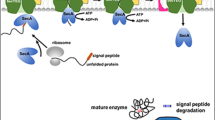
Abstract
Bacillus subtilis is a well-characterized Gram-positive bacterium and a valuable host for recombinant protein production because of its efficient secretion ability, high yield, and non-toxicity. Here, we comprehensively review the recent studies on recombinant protein production in B. subtilis to update and supplement other previous reviews. We have focused on several aspects, including optimization of B. subtilis strains, enhancement and regulation of expression, improvement of secretion level, surface display of proteins, and fermentation optimization. Among them, optimization of B. subtilis strains mainly involves undirected chemical/physical mutagenesis and selection and genetic manipulation; enhancement and regulation of expression comprises autonomous plasmid and integrated expression, promoter regulation and engineering, and fine-tuning gene expression based on proteases and molecular chaperones; improvement of secretion level predominantly involves secretion pathway and signal peptide screening and optimization; surface display of proteins includes surface display of proteins on spores or vegetative cells; and fermentation optimization incorporates medium optimization, process condition optimization, and feeding strategy optimization. Furthermore, we propose some novel methods and future challenges for recombinant protein production in B. subtilis.
Key points
• A comprehensive review on recombinant protein production in Bacillus subtilis.
• Novel techniques facilitate recombinant protein expression and secretion.
• Surface display of proteins has significant potential for different applications.

Similar content being viewed by others
References
Altenbuchner J (2016) Editing of the Bacillus subtilis genome by the CRISPR-Cas9 system. Appl Environ Microbiol 82:5421–5427
Ara K, Ozaki K, Nakamura K, Yamane K, Sekiguchi J, Ogasawara N (2007) Bacillus minimum genome factory: effective utilization of microbial genome information. Biotechnol Appl Biochem 46:169–178
Arnett C, Lange J, Boyd A, Page M, Cropek D (2019) Expression and secretion of active Moringa oleifera coagulant protein in Bacillus subtilis. Appl Microbiol Biotechnol 103:9411–9422
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Sci 315:1709–1712
Benenson Y (2012) Synthetic biology with RNA: progress report. Curr Opin Chem Biol 16:278–284
Bhavsar AP, Zhao X, Brown ED (2001) Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl Environ Microbiol 67:403–410
Biedendieck R, Gamer M, Jaensch L, Meyer S, Rohde M, Deckwer WD, Jahn D (2007) A sucrose-inducible promoter system for the intra-and extracellular protein production in Bacillus megaterium. J Biotechnol 132:426–430
Bijesh K, Denoj S (2018) Biotechnological valorization of pineapple stem for pectinase production by Bacillus subtilis BKDS1: media formulation and statistical optimization for submerged fermentation. Biocatal Agric Biotechnol 16:715–722
Blazeck J, Alper HS (2013) Promoter engineering: recent advances in controlling transcription at the most fundamental level. Biotechnol J 8:46-+
Bleichert F, Botchan MR (2017) Mechanisms for initiating cellular DNA replication. Science 355:eaah6317
Bloor AE, Cranenburgh RM (2006) An efficient method of selectable marker gene excision by Xer recombination for gene replacement in bacterial chromosomes. Appl Environ Microbiol 72:2520–2525
Boekhorst J, de Been MW, Kleerebezem M, Siezen RJ (2005) Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J Bacteriol 187(14):4928–4934
Bongers RS, Veening JW, Van Wieringen M, Kuipers OP, Kleerebezem M (2005) Development and characterization of a subtilin-regulated expression system in Bacillus subtilis: strict control of gene expression by addition of subtilin. Appl Environ Microbiol 71:8818–8824
Bouassida M, Ghazala I, Ellouze-Chaabouni S, Ghribi D (2018) Improved biosurfactant production by Bacillus subtilis SPB1 mutant obtained by random mutagenesis and Its application in enhanced oil recovery in a sand system. J Microbiol Biotechnol 28:95–104
Boutros M, Ahringer J (2008) The art and design of genetic screens: RNA interference. Nat Rev Genet 9(7):554–566
Brans A, Filée P, Chevigné A, Claessens A, Joris B (2004) New integrative method to generate Bacillus subtilis recombinant strains free of selection markers. Appl Environ Microbiol 70(12):7241–7250
Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T (2006) Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J Mol Biol 362(3):393–402
Bukau B, Weissman J, Horwich A (2006) Molecular chaperones and protein quality control. Cell 125:443–451
Chen B, Retzlaff M, Roos T, Frydman J (2011) Cellular strategies of protein quality control. Cold Spring Harb Perspect Biol 3:a004374
Chen H, Ullah J, Jia J (2017) Progress in Bacilus subtilis spore surface display technology towards environment, vaccine development, and biocatalysis. J Mol Microbiol Biotechnol 27:159–167
Chen PT, Shaw JF, Chao YP, David Ho TH, Yu SM (2010) Construction of chromosomally located T7 expression system for production of heterologous secreted proteins in Bacillus subtilis. J Agric Food Chem 58:5392–5399
Cheng JT, Guan CR, Cui WJ, Zhou L, Liu ZM, Li WJ, Zhou ZM (2016) Enhancement of a high efficient autoinducible expression system in Bacillus subtilis by promoter engineering. Protein Expr Purif 127:81–87
Cheng JX, Floer M, Ononaji P, Bryant G, Ptashne M (2002) Responses of four yeast genes to changes in the transcriptional machinery are determined by their promoters. Curr Biol 12:1828–1832
Choi YJ, Morel L, Bourque D, Mullick A, Massie B, Míguez CB (2006) Bestowing inducibility on the cloned methanol dehydrogenase promoter (PmxaF) of Methylobacterium extorquens by applying regulatory elements of Pseudomonas putida F1. Appl Environ Microbiol 72:7723–7729
