuchs-Buder T, Romero CS, Lewald H et al (2023) Peri-operative management of neuromuscular blockade: a guideline from the European society of anaesthesiology and intensive care. Eur J Anaesthesiol 40:82–94
Tessarolo E, Alkhouri H, Lelos N et al (2022) Review article: Effectiveness and risks of cricoid pressure during rapid sequence induction for endotracheal intubation in the emergency department: a systematic review. Emerg Med Australas 34:484–491
Frerk C, Mitchell VS, McNarry AF et al (2015) Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. Br J Anaesth 115:827–848
Sajayan A, Wicker J, Ungureanu N et al (2016) Current practice of rapid sequence induction of anaesthesia in the UK - a national survey. Br J Anaesth 117(Suppl 1):i69–i74
Buddeberg BS, Seeberger MD (2022) Anesthesia and oncology: friend or foe? Front Oncol 12:802210
Miller D, Lewis SR, Pritchard MW et al (2018) Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev 8:CD012317
Berger M, Schenning KJ, Brown CH 4th et al (2018) Best practices for postoperative brain health: recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg 127:1406–1413
Punjasawadwong Y, Chau-In W, Laopaiboon M et al (2018) Processed electroencephalogram and evoked potential techniques for amelioration of postoperative delirium and cognitive dysfunction following non-cardiac and non-neurosurgical procedures in adults. Cochrane Database Syst Rev 5:CD011283
Evered LA, Chan MTV, Han R et al (2021) Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth 127:704–712
Ansaloni L, Catena F, Chattat R et al (2010) Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 97:273–280
Pandit JJ, Andrade J, Bogod DG et al (2014) 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Br J Anaesth 113:549–559
Evered LA, Goldstein PA (2021) Reducing perioperative neurocognitive disorders (PND) through depth of anesthesia monitoring: a critical review. Int J Gen Med 14:153–162
Gan TJ, Belani KG, Bergese S et al (2020) Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 131:411–448
Rajan N, Joshi GP (2021) Management of postoperative nausea and vomiting in adults: current controversies. Curr Opin Anaesthesiol 34:695–702
Schlesinger T, Weibel S, Meybohm P, Kranke P (2021) Drugs in anesthesia: preventing postoperative nausea and vomiting. Curr Opin Anaesthesiol 34:421–427
Evans L, Rhodes A, Alhazzani W et al (2021) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med 49:e1063–e1143
Corcoran TB, Myles PS, Forbes AB et al (2021) Dexamethasone and surgical-site infection. N Engl J Med 384:1731–1741
The 2019 American Geriatrics Society Beers Criteria® Update Expert Panel (2019) American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 67:674–694
Feldheiser A, Aziz O, Baldini G et al (2016) Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 60:289–334
Galvão CM, Liang Y, Clark AM (2010) Effectiveness of cutaneous warming systems on temperature control: meta-analysis. J Adv Nurs 66:1196–1206
Young CC, Harris EM, Vacchiano C et al (2019) Lung-protective ventilation for the surgical patient: international expert panel-based consensus recommendations. Br J Anaesth 123:898–913
Watson X, Chereshneva M, Odor PM et al (2018) Adoption of lung protective ventilation IN patients undergoing emergency laparotomy: the ALPINE study. A prospective multicentre observational study. Br J Anaesth 121:909–917
Fernandez-Bustamante A, Frendl G, Sprung J (2017) Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA 152:157–166
Murphy GS, Szokol JW, Avram MJ et al (2015) Residual neuromuscular block in the elderly: incidence and clinical implications. Anesthesiology 123:1322–1336
Murphy GS, Szokol JW, Avram MJ et al (2013) Postoperative residual neuromuscular blockade is associated with impaired clinical recovery. Anesth Analg 117:133–141
McLean DJ, Diaz-Gil D, Farhan HN et al (2015) Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology 122:1201–1213
Naguib M, Brull SJ, Kopman AF et al (2018) Consensus statement on perioperative use of neuromuscular monitoring. Anesth Analg 127:71–80
Togioka BM, Yanez D, Aziz MF et al (2020) Randomised controlled trial of sugammadex or neostigmine for reversal of neuromuscular block on the incidence of pulmonary complications in older adults undergoing prolonged surgery. Br J Anaesth 124:553–561
Wang J-F, Zhao Z-Z, Jiang Z-Y et al (2021) Influence of sugammadex versus neostigmine for neuromuscular block reversal on the incidence of postoperative pulmonary complications: a meta-analysis of randomized controlled trials. Perioper Med (Lond) 10:32
