Abstract
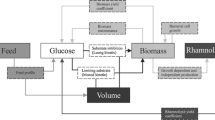
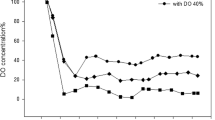
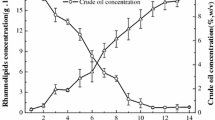
A lack of understanding of the quantitative rhamnolipid production regulation in bioreactor cultivations of Pseudomonas aeruginosa and the absence of respective comparative studies are important reasons for achieving insufficient productivities for an economic production of these biosurfactants. The Pseudomonas strains DSM 7108 and DSM 2874 are described to be good rhamnolipid over-producers. The strain PAO1 on the other hand is the best analyzed type strain for genetic regulation mechanisms in the species P. aeruginosa. These three strains were cultivated in a 30-L bioreactor with a medium containing nitrate and sunflower oil as sole C-source at 30 and 37 °C. The achieved maximum rhamnolipid concentrations varied from 7 to 38 g/L, the volumetric productivities from 0.16 to 0.43 g/(L·h), and the cellular yield from 0.67 to 3.15 g/g, with PAO1 showing the highest results for all of these variables. The molar di- to mono-rhamnolipid ratio changed during the cultivations; it was strain dependent but not significantly influenced by the temperature. This study explicitly shows that the specific rhamnolipid synthesis rate per cell follows secondary metabolite-like courses coinciding with the transition to the stationary phase of typical logistic growth behavior. However, the rhamnolipid synthesis was already induced before N-limitation occurred.



Similar content being viewed by others
References
Abdel-Mawgoud AM, Lepine F, Deziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Arino S, Marchal R, Vandecasteele JP (1996) Identification and production of a rhamnolipidic biosurfactant by a Pseudomonas species. Appl Microbiol Biotechnol 45:162–168
Babu PS, Vaidya AN, Bal AS, Kapur R, Juwarkar A, Khanna P (1996) Kinetics of biosurfactant production by Pseudomonas aeruginosa strain BS2 from industrial wastes. Biotechnol Lett 18:263–268
Bertani G (1951) Studies on lysogenisis: the mode of phage II liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Burger M, Glaser L, Burton RM (1963) The enzymatic synthesis of a rhamnose-containing glycolipid by extracts of Pseudomonas aeruginosa. Fed Proc 238:2595–2602
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Déziel E, Lepine F, Milot S, Villemur R (2000) Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa strain 57RP. Biochim Biophys Acta Mol Cell Biol Lipids 1485:145–152
Dubeau D, Deziel E, Woods DE, Lepine F (2009) Burkholderia thailandensis harbors two identical rhl gene clusters responsible for the biosynthesis of rhamnolipids. BMC Microbiol 9:263
Erkmen O, Alben E (2002) Mathematical modeling of citric acid production and biomass formation by Aspergillus niger in undersized semolina. J Food Eng 52:161–166
Giani C, Wullbrandt D, Rothert R, Meiwes J (1997) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of L-rhamnose. German Patent US005658793A
Guerra-Santos L, Kappeli O, Fiechter A (1984) Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol 48:301–305
Hancock RE, Carey AM (1979) Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol 140:902–910
Hauser G, Karnovsky ML (1957) Rhamnose and rhamnolipide biosynthesis by Pseudomonas aeruginosa. J Biol Chem 224:91–105
Hauser G, Karnovsky ML (1958) Studies on the biosynthesis of l-rhamnose. J Biol Chem 233:287–291
Häußler S, Nimtz M, Domke T, Wray V, Steinmetz I (1998) Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect Immun 66:1588–1593
Hembach T (1994) Untersuchungen zur mikrobiellen Umsetzung von Maiskeimöl zu Rhamnolipid. PhD Thesis, University of Hohenheim
Hörmann B, Müller MM, Syldatk C, Hausmann R (2010) Rhamnolipid production by Burkholderia plantarii DSM 9509 T. Eur J Lipid Sci Technol 112:674–680
Jarvis FG, Johnson MJ (1949) A glycolipid produced by Pseudomonas aeruginosa. J Am Chem Soc 71:4124–4126