Choi YJ, Morel T, Le François L, Bourque D, Bourget L, Groleau D, Massie B, Míguez C (2010) Novel, versatile, and tightly regulated expression system for Escherichia coli strains. Appl Environ Microbiol 76:5058–5066
Christen B, Abeliuk E, Collier JM, Kalogeraki VS, Passarelli B, Coller JA, Fero MJ, McAdams HH, Shapiro L (2011) The essential genome of a bacterium. Mol Syst Biol 7:528
Clancy KW, Melvin JA, McCafferty DG (2010) Sortase transpeptidases: insights into mechanism, substrate specificity, and inhibition. Biopolymers 94(4):385–396
Clarke S, Mandelstam J (1987) Regulation of stage II of sporulation in Bacillus subtilis. J Gen Microbiol 133:2371–2380
Coelho RV, Dall’Alba G, de Avilae SS, Echeverrigaray S, Longaray Delamare AP (2020) Toward algorithms for automation of postgenomic data analyses: Bacillus subtilis promoter prediction with artificial neural network. OMICS 24:300–309
Comfort D, Clubb RT (2004) A comparative genome analysis identifies distinct sorting pathways in gram-positive bacteria. Infect Immun 72(5):2710–2722
Cosby WM, Vollenbroich D, Lee O, Zuber P (1998) Altered srf expression in Bacillus subtilis resulting from changes in culture pH is dependent on the Spo0K oligopeptide permease and the ComQX system of extracellular control. J Bacteriol 180:1438–1445
Costa A, Hood IV, Berger JM (2013) Mechanisms for initiating cellular DNA replication. Annu Rev Biochem 82:25–54
Coutte F, Leclere V, Bechet M, Guez JS, Lecouturier D, Chollet-Imbert M, Dhulster P, Jacques P (2010) Effect of pps disruption and constitutive expression of srfA on surfactin productivity, spreading and antagonistic properties of Bacillus subtilis 168 derivatives. J Appl Microbiol 109:480–491
Craig NL, Craigie R, Gellert M, Lambowitz AM (eds) (2002) Mobile DNA II. ASM Press, Washington, DC
Cui WJ, Han LC, Cheng JY, Liu ZM, Zhou L, Guo JL, Zhou ZM (2016) Engineering an inducible gene expression system for Bacillus subtilis from a strong constitutive promoter and a theophylline-activated synthetic riboswitch. Microb Cell Fact 15:199
Cui WJ, Han LC, Suo FY, Liu ZM, Zhou L, Zhou ZM (2018) Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J Microbiol Biotechnol 34(10):145
de Kok S, Stanton LH, Slaby T, Durot M, Holmes VF, Patel KG, Platt D, Shapland EB, Serber Z, Dean J, Newman JD, Chandran SS (2014) Rapid and reliable DNA assembly via ligase cycling reaction. ACS Synth Biol 3:97–106
Defoor E, Kryger MB, Martinussen J (2007) The orotate transporter encoded by oroP from Lactococcus lactis is required for orotate utilization and has utility as a food-grade selectable marker. Microbiol-Sgm 153:3645–3659
Dougan D, Mogk A, Bukau B (2002) Protein folding and degradation in bacteria: to degrade or not to degrade? That is the question. CMLS 59:1607–1616
Dong H, Zhang D (2014) Current development in genetic engineering strategies of Bacillus species. Microb Cell Fact 13:63
Drake JW, Charlesworth B, Charlesworth D, Crow J (1998) Rates of spontaneous mutation. Genetics 148:1667–1686
Ebner P, Gotz F (2019) Bacterial excretion of cytoplasmic proteins (ECP): occurrence, mechanism, and function. Trends Microbiol 27:176–187
Errington J, van der Aart LT (2020) Microbe profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse. Microb 166(5):425–427
Fabret C, Ehrlich SD, Noirot P (2002) A new mutation delivery system for genome-scale approaches in Bacillus subtilis. Mol Microbiol 46(1):25–36
Fan X, Yang MM, Cao PT, Zhang W, Zhang W, Song XP, Gong YS (2011) Screening of optimal signal peptide for heterologous xylanase secretion by Bacillus subtilis. Wei Sheng Wu Xue Bao 51:979–983
Feng J, Wang Z, Pan K (2017) Application of Bacillus subtilis spore surface display of functional heterologous protein. China JAN 29:893–3898
Feng Y, Liu S, Jiao Y, Wang YH, Wang M, Du GC (2019) Gene cloning and expression of the L-asparaginase from Bacillus cereus BDRD-ST26 in Bacillus subtilis WB600. J Biosci 127:418–424
Frain KM, Robinson C, van Dijl JM (2019) Transport of folded proteins by the Tat system. Protein J 38(4):377–388
Frisby D, Zuber P (1994) Mutations in pts cause catabolite-resistant sporulation and altered regulation of Spo0h in Bacillius subtilis. J Bacteriol 176:2587–2595
Fu G, Liu J, Li J, Zhu B, Zhang D (2018) Systematic screening of optimal signal peptides for secretory production of heterologous proteins in Bacillus subtilis. J Agric Food Chem 66:13141–13151
Gao XL, Liu E, Yin YY, Yang LX, Huang QR, Chen S, Ho CT (2020) Enhancing activities of salt-tolerant proteases secreted by Aspergillus oryzae using atmospheric and room-temperature plasma mutagenesis. J Agric Food Chem 68:2757–2764
Gaudreau L, Keaveney M, Nevado J, Zaman Z, Bryant GO, Struhl K, Ptashne M (1999) Transcriptional activation by artificial recruitment in yeast is influenced by promoter architecture and downstream sequences. Proc Natl Acad Sci USA 96:2668–2673
Ge CL, Liu ZM, Cui WJ, Zhou L, Guo JL, Hu YF, Zhou ZM (2016) Efficient overexpression of recombinant nattokinase in Bacillus subtilis by tandem promoters. Modern Food Sci Technol 11:72–78
Gibson D, Young L, Chuang R, Venter J, Hutchison C, Smith H (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345
Gimenez GG, Costa H, de Lima Neto QA, Fernandez MA, Alicia Ferrarotti S, Matioli G (2019) Sequencing, cloning, and heterologous expression of cyclomaltodextrin glucanotransferase of Bacillus firmus strain 37 in Bacillus subtilis WB800. Bioprocess Biosyst Eng 42:621–629
Gruss A, Ehrlich SD (1989) The family of highly interrelated single-stranded deoxyribonucleic acid plasmids. Microbiol Mol Biol Rev 53:231–241
Gryczan TJ, Dubnau D (1978) Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci USA 75:1428–1432
Gu Y, Xu XH, Wu YK, Niu TF, Liu YF, Li JH, Du GC, Liu L (2018) Advances and prospects of Bacillus subtilis cellular factories: from rational design to industrial applications. Metab Eng 50:109–121