Holte K, Sharrock NE, Kehlet H (2002) Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth 89:622–632
Chappell D, Jacob M, Hofmann-Kiefer K et al (2008) A rational approach to perioperative fluid management. Anesthesiology 109:723–740
Peden C, Scott MJ (2015) Anesthesia for emergency abdominal surgery. Anesthesiol Clin 33:209–221
Harten J, Crozier JEM, McCreath B et al (2008) Effect of intraoperative fluid optimisation on renal function in patients undergoing emergency abdominal surgery: a randomised controlled pilot study (ISRCTN 11799696). Int J Surg 6:197–204
Aaen AA, Voldby AW, Storm N et al (2021) Goal-directed fluid therapy in emergency abdominal surgery: a randomised multicentre trial. Br J Anaesth 127:521–531
Todd SR, Malinoski D, Muller PJ, Schreiber MA (2007) Lactated Ringer’s is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma 62:636–639
Lobo DN, Awad S (2014) Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent “pre-renal”acute kidney injury?: con. Kidney Int 86:1096–1105
Chowdhury AH, Cox EF, Francis ST, Lobo DN (2012) A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 256:18–24
Weinberg L, Li M, Churilov L et al (2018) Associations of fluid amount, type, and balance and acute kidney injury in patients undergoing major surgery. Anaesth Intensive Care 46:79–87
Volta CA, Trentini A, Farabegoli L et al (2013) Effects of two different strategies of fluid administration on inflammatory mediators, plasma electrolytes and acid/base disorders in patients undergoing major abdominal surgery: a randomized double blind study. J Inflamm 10:29
Pfortmueller CA, Funk G-C, Reiterer C et al (2018) Normal saline versus a balanced crystalloid for goal-directed perioperative fluid therapy in major abdominal surgery: a double-blind randomised controlled study. Br J Anaesth 120:274–283
Semler MW, Self WH, Wanderer JP et al (2018) Balanced crystalloids versus saline in critically Ill adults. N Engl J Med 378:829–839
Semler MW, Kellum JA (2019) Balanced crystalloid solutions. Am J Respir Crit Care Med 199:952–960
Semler MW, Wanderer JP, Ehrenfeld JM et al (2017) Balanced crystalloids versus saline in the intensive care unit. The salt randomized trial. Am J Respir Crit Care Med 195:1362–1372
Futier E, Garot M, Godet T et al (2020) Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery: the flash randomized clinical trial. JAMA 323:225–236
Zarychanski R, Abou-Setta AM, Turgeon AF et al (2013) Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA 309:678–688
Funk G-C, Lindner G, Druml W et al (2010) Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 36:304–311
Stelfox HT, Ahmed SB, Khandwala F et al (2008) The epidemiology of intensive care unit-acquired hyponatraemia and hypernatraemia in medical-surgical intensive care units. Crit Care 12:R162
Buckley MS, Leblanc JM, Cawley MJ (2010) Electrolyte disturbances associated with commonly prescribed medications in the intensive care unit. Crit Care Med 38:S253–S264
Martyn JAJ, Richtsfeld M (2006) Succinylcholine-induced hyperkalemia in acquired pathologic states: etiologic factors and molecular mechanisms. Anesthesiology 104:158–169
Bugg NC, Jones JA (1998) Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia 53:895–902
Zaloga GP (1992) Hypocalcemia in critically ill patients. Crit Care Med 20:251–262
Besunder JB, Smith PG (1991) Toxic effects of electrolyte and trace mineral administration in the intensive care unit. Crit Care Clin 7:659–693
Lee JW (2010) Fluid and electrolyte disturbances in critically ill patients. Electrolyte Blood Press 8:72–81
Chong MA, Wang Y, Berbenetz NM, McConachie I (2018) Does goal-directed haemodynamic and fluid therapy improve peri-operative outcomes?: A systematic review and meta-analysis. Eur J Anaesthesiol 35:469–483
Cecconi M, Corredor C, Arulkumaran N et al (2013) Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care 17:209
Grocott MPW, Dushianthan A, Hamilton MA et al (2012) Perioperative increase in global blood flow to explicit defined goals and outcomes following surgery. Cochrane Database Syst Rev 11:CD004082
Challand C, Struthers R, Sneyd JR et al (2012) Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth 108:53–62
Rollins KE, Lobo DN (2016) Intraoperative goal-directed fluid therapy in elective major abdominal surgery: a meta-analysis of randomized controlled trials. Ann Surg 263:465–476
Pearse RM, Harrison DA, MacDonald N et al (2014) Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 311:2181–2190
Rollins KE, Mathias NC, Lobo DN (2019) Meta-analysis of goal-directed fluid therapy using transoesophageal Doppler monitoring in patients undergoing elective colorectal surgery. BJS Open 3:606–616
