Abstract
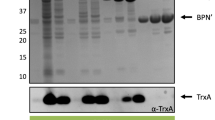
The Bacillus subtilis lipoprotein PrsA enhances the yield of several homologous and heterologous exported proteins in B. subtilis by being involved in the posttranslocational stage of the secretion process. In this work, we have studied the effect of B. subtilis PrsA on the secretion of Bacillus amyloliquefaciens α-amylase (AmyQ), a target protein for PrsA, and Bacillus licheniformis penicillinase (PenP) a nontarget protein for PrsA, in Lactococcus lactis. Two compatible plasmids were constructed and introduced into L. lactis strain NZ9000: one high copy plasmid, expressing the AmyQ gene (amyQ) or the PenP gene (penP), and one low copy plasmid, expressing the PrsA encoding gene (prsA). When amyQ and prsA were simultaneously expressed under the nisin-inducible promoter PnisA, Western blotting experiments revealed a 15- to 20-fold increase in the total yield of AmyQ and a sixfold increase in secreted AmyQ activity, compared to a control strain lacking prsA. When expressed under the same induction conditions, PrsA had no effect on the secretion or total yield of PenP. These results show that the secretion yield of some heterologous proteins can be significantly increased in L. lactis when coproduced with the B. subtilis PrsA protein.





Similar content being viewed by others
References
Bermudez-Humaran LG, Langella P, Commissaire J, Gilbert S, Le Loir Y, L’Haridon R, Corthier G (2003) Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis. FEMS Microbiol Lett 224:307–313
Bolhuis A, Tjalsma H, Smith HE, de Jong A, Meima R, Venema G, Bron S, van Dijl JM (1999) Evaluation of bottlenecks in the late stages of protein secretion in Bacillus subtilis. Appl Environ Microbiol 65:2934–2941
Bonifacino JS, Dell’Angelica EC, Springer TA (eds) (1999) Analysis of proteins. Immunoprecipitation. Green Publishing Associates, New York, pp 10.16.1–10.16.29
Colomer-Pallas A, Petit-Glatron MF, Chambert R (2004) Bacillus subtilis alpha-amylase: interactions of a partially folded conformer with small unilamellar vesicles. Biochim Biophys Acta 1660:16–23
de Ruyter PG, Kuipers OP, Beerthuyzen MM, van Alen-Boerrigter I, de Vos WM (1996a) Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol 178:3434–3439
de Ruyter PG, Kuipers OP, de Vos WM (1996b) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62:3662–3667
Drouault S, Anba J, Bonneau S, Bolotin A, Ehrlich SD, Renault P (2002) The peptidyl-prolyl isomerase motif is lacking in PmpA, the PrsA-like protein involved in the secretion machinery of Lactococcus lactis. Appl Environ Microbiol 68:3932–3942
Holo H, Nes IF (1995) Transformation of Lactococcus by electroporation. Methods Mol Biol 47:195–199
Kontinen VP, Sarvas M (1988) Mutants of Bacillus subtilis defective in protein export. J Gen Microbiol 134:2333–2344
Kontinen VP, Sarvas M (1993) The PrsA lipoprotein is essential for protein secretion in Bacillus subtilis and sets a limit for high-level secretion. Mol Microbiol 8:727–737
Kontinen VP, Saris P, Sarvas M (1991) A gene (prsA) of Bacillus subtilis involved in a novel, late stage of protein export. Mol Microbiol 5:1273–1283
Le Loir Y, Azevedo V, Oliveira SC, Freitas DA, Miyoshi A, Bermudez-Humaran LG, Nouaille S, Ribeiro LA, Leclercq S, Gabriel JE, Guimaraes VD, Oliveira MN, Charlier C, Gautier M, Langella P (2005) Protein secretion in Lactococcus lactis: an efficient way to increase the overall heterologous protein production. Microb Cell Fact 4:2