Guan CR, Cui WJ, Cheng JT, Zhou L, Guo JL, Hu X, Xiao GP, Zhou ZM (2015) Construction and development of an auto-regulatory gene expression system in Bacillus subtilis. Microb Cell Fact 14:150
Guérout-Fleury AM, Shazand K, Frandsen N, Stragier P (1995) Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335–336
Guiziou S, Sauveplane V, Chang HJ, Clerté C, Declerck N, Jules M, Bonnet J (2016) A part toolbox to tune genetic expression in Bacillus subtilis. Nucleic Acids Res 44:7495–7508
Gupta M, Rao KK (2014) Phosphorylation of DegU is essential for activation of amyE expression in Bacillus subtilis. J Biosci 39:747–752
Han LC, Cui WJ, Suo FY, Miao SN, Hao WL, Chen QQ, Guo JL, Liu ZM, Zhou L, Zhou ZM (2019) Development of a novel strategy for robust synthetic bacterial promoters based on a stepwise evolution targeting the spacer region of the core promoter in Bacillus subtilis. Microb Cell Fact 18:96
Harwood CR, Cranenburgh R (2008) Bacillus protein secretion: an unfolding story. Trends Microbiol 16:73–79
He W, Jiang B, Mu WM, Zhang T (2016) Production of D-allulose with D-Psicose 3-epimerase expressed and displayed on the surface of Bacillus subtilis spores. J Agric Food Chem 64:7201–7207
Helmann JD (1995) Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res 23(13):2351–2360
Hemmerich J, Rohe P, Kleine B, Jurischka S, Wiechert W, Freudl R, Oldiges M (2016) Use of a Sec signal peptide library from Bacillus subtilis for the optimization of cutinase secretion in Corynebacterium glutamicum. Microb Cell Fact 15:208
Hendrick JP, Hartl FU (1993) Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem 62:349–384
Hinc K, Isticato R, Dembek M, Karczewska J, Iwanicki A, Peszynska-Sularz G, De Felice M, Obuchowski M, Ricca E (2010) Expression and display of UreA of Helicobacter acinonychis on the surface of Bacillus subtilis spores. Microb Cell Fact 9:2
Hinc K, Iwanicki A, Obuchowski M (2013) New stable anchor protein and peptide linker suitable for successful spore surface display in B. subtilis. Microb Cell Fact 12:22
Hoffmann T, Bleisteiner M, Sappa PK, Steil L, Maeder U, Voelker U, Bremer E (2017) Synthesis of the compatible solute proline by Bacillus subtilis: point mutations rendering the osmotically controlled proHJ promoter hyperactive. Environ Microbiol 19:3700–3720
Hong KQ, Liu DY, Chen T, Wang ZW (2018) Recent advances in CRISPR/Cas9 mediated genome editing in Bacillus subtilis. World J Microbiol Biotechnol 34(10):153
Horbal L, Fedorenko V, Luzhetskyy A (2014) Novel and tightly regulated resorcinol and cumate-inducible expression systems for Streptomyces and other actinobacteria. Appl Microbiol Biotechnol 98:8641–8655
Huang K, Zhang T, Jiang B, Yan X, Mu W, Miao M (2017) Overproduction of Rummeliibacillus pycnus arginase with multi-copy insertion of the argR.pyc cassette into the Bacillus subtilis chromosome. Appl Microbiol Biotechnol 101:6039–6048
Isaacs FJ, Dwyer DJ, Collins JJ (2006) RNA synthetic biology. Nat Biotechnol 24:545–554
Isticato R, Ricca E (2014) Spore Surface Display. Microbiol Spectr 2
Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C (2003) Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 100(24):14339–14344
Jannière L, Bruand C, Ehrlich SD (1990) Structurally stable Bacillus subtilis cloning vectors. Gene 87:53–61
Jeong DE, Park SH, Pan JG, Kim EJ, Choi SK (2015) Genome engineering using a synthetic gene circuit in Bacillus subtilis. Nucleic Acids Res 43(6):e42
Jewett MC, Forster AC (2010) Update on designing and building minimal cells. Curr Opin Biotechnol 21:697–703
Ji S, Li W, Rasheed Baloch A, Wang M, Li H, Cao B, Zhang H (2017) Efficient biosynthesis of a cecropin A-melittin mutant in Bacillus subtilis WB700. Sci Rep 7:40587
Juhas M, Eberl L, Church GM (2012) Essential genes as antimicrobial targets and cornerstones of synthetic biology. Trends Biotechnol 30:601–607
Juhas M, Reuß DR, Zhu BY, Commichau FM (2014) Bacillus subtilis and Escherichia coli essential genes and minimal cell factories after one decade of genome engineering. Microbiol-Sgm 160:2341–2351
Kaczmarczyk A, Vorholt JA, Francez-Charlot A (2013) Cumate-inducible gene expression system for Sphingomonads and other Alphaproteobacteria. Appl Environ Microbiol 79:6795–6802
Kang HK, Jang JH, Shim JH, Park JT, Kim YW, Park KH (2010) Efficient constitutive expression of thermostable 4-alpha-glucanotransferase in Bacillus subtilis using dual promoters. World J Microbiol Biotechnol 26:1915–1918
Karata AY, Çetin S, Özcengiz G (2003) The effects of insertional mutations in comQ, comP, srfA, spo0H, spo0A and abrB genes on bacilysin biosynthesis in Bacillus subtilis. Biochim Biophys Acta 1626:51–56
Kawabata Y, Kimura K, Funane K (2012) Extracellular production of cycloisomaltooligosaccharide glucanotransferase and cyclodextran by a protease-deficient Bacillus subtilis host–vector system. Appl Microbiol Biotechnol 93:1877–1884
Kearns DB, Losick R (2005) Cell population heterogeneity during growth of Bacillus subtilis. Genes Dev 19(24):3083–3094
Kleckner N, Bender J, Gottesman S (1991) Uses of transposons with emphasis on Tn10. Methods Enzymol 204:139–180
Kosuri S, Eroshenko N, Leproust EM, Super M, Way J, Li JB, Church GM (2010) Scalable gene synthesis by selective amplification of DNA pools from high-fidelity microchips. Nat Biotechnol 28:1295–1299
Kramer MG, Espinosa M, Misra TK, Khan SA (2010) Characterization of a single-strand origin, ssoU, required for broad host range replication of rolling-circle plasmids. Mol Microbiol 33:466–475
Krishnappa L, Dreisbach A, Otto A, Goosens VJ, Cranenburgh R, Harwood CR, Becher D, van Dijl JM (2013) Extracytoplasmic proteases determining the cleavage and release of secreted proteins, lipoproteins, and membrane proteins in Bacillus subtilis. J Proteome Res 12:4101-4110
Kwon E, S Devkota R, Pathak D, Dahal P, Kim DY (2019) Structural analysis of the recognition of the-35 promoter element by SigW from Bacillus subtilis. PLoS One 14:e0221666