Tengberg LT, Bay-Nielsen M, Bisgaard T et al (2017) Multidisciplinary perioperative protocol in patients undergoing acute high-risk abdominal surgery. Br J Surg 104:463–471
Salmasi V, Maheshwari K, Yang D et al (2017) Relationship between Intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 126:47–65
Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG et al (2018) Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial). Br J Anaesth 120:734–744
Futier E, Lefrant J-Y, Guinot P-G et al (2017) Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA 318:1346–1357
Vieillard-Baron A, Millington SJ, Sanfilippo F et al (2019) A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 45:770–788
Marik PE (2013) Noninvasive cardiac output monitors: a state-of the-art review. J Cardiothorac Vasc Anesth 27:121–134
Rhodes A, Evans LE, Alhazzani W et al (2017) Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45:486–552
Lamontagne F, Richards-Belle A, Thomas K et al (2020) Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA 323:938–949
Lamontagne F, Day AG, Meade MO et al (2018) Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 44:12–21
French WB, Rothstein WB, Scott MJ (2021) Time to use peripheral norepinephrine in the operating room. Anesth Analg 133:284–288
Kotagal M, Symons RG, Hirsch IB et al (2015) Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg 261:97–103
Frisch A, Chandra P, Smiley D et al (2010) Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33:1783–1788
Farrokhi F, Smiley D, Umpierrez GE (2011) Glycemic control in non-diabetic critically ill patients. Best Pract Res Clin Endocrinol Metab 25:813–824
Umpierrez GE, Smiley D, Jacobs S et al (2011) Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 34:256–261
Duggan EW, Carlson K, Umpierrez GE (2017) Perioperative hyperglycemia management: an update. Anesthesiology 126:547–560
Schack A, Ekeloef S, Ostrowski SR et al (2021) Blood transfusion in major emergency abdominal surgery. Eur J Trauma Emerg Surg 48:121–131
Narasimhan V, Spychal R, Pilgrim C (2017) Blood transfusions for emergency laparotomies in general surgery. J Trauma Acute Care Surg 7:15–22
American Society of Anesthesiologists Task Force on Perioperative Blood Management (2015) Practice guidelines for perioperative blood management: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 122:241–275
Kozek-Langenecker SA, Ahmed AB, Afshari A et al (2017) Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol 34:332–395
Ferraris VA, Hochstetler M, Martin JT et al (2015) Blood transfusion and adverse surgical outcomes: The good and the bad. Surgery 158:608–617
Baker L, Park L, Gilbert R et al (2021) Intraoperative red blood cell transfusion decision-making: a systematic review of guidelines. Ann Surg 274:86–96
Wick EC, Grant MC, Wu CL (2017) Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques. JAMA Surg 152:691
Soffin EM, YaDeau JT (2016) Enhanced recovery after surgery for primary hip and knee arthroplasty: a review of the evidence. Br J Anaesth 117:iii62–iii72
Grant MC, Sommer PM, He C et al (2017) Preserved analgesia with reduction in opioids through the use of an acute pain protocol in enhanced recovery after surgery for open hepatectomy. Reg Anesth Pain Med 42:451–457
Ban KA, Gibbons MM, Ko CY et al (2019) Evidence review conducted for the agency for healthcare research and quality safety program for improving surgical care and recovery: focus on anesthesiology for colorectal surgery. Anesth Analg 128:879
Arron MNN, Lier EJ, de Wilt JHW et al (2020) Postoperative administration of non-steroidal anti-inflammatory drugs in colorectal cancer surgery does not increase anastomotic leak rate; a systematic review and meta-analysis. Eur J Surg Oncol 46:2167–2173
Park CM, Inouye SK, Marcantonio ER et al (2022) Perioperative gabapentin use and in-hospital adverse clinical events among older adults after major surgery. JAMA Intern Med 182:1117–1127
Pöpping DM, Elia N, Marret E et al (2008) Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 143:990–999 (discussion 1000)
Guay J, Nishimori M, Kopp S (2016) Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev 7:CD001893
Werawatganon T, Charuluxanun S (2005) Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Syst Rev CD004088
De Oliveira Jr GS, Castro-Alves LJ, Nader A et al (2014) Transversus abdominis plane block to ameliorate postoperative pain outcomes after laparoscopic surgery: a meta-analysis of randomized controlled trials. Anesth Analg 118:454–463