Leskela S, Wahlstrom E, Kontinen VP, Sarvas M (1999) Lipid modification of prelipoproteins is dispensable for growth but essential for efficient protein secretion in Bacillus subtilis: characterization of the lgt gene. Mol Microbiol 31:1075–1085
Lindholm A, Smeds A, Palva A (2004) Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl Environ Microbiol 70:2061–2071
Neugebauer K, Sprengel R, Schaller H (1981) Penicillinase from Bacillus licheniformis: nucleotide sequence of the gene and implications for the biosynthesis of a secretory protein in a gram-positive bacterium. Nucleic Acids Res 9:2577–2588
Novotny R, Scheberl A, Giry-Laterriere M, Messner P, Schaffer C (2005) Gene cloning, functional expression and secretion of the S-layer protein SgsE from Geobacillus stearothermophilus NRS 2004/3a in Lactococcus lactis. FEMS Microbiol Lett 242:27–35
Otto R, Brink Bt, Veldkamp H, Konings WN (1983) The relation between growth rate and electrochemical proton gradient of Streptococcus cremoris. FEMS Microbiol Lett 16:69–74
Palva I (1982) Molecular cloning of alpha-amylase gene from Bacillus amyloliquefaciens and its expression in B. subtilis. Gene 19:81–87
Poolman B, Konings WN (1988) Relation of growth of Streptococcus lactis and Streptococcus cremoris to amino acid transport. J Bacteriol 170:700–707
Sarvas M, Harwood CR, Bron S, van Dijl JM (2004) Post-translocational folding of secretory proteins in gram-positive bacteria. Biochim Biophys Acta 1694:311–327
Savijoki K, Kahala M, Palva A (1997) High level heterologous protein production in Lactococcus and Lactobacillus using a new secretion system based on the Lactobacillus brevis S-layer signals. Gene 186:255–262
Simon D, Chopin A (1988) Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559–566
Simons K, Sarvas M, Garoff H, Helenius A (1978) Membrane-bound and secreted forms of penicillinase from Bacillus licheniformis. J Mol Biol 126:673–690
Takkinen K, Pettersson RF, Kalkkinen N, Palva I, Soderlund H, Kaariainen L (1983) Amino acid sequence of alpha-amylase from Bacillus amyloliquefaciens deduced from the nucleotide sequence of the cloned gene. J Biol Chem 258:1007–1013
Vitikainen M, Pummi T, Airaksinen U, Wahlstrom E, Wu H, Sarvas M, Kontinen VP (2001) Quantitation of the capacity of the secretion apparatus and requirement for PrsA in growth and secretion of alpha-amylase in Bacillus subtilis. J Bacteriol 183:1881–1890
Vitikainen M, Hyyrylainen HL, Kivimaki A, Kontinen VP, Sarvas M (2005) Secretion of heterologous proteins in Bacillus subtilis can be improved by engineering cell components affecting post-translocational protein folding and degradation. J Appl Microbiol 99:363-375
Wahlstrom E, Vitikainen M, Kontinen VP, Sarvas M (2003) The extracytoplasmic folding factor PrsA is required for protein secretion only in the presence of the cell wall in Bacillus subtilis. Microbiology 149:569–577
Acknowledgements
This work was supported by the Academy of Finland (57909). We thank Dr. Ilkka Palva for valuable discussions and critical reading of the manuscript and for providing the specific antibody against AmyQ. We also thank Dr. Matti Sarvas for valuable discussions and Dr. Vesa Kontinen for providing the purified PrsA protein and the anti-PrsA and anti-PenP serums. Finally, we thank Anja Osola for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindholm, A., Ellmén, U., Tolonen-Martikainen, M. et al. Heterologous protein secretion in Lactococcus lactis is enhanced by the Bacillus subtilis chaperone-like protein PrsA. Appl Microbiol Biotechnol 73, 904–914 (2006). https://doi.org/10.1007/s00253-006-0551-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0551-y