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lam K, Chow K, Wong W (1998) Construction of an efficient Bacillus subtilis system for extracellular production of heterologous proteins. J Biotechnol 63(3):167–177
Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, Haase J, Charles I, Maskell DJ, Peters SE, Dougan G, Wain J, Parkhill J, Turner AK (2009) Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res 19(12):2308–2316
Le ATT, Schumann W (2007) A novel cold-inducible expression system for Bacillus subtilis. Protein Expr Purif 53:264–269
Le Breton Y, Mohapatra NP, Haldenwang WG (2006) In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72:327–333
Le VD, Phan TTP, Nguyen TM, Luc B, Wolfgang S, Nguyen HD (2019) Using the IPTG-inducible Pgrac212 promoter for overexpression of human Rhinovirus 3C protease fusions in the cytoplasm of Bacillus subtilis cells. Curr Microbiol 76(12):1477–1486
Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Desiderio DM, Kim YK, Kim KP, Gho YS (2009) Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9:5425–5436
Lee SJ, Pan JG, Park SH, Choi SK (2010) Development of a stationary phase-specific autoinducible expression system in Bacillus subtilis. J Biotechnol 149:16–20
Lee SC, Olins PO (1992) Effect of overproduction of heat-shock chaperones groesl and dnak on human procollagenase production in Escherichia-coli. J Biol Chem 267:2849–2852
Łęga T, Weiher P, Obuchowski M, Nidzworski D (2016) Presenting influenza A M2e antigen on recombinant spores of Bacillus subtilis. PLoS One 11(11):e0167225.
Liew PX, Wang CLC, Wong SL (2012) Functional characterization and localization of a Bacillus subtilis sortase and its substrate and use of this sortase system to covalently anchor a heterologous protein to the B. subtilis cell wall for surface display. J Bacteriol 194(1):161–175
Lin P, Yuan H, Du J, Liu K, Liu H, Wang T (2020) Progress in research and application development of surface display technology using Bacillus subtilis spores. Appl Microbiol Biotechnol 104:2319–2331
Lindholm A, Ellmen U, Tolonen-Martikainen M, Palva A (2006) Heterologous protein secretion in Lactococcus lactis is enhanced by the Bacillus subtilis chaperone-like protein PrsA. Appl Microbiol Biotechnol 73:904–914
Liu D, Huang C, Guo J, Zhang P, Chen T, Wang Z, Zhao X (2019a) Development and characterization of a CRISPR/Cas9n-based multiplex genome editing system for Bacillus subtilis. Biotechnol Biofuels 12:197
Liu H, Wang X, Yang S, Wang R, Wang T (2019b) Saturation mutagenesis and self-inducible expression of trehalose synthase in Bacillus subtilis. Biotechnol Prog 35:e2826
Liu JH, Chen YT, Li H, Jia YP, Xu RD, Wang J (2015) Optimization of fermentation conditions for biosurfactant production by Bacillus subtilis strains CCTCC M201162 from oilfield wastewater. Environ Progress Sustain Ene 34:548–554
Liu S, Endo K, Ara K, Ozaki K, Ogasawara N (2008) Introduction of marker-free deletions in Bacillus subtilis using the AraR repressor and the ara promoter. Microbiology 154:2562–2570
Liu S, Wang J, Zhu Z, Shi T, Zhang YHPJ (2020) Efficient secretory production of large-size heterologous enzymes in Bacillus subtilis: a secretory partner and directed evolution. Biotechnol Bioeng 117:2957–2968
Liu SL, Du K (2012) Enhanced expression of an endoglucanase in Bacillus subtilis by using the sucrose-inducible sacB promoter and improved properties of the recombinant enzyme. Protein Expr Purif 83:164–168
Liu X, Guo LN, Ma HL, Wang K, Wu P, Hong C, Zhao X, Zhang C (2018a) Optimization of the process conditions of walnut meal peptides by solid state fermentation-Bacillus subtilis and Aspergillus niger. Modern Food Sci Technol 34:130–137
Liu X, Wang H, Wang B, Pan L (2018b) Efficient production of extracellular pullulanase in Bacillus subtilis ATCC6051 using the host strain construction and promoter optimization expression system. Microb Cell Fact 17:163
Liu X, Wang H, Wang B, Pan L (2018c) High-level extracellular protein expression in Bacillus subtilis by optimizing strong promoters based on the transcriptome of Bacillus subtilis and Bacillus megaterium. Protein Expr Purif 151:72–77
Liu YF, Liu L, Li JH, Du GC, Chen J (2019c) Synthetic biology toolbox and chassis development in Bacillus subtilis. Trends Biotechnol 37(5):548–562
Liu Y, Shi C, Li D, Chen X, Li J, Zhang Y, Yuan H, Li Y, Lu FP (2019d) Engineering a highly efficient expression system to produce BcaPRO protease in Bacillus subtilis by an optimized promoter and signal peptide. Int J Biol Macromol 138:903–911
Lu Z, Yang S, Yuan X, Shi Y, Ouyang L, Jiang S, Yi L, Zhang GM (2019). CRISPR-assisted multi-dimensional regulation for fine-tuning gene expression in Bacillus subtilis. Nucleic Acids Res 47:e40
Ma YF, Shen W, Chen XZ, Liu L, Zhou ZM, Xu F, Yang HQ (2016) Significantly enhancing recombinant alkaline amylase production in Bacillus subtilis by integration of a novel mutagenesis-screening strategy with systems-level fermentation optimization. J Biol Eng 10:13
Ma YF, Yang HQ, Chen XZ, Sun B, Du GC, Zhou ZM, Song JN, Fan Y, Shen W (2015) Significantly improving the yield of recombinant proteins in Bacillus subtilis by a novel powerful mutagenesis tool (ARTP): Alkaline alpha-amylase as a case study. Protein Expr Purif 114:82–88
Maeda T, Sanchez-Torres V, Wood TK (2012) Hydrogen production by recombinant Escherichia coli strains. Microb Biotechnol 5:214–225
Mahidsanan T, Gasaluck P (2016) Improvement of poly-gamma-glutamic acid (PGA) producing Bacillus subtilis SB-MYP-1 by N-methyl-N’-nitro-N-nitrosoguanidine (NTG) mutagenesis. Int Food Res J 23:751–755
Maloy SR (2007) Use of antibiotic-resistant transposons for mutagenesis. Methods Enzymol 421:11–17
Man LJ, Xiang DJ (2019) Optimization of fermentation conditions of nattokinase production in Bacillus subtilis MX-6 by response surface methodology. Food Res Develop 40:214–219