Oh TK, Lee S-J, Do S-H, Song I-A (2018) Transversus abdominis plane block using a short-acting local anesthetic for postoperative pain after laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc 32:545–552
Baeriswyl M, Kirkham KR, Kern C, Albrecht E (2015) The analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: a meta-analysis. Anesth Analg 121:1640–1654
Mrunalini P, Raju NVR, Nath VN, Saheb SM (2014) Efficacy of transversus abdominis plane block in patients undergoing emergency laparotomies. Anesth Essays Res 8:377–382
Sanderson BJ, Doane MA (2018) Transversus abdominis plane catheters for analgesia following abdominal surgery in adults. Reg Anesth Pain Med 43:5–13
Bamigboye AA, Hofmeyr GJ (2009) Local anaesthetic wound infiltration and abdominal nerves block during caesarean section for postoperative pain relief. Cochrane Database Syst Rev CD006954
Yu N, Long X, Lujan-Hernandez JR et al (2014) Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol 14:121
Karthikesalingam A, Walsh SR, Markar SR et al (2008) Continuous wound infusion of local anaesthetic agents following colorectal surgery: systematic review and meta-analysis. World J Gastroenterol 14:5301–5305
Marks JL, Ata B, Tulandi T (2012) Systematic review and metaanalysis of intraperitoneal instillation of local anesthetics for reduction of pain after gynecologic laparoscopy. J Minim Invasive Gynecol 19:545–553
Weibel S, Jokinen J, Pace NL et al (2016) Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: a systematic review with trial sequential analysis. Br J Anaesth 116:770–783
Sun Y, Li T, Wang N et al (2012) Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 55:1183–1194
McCarthy GC, Megalla SA, Habib AS (2010) Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 70:1149–1163
Ventham NT, Hughes M, O’Neill S et al (2013) Systematic review and meta-analysis of continuous local anaesthetic wound infiltration versus epidural analgesia for postoperative pain following abdominal surgery. Br J Surg 100:1280–1289
Rollins KE, Javanmard-Emamghissi H, Scott MJ, Lobo DN (2020) The impact of peri-operative intravenous lidocaine on postoperative outcome after elective colorectal surgery: a meta-analysis of randomised controlled trials. Eur J Anaesthesiol 37:659–670
Levy N, Quinlan J, El-Boghdadly K et al (2021) An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia 76:520–536
Schwenk ES, Viscusi ER, Buvanendran A et al (2018) Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the american society of regional anesthesia and pain medicine, the american academy of pain medicine, and the american society of anesthesiologists. Reg Anesth Pain Med 43:456–466
Brueckmann B, Villa-Uribe JL, Bateman BT et al (2013) Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 118:1276–1285
Neto AS, da Costa LGV, Hemmes SNT et al (2018) The LAS VEGAS risk score for prediction of postoperative pulmonary complications: an observational study. Eur J Anaesthesiol 35:691–701
Fernandez-Bustamante A, Frendl G, Sprung J et al (2017) Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg 152:157–166
The Royal College of Surgeons and Department of Health (2011) The Higher Risk General Surgical Patient: Towards Improved Care for a Forgotten Group https://www.rcseng.ac.uk/library-and-publications/rcs-publications/docs/the-higher-risk-general-surgical-patient/
Canet J, Mazo V (2010) Postoperative pulmonary complications. Minerva Anestesiol 76:138–143
Canet J, Gallart L, Gomar C et al (2010) Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 113:1338–1350
Leone M, Einav S, Chiumello D et al (2020) Noninvasive respiratory support in the hypoxaemic peri-operative/periprocedural patient: a joint ESA/ESICM guideline. Intensive Care Med 46:697–713
Squadrone V, Coha M, Cerutti E et al (2005) Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA 293:589–595
Jaber S, Lescot T, Futier E et al (2016) Effect of noninvasive ventilation on tracheal reintubation among patients with hypoxemic respiratory failure following abdominal surgery: a randomized clinical trial. JAMA 315:1345–1353
Ireland CJ, Chapman TM, Mathew SF, et al (2014) Continuous positive airway pressure (CPAP) during the postoperative period for prevention of postoperative morbidity and mortality following major abdominal surgery. Cochrane Database Syst Rev CD008930
Pearse R, Ranieri M, Abbott T et al (2021) Postoperative continuous positive airway pressure to prevent pneumonia, re-intubation, and death after major abdominal surgery (PRISM): a multicentre, open-label, randomised, phase 3 trial. Lancet Respir Med 9:1221–1230