Leitermann F (2008) Biotechnologische Herstellung mikrobieller Rhamnolipide. PhD Thesis, Karlsruhe Institute of Technology (KIT)
Linhardt RJ, Bakhit R, Daniels L, Mayerl F, Pickenhagen W (1989) Microbially produced rhamnolipid as a source of rhamnose. Biotechnol Bioeng 33:365–368
Manresa M, Bastida J, Mercade M, Robert M, Deandres C, Espuny M, Guinea J (1991) Kinetic studies on surfactant production by Pseudomonas aeruginosa 44T1. J Ind Microbiol 8:133–136
Marsudi S, Unno H, Hori K (2008) Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78:955–961
Medina G, Juarez K, Diaz R, Soberon-Chavez G (2003) Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiol-SGM 149:3073–3081
Müller MM, Hörmann B, Syldatk C, Hausmann R (2010) Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor cultivations. Appl Microbiol Biotechnol 87:167–174
Mulligan CN, Mahmourides G, Gibbs BF (1989) The influence of phosphate metabolism on biosurfactant production by Pseudomonas aeruginosa. J Biotechnol 12:37–43
Nitschke M, Costa SGVAO, Haddad R, Goncalves LAG, Eberlin MN, Contiero J (2005) Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnol Prog 21:1562–1566
Ochsner UA, Reiser J (1995) Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92:6424–6428
Pajarron AM, Dekoster CG, Heerma W, Schmidt M, Haverkamp J (1993) Structure identification of natural rhamnolipid mixtures by fast-atom-bombardment tandem mass-spectrometry. Glycoconj J 10:219–226
Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, Lam JS, Soberon-Chavez G (2001) Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyltransferase 2, an enzyme responsible for di-rhamnolipid biosynthesis. Mol Microbiol 40:708–718
Rahman KSM, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Ramana KV, Karanth NG (1989) Factors affecting biosurfactant production using Pseudomonas aeruginosa CFTR-6 under submerged conditions. J Chem Technol Biotechnol 45:249–257
Ramana KV, Charyulu N, Karanth NG (1991) A mathematical model for the production of biosurfactants of Pseudomonas aeruginosa CFTR-6: production of biomass. J Chem Technol Biotechnol 51:525–538
Robert M, Mercadé ME, Bosch MP, Parra JL, Espuny MJ, Manresa A, Guinea J (1989) Effect of the carbon source on biosurfactant production by Pseudomonas aeruginosa 44T1. Biotechnol Lett 11:871–874
Schenk T, Schuphan I, Schmidt B (1995) High-performance liquid-chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J Chromatogr A 693:7–13
Soberón-Chávez G, Lépine F, Déziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68:718–725
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Syldatk C, Lang S, Matulovic U, Wagner F (1985a) Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Z Naturforsch C 40:61–67
Syldatk C, Lang S, Wagner F, Wray V, Witte L (1985b) Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas sp. DSM 2874 grown on n-alkanes. Z Naturforsch [C] 40:51–60
Trummler K, Effenberger F, Syldatk C (2003) An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur J Lipid Sci Technol 105:563–571
Van Delden C, Pesci EC, Pearson JP, Iglewski BH (1998) Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun 66:4499–4502
Wei Y-H, Chou C-L, Chang J-S (2005) Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem Eng J 27:146
Williams P, Camara M (2009) Quorum-sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191
Zwietering MH, Jongenburger I, Rombouts FM, Vantriet K (1990) Modeling of bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Acknowledgement
We want to thank the Fachagentur Nachwachsende Rohstoffe e.V. (FNR) for funding the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, M.M., Hörmann, B., Kugel, M. et al. Evaluation of rhamnolipid production capacity of Pseudomonas aeruginosa PAO1 in comparison to the rhamnolipid over-producer strains DSM 7108 and DSM 2874. Appl Microbiol Biotechnol 89, 585–592 (2011). https://doi.org/10.1007/s00253-010-2901-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2901-z