Marimuthu M, Sorimuthu A, Muruganantham S (2019) Production and optimization of xylanase enzyme from Bacillus subtilis using agricultural wastes by solid state fermentation. Int J Pharm Investig 9:169–173
Marraffini LA, Dedent AC, Schneewind O (2006) Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol Mol Biol Rev 70(1):192–221
Matzas M, Stähler PF, Kefer N, Siebelt N, Boisguérin V, Leonard JT, Keller A, Stähler CF, Häberle P, Gharizadeh B, Babrzadeh F, Church GM (2010) High-fidelity gene synthesis by retrieval of sequence-verified DNA identified using high-throughput pyrosequencing. Nat Biotechnol 28:1291–1294
Mauriello EM, le Duc H, Isticato R, Cangiano G, Hong HA, De Felice M, Ricca E, Cutting SM (2004) Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22(9–10):1177–1187
Mayer M, Bukau B (2005) Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 62:670
Meijer WJ, Van der Lelie D, Venema G, Bron S (1995) Effects of the generation of single-stranded DNA on the maintenance of plasmid pMV158 and derivatives in Lactococcus lactis. Plasmid 33:91–99
Meng FQ, Zhu XY, Nie T, Lu FX, Bie XM, Lu YJ, Trouth F, Lu ZX (2018) Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination. Front Microbiol 9:2635
Ming YM, Wei ZW, Lin CY, Sheng GY (2010) Development of a Bacillus subtilis expression system using the improved Pglv promoter. Microb Cell Fact 9:55
Moliere N, Turgay K (2009) Chaperone-protease systems in regulation and protein quality control in Bacillus subtilis. Res Microbiol 160:637–644
Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, Manabe K, Kanaya S, Ara K, Ozaki K, Ogasawara N (2008) Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res 15(2):73–81
Mu DD, Lu JJ, Qiao MQ, Kuipers OP, Zhu J, Li XJ, Yang PZ, Zhao YY, Luo SZ, Wu XF, Jiang ST, Zheng Z (2018) Heterologous signal peptides-directing secretion of Streptomyces mobaraensis transglutaminase by Bacillus subtilis. Appl Microbiol Biotechnol 102:5533–5543
Neef J, van Dijl JM, Buist G (2021) Recombinant protein secretion by Bacillus subtilis and Lactococcus lactis: pathways, applications, and innovation potential. Essays Biochem 6:EBC20200171
Negri A, Potocki W, Iwanicki A, Obuchowski M, Hinc K (2013) Expression and display of Clostridium difficile protein FliD on the surface of Bacillus subtilis spores. J Med Microbiol 62:1379–1385
Nguyen ATV, Pham CK, Pham HTT, Pham HL, Nguyen AH, Dang LT, Huynh HA, Cutting SM, Tuan-Nghia P (2014) Bacillus subtilis spores expressing the VP28 antigen: a potential oral treatment to protect Litopenaeus vannamei against white spot syndrome. FEMS Microbiol Lett 358:202–208
Nguyen HD, Nguyen QA, Ferreira RC, Ferreira LC, Tran LT, Schumanna W (2005) Construction of plasmid-based expression vectors for Bacillus subtilis exhibiting full structural stability. Plasmid 54:241–248
Nguyen HD, Phan TTP, Wolfgang S (2011) Analysis and application of Bacillus subtilis sortases to anchor recombinant proteins on the cell wall. AMB Express 1:22
Nguyen HD, Schumann W (2006) Establishment of an experimental system allowing immobilization of proteins on the surface of Bacillus subtilis cells. J Biotechnol 122(4):473–482
Nijland R, Kuipers OP (2008) Optimization of protein secretion by Bacillus subtilis. Recent Pat Biotechnol 2:79–87
Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, Kondo A (2016) Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353(6305):aaf8729
Ohta Y, Maeda M, Kudo T (2001) Pseudomonas putida CE2010 can degrade biphenyl by a mosaic pathway encoded by the tod operon and cmtE, which are identical to those of P. putida F1 except for a single base difference in the operator–promoter region of the cmt operon. Microbiol 147:31–41
Ӧztürk S, Ӧalık P, Ӧzdamar TH (2016) Fed-Batch biomolecule production by Bacillus subtilis: A state of the art review. Trends Biotechnol 34(4):329–345
Pallen MJ, Lam AC, Antonio M, Dunbar K (2001) An embarrassment of sortases-a richness of substrates? Trends Microbiol 9(3):97–102
Panahi R, Vasheghani-Farahani E, Shojaosadati SA, Bambai B (2014) Induction of Bacillus subtilis expression system using environmental stresses and glucose starvation. Ann Microbiol 64:879–882
Park S, Schumann W (2015) Optimization of the secretion pathway for heterologous proteins in Bacillus subtilis. Biotechnol Bioprocess Eng 20:623–633
Park SA, Bhatia SK, Park HA, Kim SY, Sudheer PDVN, Yang YH, Choi KY (2021) Bacillus subtilis as a robust host for biochemical production utilizing biomass. Crit Rev Biotechnol 41(6):827–848
Petit M, Bruand C, Jannière L, Ehrlich D (1990) Tn10 derived transposons active in Bacillus subtilis. J Bacteriol 172:6736–6740
Phan TTP, Nguyen HD, Schumann W (2006) Novel plasmid-based expression vectors for intra- and extracellular production of recombinant proteins in Bacillus subtilis. Protein Expr Purif 46:189–195
Phan TTP, Nguyen HD, Schumann W (2013) Construction of a 5’-controllable stabilizing element (CoSE) for over-production of heterologous proteins at high levels in Bacillus subtilis. J Biotechnol 168(1):32–39
Phan TTP, Nguyen HD, Schumann W (2012) Development of a strong intracellular expression system for Bacillus subtilis by optimizing promoter elements. J Biotechnol 157(1):167–172
Philibert T, Rao Z, Yang T, Zhou J, Huang G, Irene K, Samuel N (2016) Heterologous expression and characterization of a new heme-catalase in Bacillus subtilis 168. J Ind Microbiol Biotechnol 43:729–740
Ploss TN, Reilman E, Monteferrante CG, Denham EL, Piersma S, Lingner A, Vehmaanpera J, Lorenz P, van Dijl JM (2016) Homogeneity and heterogeneity in amylase production by Bacillus subtilis under different growth conditions. Microb Cell Fact 15:57
Pluta R, Espinosa M (2018) Antisense and yet sensitive: copy number control of rolling circle-replicating plasmids by small RNAs. WIREs RNA 9:e1500.