Odor PM, Bampoe S, Gilhooly D et al (2020) Perioperative interventions for prevention of postoperative pulmonary complications: systematic review and meta-analysis. BMJ 368:m540
Fagevik Olsén M, Hahn I, Nordgren S et al (1997) Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. Br J Surg 84:1535–1538
Boden I, Sullivan K, Hackett C et al (2018) ICEAGE (incidence of complications following emergency abdominal surgery: get exercising): study protocol of a pragmatic, multicentre, randomised controlled trial testing physiotherapy for the prevention of complications and improved physical recovery after emergency abdominal surgery. World J Emerg Surg 13:29
Boden I, Sullivan K, Hackett C et al (2022) Intensive physical therapy after emergency laparotomy: pilot phase of the Incidence of Complications following Emergency Abdominal surgery Get Exercising randomized controlled trial. J Trauma Acute Care Surg 92:1020–1030
Rochwerg B, Einav S, Chaudhuri D et al (2020) The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med 46:2226–2237
Lissauer ME, Galvagno SM Jr, Rock P et al (2014) Increased ICU resource needs for an academic emergency general surgery service. Crit Care Med 42:910–917
Sobol JB, Wunsch H (2011) Triage of high-risk surgical patients for intensive care. Crit Care 15:217
Pearse RM, Moreno RP, Bauer P et al (2012) Mortality after surgery in Europe: a 7 day cohort study. Lancet 380:1059–1065
Wunsch H, Gershengorn HB, Cooke CR et al (2016) Use of intensive care services for medicare beneficiaries undergoing major surgical procedures. Anesthesiology 124:899–907
Aggarwal G, Broughton KJ, Williams LJ et al (2020) Early postoperative death in patients undergoing emergency high-risk surgery: towards a better understanding of patients for whom surgery may not be beneficial. J Clin Med 9:1288
Tengberg LT, Cihoric M, Foss NB et al (2017) Complications after emergency laparotomy beyond the immediate postoperative period - a retrospective, observational cohort study of 1139 patients. Anaesthesia 72:309–316
Vester-Andersen M, Lundstrøm LH, Møller MH et al (2014) Mortality and postoperative care pathways after emergency gastrointestinal surgery in 2904 patients: a population-based cohort study. Br J Anaesth 112:860–870
Saunders DI, Murray D, Pichel AC et al (2012) Variations in mortality after emergency laparotomy: the first report of the UK Emergency Laparotomy Network. Br J Anaesth 109:368–375
Akyar S, Armenia SJ, Ratnani P, Merchant AM (2018) The impact of frailty on postoperative cardiopulmonary complications in the emergency general surgery population. Surg J (N Y) 4:e66–e77
McIsaac DI, Moloo H, Bryson GL, van Walraven C (2017) The association of frailty with outcomes and resource use after emergency general surgery: a population-based cohort study. Anesth Analg 124:1653–1661
Gillies MA, Harrison EM, Pearse RM et al (2017) Intensive care utilization and outcomes after high-risk surgery in Scotland: a population-based cohort study. Br J Anaesth 118:123–131
Chana P, Joy M, Casey N et al (2017) Cohort analysis of outcomes in 69 490 emergency general surgical admissions across an international benchmarking collaborative. BMJ Open 7:e014484
Ingraham A, Nathens A, Peitzman A et al (2017) Assessment of emergency general surgery care based on formally developed quality indicators. Surgery 162:397–407
Update to the high-risk surgical patient released by RCS England. https://www.nela.org.uk/RCS-Update-The-High-Risk-Surgical-Patient-Released. Accessed 19 Nov 2022
Saravana-Bawan B, Warkentin LM, Rucker D et al (2019) Incidence and predictors of postoperative delirium in the older acute care surgery population: a prospective study. Can J Surg 62:33–38
de Castro SMM, Ünlü Ç, Tuynman JB et al (2014) Incidence and risk factors of delirium in the elderly general surgical patient. Am J Surg 208:26–32
Saljuqi AT, Hanna K, Asmar S et al (2020) Prospective evaluation of delirium in geriatric patients undergoing emergency general surgery. J Am Coll Surg 230:758–765
Peden CJ, Miller TR, Deiner SG et al (2021) Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. Br J Anaesth 126:423–432
Soiza RL, Myint PK (2019) The Scottish intercollegiate guidelines network (SIGN) 157: guidelines on risk reduction and management of delirium. Medicina 55:491
Culley DJ, Flaherty D, Fahey MC et al (2017) Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology 127:765–774
Mohanty S, Rosenthal RA, Russell MM et al (2016) Optimal perioperative management of the geriatric patient: a best practices guideline from the American college of surgeons nsqip and the american geriatrics society. J Am Coll Surg 222:930–947
Arias F, Wiggins M, Urman RD et al (2020) Rapid in-person cognitive screening in the preoperative setting: test considerations and recommendations from the society for perioperative assessment and quality improvement (SPAQI). Perioper Care Oper Room Manage 19:100089
Hughes CG, Boncyk CS, Culley DJ et al (2020) American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg 130:1572–1590
Hshieh TT, Yue J, Oh E et al (2015) Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med 175:512–520
Chen CC-H, Li H-C, Liang J-T et al (2017) Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surg 152:827–834
Wang Y-Y, Yue J-R, Xie D-M et al (2020) Effect of the tailored, family-involved hospital elder life program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Intern Med 180:17–25
Guideline for Perioperative Care for People Living with Frailty Undergoing Elective and Emergency Surgery (2021) Centre for Perioperative Care (CPOC) https://www.cpoc.org.uk/sites/cpoc/files/documents/2021-09/CPOC-BGS-Frailty-Guideline-2021.pdf
Gou RY, Hshieh TT, Marcantonio ER et al (2021) One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surg 156:430–442
Deeken F, Sánchez A, Rapp MA et al (2021) Outcomes of a delirium prevention program in older persons after elective surgery: a stepped-wedge cluster randomized clinical trial. JAMA Surg 157:e216370
Murphy PB, Vogt KN, Lau BD et al (2018) Venous thromboembolism prevention in emergency general surgery: a review. JAMA Surg 153:479–486
Ross SW, Kuhlenschmidt KM, Kubasiak JC et al (2020) Association of the Risk of a venous thromboembolic event in emergency vs elective general surgery. JAMA Surg 155:503–511
Mallick S, Aiken T, Varley P et al (2022) Readmissions from venous thromboembolism after complex cancer surgery. JAMA Surg 157:312–320
Fleming F, Gaertner W, Ternent CA et al (2018) The American society of colon and rectal surgeons clinical practice guideline for the prevention of venous thromboembolic disease in colorectal surgery. Dis Colon Rectum 61:14–20
National Institute for Health and Care Excellence (2018) Venous Thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. NICE guideline [NG89]. https://www.nice.org.uk/guidance/ng89
Kearon C, Akl EA, Comerota AJ et al (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest 141:e419S-e496S
Anderson DR, Morgano GP, Bennett C et al (2019) American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 3:3898–3944
Sweetland S, Green J, Liu B et al (2009) Million Women Study collaborators. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ 339:b4583
Streiff MB, Holmstrom B, Angelini D et al (2021) Cancer-associated venous thromboembolic disease, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:1181–1201
Gould MK, Garcia DA, Wren SM et al (2012) Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:e227S-e277S
DeWane MP, Davis KA, Schuster KM et al (2018) Venous thromboembolism-related readmission in emergency general surgery patients: a role for prophylaxis on discharge? J Am Coll Surg 226:1072-1077.e3
Ljungqvist O, Scott M, Fearon KC (2017) Enhanced recovery after surgery: a review. JAMA Surg 152:292–298
Wald HL, Ma A, Bratzler DW, Kramer AM (2008) Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data. Arch Surg 143:551–557
Lee Y, McKechnie T, Springer JE et al (2019) Optimal timing of urinary catheter removal following pelvic colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis 34:2011–2021
Patel DN, Felder SI, Luu M et al (2018) Early urinary catheter removal following pelvic colorectal surgery: a prospective, randomized, noninferiority trial. Dis Colon Rectum 61:1180–1186
Sadeghi M, Leis JA, Laflamme C et al (2019) Standardisation of perioperative urinary catheter use to reduce postsurgical urinary tract infection: an interrupted time series study. BMJ Qual Saf 28:32–38
Okrainec A, Aarts M-A, Conn LG et al (2017) Compliance with urinary catheter removal guidelines leads to improved outcome in enhanced recovery after surgery patients. J Gastrointest Surg 21:1309–1317
Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Committee HICPA (2010) Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol 31:319–326
Meddings J, Saint S, Fowler KE et al (2015) The ann arbor criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med 162:S1-34
Nelson R, Edwards S, Tse B (2007) Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database Syst Rev CD004929
Vermeulen H, Storm-Versloot MN, Busch ORC, Ubbink DT (2006) Nasogastric intubation after abdominal surgery: a meta-analysis of recent literature. Arch Surg 141:307–314