Potot S, Serra CR, Henriques AO, Schyns G (2010) Display of recombinant proteins on Bacillus subtilis spores, using a coat-associated enzyme as the carrier. Appl Environ Microbiol 76:5926–5933
Pozsgai ER, Blair KM, Kearns DB (2012) Modified mariner transposons for random inducible-expression insertions and transcriptional reporter fusion insertions in Bacillus subtilis. Appl Environ Microbiol 78(3):778–785
Promchai R, Promdonkoy B, Tanapongpipat S, Visessanguan W, Eurwilaichitr L, Luxananil P (2016) A novel salt-inducible vector for efficient expression and secretion of heterologous proteins in Bacillus subtilis. J Biotechnol 222:86–93
Ptashne M, Gann A (1997) Transcriptional activation by recruitment. Nature 386:569–577
Quan J, Tian J (2011) Circular polymerase extension cloning for high-throughput cloning of complex and combinatorial DNA libraries. Nat Protoc 6:242–251
Reuß DR, Altenbuchner J, Mäder U, Rath H, Ischebeck T, Sappa PK, Thürmer A, Guérin C, Nicolas P, Steil L, Zhu B, Feussner I, Klumpp S, Daniel R, Commichau FM, Völker U, Stülke J (2017) Large-scale reduction of the Bacillus subtilis genome: consequences for the transcriptional network, resource allocation, and metabolism. Genome Res 27(2):289–299
Ricca E, Baccigalupi L, Isticato R (2021) Spore-adsorption: mechanism and applications of a non-recombinant display system. Biotechnol Adv 47:107693
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950
Samuelson P, Gunneriusson E, Nygren PÅ, Stahl S (2002) Display of proteins on bacteria. J Biotechnol 96(2):129–154
Sargent F, Bogsch EG, Stanley NR, Wexler M, Robinson C, Berks BC, Palmer T (1998) Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J 17:3640–3650
Schumann W (2007) Production of recombinant proteins in Bacillus subtilis. Adv Appl Microbiol 62:137–189
Selle K, Barrangou R (2015) Harnessing CRISPR-Cas systems for bacterial genome editing. Trends Microbiol 23:225–232
Seo SO, Schmidt-Dannert C (2019) Development of a synthetic cumate-inducible gene expression system for Bacillus. Appl Microbiol Biotechnol 103:303–313
Shao HH, Cao QH, Zhao HY, Tan XM, Feng H (2015) Construction of novel shuttle expression vectors for gene expression in Bacillus subtilis and Bacillus pumilus. J Gen Appl Microbiol 61(4):124–131
Shaw GC, Wu MY, Lee TR, Hsu CW (2005) The influence of nucleotide sequences at and near ribosome-binding site on translational efficiency of the Bacillus subtilis rho gene. Biochim Biophys Acta 1729:10–13
Shu L, Si X, Yang X, Ma W, Sun J, Zhang J, Xue X, Wang D, Gao Q (2020) Enhancement of acid protease activity of Aspergillus oryzae using atmospheric and room temperature plasma. Front Microbiol 11:1418
Simonen M, Palva I (1993) Protein secretion in Bacillus species. Microbiol Rev 57:109–137
Sone Y, Nikoloff JM, Zhang D (2015) Improving protein production on the level of regulation of both expression and secretion pathways in Bacillus subtilis. J Microbiol Biotechnol 25:963–977
Song W, Nie Y, Mu XQ, Xu Y (2016) Enhancement of extracellular expression of Bacillus naganoensis pullulanase from recombinant Bacillus subtilis: effects of promoter and host. Protein Expr Purif 124:23–31
Stefanic P, Decorosi F, Viti C, Petito J, Cohan FM, Mandic-Mulec I (2012) The quorum sensing diversity within and between ecotypes of Bacillus subtilis. Environ Microbiol 14:1378–1389
Steinmetz M, Richter R (1994) Easy cloning of Mini-Tn10 insertions from the Bacillus subtilis chromosome. J Bacteriol 176:1761–1763
Su Y, Liu C, Fang H, Zhang DW (2020) Bacillus subtilis: a universal cell factory for industry, agriculture, biomaterials and medicine. Microb Cell Fact 19(1):173
Sun T, Altenbuchner J (2010) Characterization of a mannose utilization system in Bacillus subtilis. J Bacteriol 192:2128–2139
Sun T, Tian R, Zhang T, Chen H (2014) Surface display of heterologous proteins on Bacillus subtilis spores. J Microbiol 34:82–87
Sun WF, Wu YM, Ding WW, Wang L, Wu LJ, Lin L, Che ZM, Zhu LB, Liu Y, Chen XH (2020) An auto-inducible expression and high cell density fermentation of beefy meaty peptide with Bacillus subtilis. Bioprocess Biosyst Eng 43:701–710
Tang X, Zhang C, Zhou W, Sui M, Shu X (2019) Optimization of the fermentation process for protease production in Bacillus subtilis by response surface methodology. Cereal Food Ind 4:29–35
Tian J, Long XF, Tian YQ, Shi B (2019) Enhanced extracellular recombinant keratinase activity in Bacillus subtilis SCK6 through signal peptide optimization and site-directed mutagenesis. Rsc Adv 9(57):33337–33344
Tjalsma H, Antelmann H, Jongbloed JDH, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM (2004) Proteomics of protein secretion by Bacillus subtilis: separating the secrets of the secretome. Microbiol Mol Biol Rev 68:207–233
Tjalsma H, Bolhuis A, Jongbloed JDH, Bron S, van Dijl JM (2000) Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev 64:515–547
Tomich PK, An FY, Clewell DB (1980) Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol 141:1366–1374
Toymentseva AA, Schrecke K, Sharipova MR, Mascher T (2012) The LIKE system, a novel protein expression toolbox for Bacillus subtilis based on the liaI promoter. Microb Cell Fact 11:143