Nelson R, Tse B, Edwards S (2005) Systematic review of prophylactic nasogastric decompression after abdominal operations. Br J Surg 92:673–680
Sapkota R, Bhandari RS (2013) Prophylactic nasogastric decompression after emergency laparotomy. JNMA J Nepal Med Assoc 52:437–442
Venara A, Barbieux J, Mucci S et al (2018) Short-term outcomes of colorectal resection for cancer in elderly in the era of enhanced recovery. Scand J Surg 107:31–37
Weimann A, Braga M, Carli F et al (2017) ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 36:623–650
Lobo DN, Gianotti L, Adiamah A et al (2020) Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr 39:3211–3227
Klappenbach RF, Yazyi FJ, Alonso Quintas F et al (2013) Early oral feeding versus traditional postoperative care after abdominal emergency surgery: a randomized controlled trial. World J Surg 37:2293–2299
Lee SH, Jang JY, Kim HW et al (2014) Effects of early enteral nutrition on patients after emergency gastrointestinal surgery: a propensity score matching analysis. Medicine 93:e323
Lobo DN, Williams RN, Welch NT et al (2006) Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: a prospective, randomized, controlled, double-blind study. Clin Nutr 25:716–726
Weimann A, Braga M, Carli F et al (2021) ESPEN practical guideline: Clinical nutrition in surgery. Clin Nutr 40:4745–4761
Marimuthu K, Varadhan KK, Ljungqvist O, Lobo DN (2012) A meta-analysis of the effect of combinations of immune modulating nutrients on outcome in patients undergoing major open gastrointestinal surgery. Ann Surg 255:1060–1068
Schuetz P, Seres D, Lobo DN et al (2021) Management of disease-related malnutrition for patients being treated in hospital. Lancet 398:1927–1938
Chapuis PH, Bokey L, Keshava A et al (2013) Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg 257:909–915
Bragg D, El-Sharkawy AM, Psaltis E et al (2015) Postoperative ileus: Recent developments in pathophysiology and management. Clin Nutr 34:367–376
Barletta JF, Senagore AJ (2014) Reducing the burden of postoperative ileus: evaluating and implementing an evidence-based strategy. World J Surg 38:1966–1977
Grass F, Slieker J, Jurt J et al (2017) Postoperative ileus in an enhanced recovery pathway—a retrospective cohort study. Int J Colorectal Dis 32:675–681
Lobo DN (2004) Fluid, electrolytes and nutrition: physiological and clinical aspects. Proc Nutr Soc 63:453–466
Lobo DN, Bostock KA, Neal KR et al (2002) Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 359:1812–1818
Chowdhury AH, Lobo DN (2011) Fluids and gastrointestinal function. Curr Opin Clin Nutr Metab Care 14:469–476
Kummer A, Slieker J, Grass F et al (2016) Enhanced recovery pathway for right and left colectomy: comparison of functional recovery. World J Surg 40:2519–2527
Branco BC, Barmparas G, Schnüriger B et al (2010) Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg 97:470–478
Traut U, Brügger L, Kunz R, et al (2008) Systemic prokinetic pharmacologic treatment for postoperative adynamic ileus following abdominal surgery in adults. Cochrane Database Syst Rev CD004930
Gustafsson UO, Scott MJ, Hubner M et al (2019) Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS®) society recommendations: 2018. World J Surg 43:659–695
Du Y, Karvellas CJ, Baracos V et al (2014) Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery 156:521–527
Engelhardt KE, Reuter Q, Liu J et al (2018) Frailty screening and a frailty pathway decrease length of stay, loss of independence, and 30-day readmission rates in frail geriatric trauma and emergency general surgery patients. J Trauma Acute Care Surg 85:167–173
Tan HL, Chia STX, Nadkarni NV et al (2019) Frailty and functional decline after emergency abdominal surgery in the elderly: a prospective cohort study. World J Emerg Surg 14:62
Khadaroo RG, Warkentin LM, Wagg AS, et al (2020) Clinical Effectiveness of the Elder-Friendly Approaches to the Surgical Environment Initiative in Emergency General Surgery. JAMA Surg e196021
Castelino T, Fiore JF Jr, Niculiseanu P et al (2016) The effect of early mobilization protocols on postoperative outcomes following abdominal and thoracic surgery: a systematic review. Surgery 159:991–1003
Balvardi S, Pecorelli N, Castelino T et al (2021) Impact of facilitation of early mobilization on postoperative pulmonary outcomes after colorectal surgery: a randomized controlled trial. Ann Surg 273:868–875
Schaller SJ, Anstey M, Blobner M et al (2016) Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 388:1377–1388
Funding
None.
Author information
Authors and Affiliations
Contributions
MS contributed to literature review and grading of evidence and drafted, edited, and made a substantial contribution to finalizing the manuscript. CP performed literature review and grading of evidence and also contributed to the writing of the manuscript. Both MS and CP organized the modified Delphi method among the authors. WBF and JK contributed to literature review, reference organization, and to writing the manuscript. DL, AB, CJ, CW, MH, NL, OL, IA, RU, RA, MG, FH, SH, JH, CJ, CO, GA, SM, CS NQ, DH, ZC, T Y-F, and JD contributed to writing and revising the paper, critical appraisal, and grading of evidence.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Scott has honoraria from and serves on advisory boards of Baxter, Edwards Lifesciences, Deltex, Trevena, and Merck. He also receives travel reimbursement from these companies and is President of ERAS®, USA. Dr. Peden has received consultancy fees from the American College of Surgeons Improving Care and Surgical Recovery program and from Medtronic for unrelated work and is Executive Medical Director for Clinical Quality for the Blue Cross Blue Shield Association, USA. Dr. Wu has nothing to disclose. Dr. Hübner has nothing to disclose. Dr. Lees has nothing to disclose. Dr. Lobo has received an unrestricted educational grant from B. Braun and speaker’s honoraria from Abbott, Corza, and Nestlé for unrelated work. He is the Scientific Chair of the ERAS® Society. Dr. Urman has received research funding or fees from Merck, Covidien/Medtronic, AcelRx, and Pfizer outside the submitted work, as well as federal funding from NIH, AHRQ, and NSF. Dr. Aitken has nothing to disclose. Dr. Grant has nothing to disclose. Dr. Hammarqvist has nothing to disclose. Dr. Hare has nothing to disclose. Dr. Havens has research grant funding from Johnson and Johnson outside the submitted work. Dr. Johnston has nothing to disclose, and Dr. Ljungqvist is the Chairman of the ERAS® Society, founded and owns stock in Encare AB, and has received honoraria for advice, lecturing including travel support from Nutricia, Fresenius-Kabi, Pharmacosmos, Encare AB, and lecturing honoraria from Medtronic and B. Braun outside the related work. Dr. Ljungqvist previously held a now expired patent for a preoperative carbohydrate drink. Angie Balfour—ERAS® Coach, Encare®. Co-Director of The Enhanced Recovery after Surgery Society (UK) C.I.C. (not-for-profit organization—Company No. 10932208). No relevant conflict of interest related to this work.
Dr. Ordoñez has nothing to disclose. Dr. Kim has nothing to disclose. Dr. French has nothing to disclose. Dr. Aggarwal has nothing to disclose. Dr. Quiney has nothing to disclose. Dr. Holena has nothing to disclose. Dr. Cooper has funding from the National Institute on Aging and the John A. Hartford Foundation outside of the submitted work. Dr. Wick has funding from the Agency for Healthcare Research and Quality outside the submitted work. Dr. Bang Foss has nothing to disclose. Dr. Young-Fadok has nothing to disclose. Dr. Mohseni has nothing to disclose. Dr. Dhesi has nothing to disclose. Dr. Sharoky has nothing to disclose. Dr. Anderson has nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scott, M.J., Aggarwal, G., Aitken, R.J. et al. Consensus Guidelines for Perioperative Care for Emergency Laparotomy Enhanced Recovery After Surgery (ERAS®) Society Recommendations Part 2—Emergency Laparotomy: Intra- and Postoperative Care. World J Surg 47, 1850–1880 (2023). https://doi.org/10.1007/s00268-023-07020-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-023-07020-6