Tran DTM, Phan TTP, Doan TTN, Tran TL, Schumann W, Nguyen HD (2020) Integrative expression vectors with Pgrac promoters for inducer-free overproduction of recombinant proteins in Bacillus subtilis. Biotechnol Rep (Amst) 28:e00540–e00540
Tran DTM, Phan TTP, Huynh TK, Dang NTK, Huynh PTK, Nguyen TM, Truong TTT, Tran TL, Schumann W, Nguyen HD (2017) Development of inducer-free expression plasmids based on IPTG-inducible promoters for Bacillus subtilis. Microb Cell Fact 16:130
Valvano MA, Eberl L (2012) High confidence prediction of essential genes in Burkholderia cenocepacia. PLoS ONE 7:e40064
Venkateswarulu TC, Prabhakar KV, Kumar RB, Krupanidhi S (2017) Modeling and optimization of fermentation variables for enhanced production of lactase by isolated Bacillus subtilis strain VUVD001 using artificial neural networking and response surface methodology. Biotech 7:186
Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS (2002) Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res 30:3141–3151
Wang H, Wang Y, Yang R (2017) Recent progress in Bacillus subtilis spore-surface display: concept, progress, and future. Appl Microbiol Biotechnol 101:933–949
Wang H, Yang R, Hua X, Zhao W, Zhang W (2015) Functional display of active beta-galactosidase on Bacillus subtilis spores using crust proteins as carriers. Food Sci Biotechnol 24:1755–1759
Wang N, Guan F, Lv X, Han D, Zhang Y, Wu N, Xia X, Tian J (2020a) Enhancing secretion of polyethylene terephthalate hydrolase PETase in Bacillus subtilis WB600 mediated by the SP(amy) signal peptide. Lett Appl Microbiol 71:235–241
Wang PZ, Doi RH (1984) Overlapping promoters transcribed by Bacillus subtilis sigma 55 and sigma 37 RNA polymerase holoenzymes during growth and stationary phases. J Biol Chem 259(13):8619–8625
Wang ST, Yang ZG, Li ZJ, Tian YQ (2020b) Heterologous expression of recombinant transglutaminase in Bacillus subtilis sck6 with optimized signal peptide and codon, and its impact on gelatin properties. J Microbiol Biotechnol 30:1082–1091
Wang XC, Zhao HY, Liu G, Cheng XJ, Feng H (2016) Improving production of extracellular proteases by random mutagenesis and biochemical characterization of a serine protease in Bacillus subtilis S1–4. Genet Mol Res 15
Wang Y, Shi Y, Hu L, Du G, Chen J, Kang Z (2019) Engineering strong and stress-responsive promoters in Bacillus subtilis by interlocking sigma factor binding motifs. Synth Syst Biotechnol 4:197–203
Wang Y, Weng J, Waseem R, Yin XH, Zhang RF, Shen QR (2012) Bacillus subtilis genome editing using ssDNA with short homology regions. Nucleic Acids Res 40(12):e91
Wenzel M, Müller A, Siemann-Herzberg M, Altenbuchner J (2011) Self-inducible Bacillus subtilis expression system for reliable and inexpensive protein production by high-cell-density fermentation. Appl Environ Microbiol 77:6419–6425
Westers H (2004) Genome engineering and protein secretion stress in the BACELL factory. Dissertation. University of Groningen, The Netherlands
Westers H, Dorenbos R, van Dijl JM, Kabel J, Flanagan T, Devine KM, Jude F, Seror SJ, Beekman AC, Darmon E, Eschevins C, de Jong A, Bron S, Kuipers OP, Albertini AM, Antelmann H, Hecker M, Zamboni N, Sauer U, Bruand C, Ehrlich DS, Alonso JC, Salas M, Quax WJ (2003) Genome engineering reveals large dispensable regions in Bacillus subtilis. Mol Biol Evol 20:2076–2090
Westers L, Westers H, Quax WJ (2004) Bacillus subtilis as cell factory for pharmaceutical proteins: a biotechnological approach to optimize the host organism. Biochim Biophys Acta Mol Cell Res 1694:299–310
Winkler WC, Breaker RR (2005) Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59:487–517
Wu SC, Wong SL (1999) Development of improved pUB110-based vectors for expression and secretion studies in Bacillus subtilis. J Biotechnol 72:185–195
Wu SC, Ye RQ, Wu XC, Ng SC, Wong SL (1998) Enhanced secretory production of a single-chain antibody fragment from Bacillus subtilis by coproduction of molecular chaperones. J Bacteriol 180:2830–2835
Wu XC, Lee W, Tran L, Wong S (1991) Engineering a Bacillus subtilis expression-secretion system with a strain deficient in six extracellular proteases. J Bacteriol 173:4952–4958
Xiang MJ, Kang Q, Zhang DW (2020) Advances on systems metabolic engineering of Bacillus subtilis as a chassis cell. Synth Syst Biotechnol 5:245–251
Xu H, Xiao T, Chen CH, Li W, Liu SX (2015) Sequence determinants of improved CRISPR sgRNA design. Genome Res 25:1147–1157
Xu J, Liu X, Yu X, Chu X, Tian J, Wu N (2020) Identification and characterization of sequence signatures in the Bacillus subtilis promoter P-ylb for tuning promoter strength. Biotechnol Lett 42:115–124
Yadav J, Balabantaray S, Patra N (2017) Statistical optimization of fermentation conditions for the improved production of poly-hydroxybutyrate from Bacillus subtilis. Chem Eng Commun 204:1122–1128
Yamane T, Shimizu S (1984) Fed-batch techniques in microbial processes. Adv Biochem Eng Biotechnol 30:147–194
Yan X, Yu HJ, Hong Q, Li SP (2008) Cre/lox system and PCR-based genome engineering in Bacillus subtilis. Appl Environ Microbiol 74:5556–5562
Yang CK, Ewis HE, Zhang X, Lu CD, Hu HJ, Pan Y, Abdelal AT, Tai PC (2011) Nonclassical protein secretion by Bacillus subtilis in the stationary phase is not due to cell lysis. J Bacteriol 193:5607–5615
Yang HQ, Ma YF, Zhao Y, Shen W, Chen XZ (2020a) Systematic engineering of transport and transcription to boost alkaline α-amylase production in Bacillus subtilis. Appl Microbiol Biotechnol 104:2973–2985
Yang L, Zhou N, Tian Y (2019) Characterization and application of dextranase produced by Chaetomium globosum mutant through combined application of atmospheric and room temperature plasma and ethyl methyl sulfone. Process Biochem 85:116–124
Yang S, Du GC, Chen J, Kang Z (2017) Characterization and application of endogenous phase-dependent promoters in Bacillus subtilis. Appl Microbiol Biotechnol 101:4151–4161
Yang XP, Ci S, Zhang WH, Liu Y, Zhou W, Xue ZL (2020b) Combined mutagenesis of ARTP and 5-BU for improving production of adenosine in Bacillus subtilis. Food Fermentation Ind 9:73–77
Yao DB, Su LQ, Li N, Wu J (2019) Enhanced extracellular expression of Bacillus stearothermophilus alpha-amylase in Bacillus subtilis through signal peptide optimization, chaperone overexpression and alpha-amylase mutant selection. Microb Cell Fact 18:69
Ying L, Jin X, Sha HT, Fan ZY, Sun HG (2016) Optimization of fermentation conditions of Bacillus subtilis SH-1 and stability of antimicrobial substances. Food Sci Technol 41:25–29
Yomantas YA, Abalakina EG, Golubeva LI, Gorbacheva LY, Mashko SV (2011) Overproduction of Bacillus amyloliquefaciens extracellular glutamyl-endopeptidase as a result of ectopic multi-copy insertion of an efficiently-expressed mpr gene into the Bacillus subtilis chromosome. Microb Cell Fact 10:64
Young JC, Agashe VR, Siegers K, Hartl FU (2004) Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 5:781–791
Youngman PJ, Perkins JB, Losick R (1983) Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci USA 80:2305–2309
Yu BJ, Kang KH, Lee JH, Sung BH, Kim MS, Kim SC (2008) Rapid and efficient construction of markerless deletions in the Escherichia coli genome. Nucleic Acids Res 36(14):e84.
Zanen G, Houben ENG, Meima R, Tjalsma H, Jongbloed JDH, Westers H, Oudega B, Luirink J, van Dijl JM, Quax WJ (2005) Signal peptide hydrophobicity is critical for early stages in protein export by Bacillus subtilis. FEBS J 272:4617–4630
Zhang AL, Liu H, Yang MM, Gong YS, Chen H (2007) Assay and characterization of a strong promoter element from B. subtilis. Biochem Biophys Res Commun 354:90–95
Zhang G, An Y, Zabed H, Guo Q, Yang MM, Yuan J, Wen L, Sun WJ, Qi XH (2019) Bacillus subtilis spore surface display technology: a review of its development and applications. J Microbiol Biotechnol 29:179–190
Zhang K, Duan XG, Wu J (2016a) Multigene disruption in undomesticated Bacillus subtilis ATCC 6051a using the CRISPR/Cas9 system. Sci Rep 6:27943
Zhang WW, Gao QR, Yang MM, Liu H, Wang D (2012a) Assay and characterization of an osmolarity inducible promoter newly isolated from Bacillus subtilis. Mol Biol Rep 39:7347–7353
Zhang W, Yang M, Yang Y, Zhan J, Zhou Y, Zhao X (2016b) Optimal secretion of alkali-tolerant xylanase in Bacillus subtilis by signal peptide screening. Appl Microbiol Biotechnol 100:8745–8756
Zhang XZ, Cui ZL, Hong Q, Li SP (2005) High-level expression and secretion of methyl parathion hydrolase in Bacillus subtilis WB800. Appl Environ Microbiol 71:4101–4103
Zhang XZ, Yan X, Cui ZL, Hong Q, Li SP (2006) MazF, a novel counter-selectable marker for unmarked chromosomal manipulation in Bacillus subtilis. Nucleic Acids Res 34:e71
Zhang Y, Nie Y, Zhou X, Bi J, Xu Y (2020) Enhancement of pullulanase production from recombinant Bacillus subtilis by optimization of feeding strategy and fermentation conditions. AMB Express 10:11
Zhang Y, Werling U, Edelmann W (2012) SLiCE: a novel bacterial cell extract-based DNA cloning method. Nucleic Acids Res 40:e55
Zhao X, Xu JY, Tan M, Zhen J, Shu WJ, Yang SB, Ma YH, Zheng HC, Song H (2020) High copy number and highly stable Escherichia coli-Bacillus subtilis shuttle plasmids based on pWB980. Microb Cell Fact 19:25
Zhou C, Ye B, Cheng S, Zhao L, Liu Y, Jiang J, Yan X (2019) Promoter engineering enables overproduction of foreign proteins from a single copy expression cassette in Bacillus subtilis. Microb Cell Fact 18:111
Zhu H, Liang C (2019) CRISPR-DT: designing gRNAs for the CRISPR-Cpf1 system with improved target efficiency and specificity. Bioinformatics 35:2783–2789
Zhao G, Miao Y, Guo Y, Qiu H, Sun S, Kou Z, Yu H, Li J, Chen Y, Jiang S, Du L, Zhou Y (2014) Development of a heat-stable and orally delivered recombinant M2e-expressing B. subtilis spore-based influenza vaccine. Hum Vaccin Immunother 1(12):3649–3658
Funding
This work was funded by the Key Research and Development Program of China (2021YFC2100200), the Open Project Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (KLIB-KF201907), Natural Science Foundation of Jiangsu Province (BK20191185), and 111 Project (111–2-06).
Author information
Authors and Affiliations
Contributions
H.Y. and X.C. designed the manuscript. H.Y., J.Q., W.Z., and W.S. wrote the manuscript. H.Y. drew figures. W.S. and X.C. revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All studies without human participants were performed in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, H., Qu, J., Zou, W. et al. An overview and future prospects of recombinant protein production in Bacillus subtilis. Appl Microbiol Biotechnol 105, 6607–6626 (2021). https://doi.org/10.1007/s00253-021-11533-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11533-2