Abstract
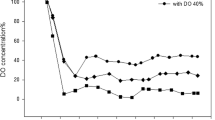
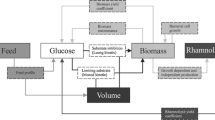
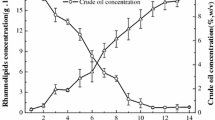
Rhamnolipids are biosurfactants with interesting physico-chemical properties. However, the main obstacles towards an economic production are low productivity, high raw-material costs, relatively expensive downstream processing, and a lack of understanding the rhamnolipid production regulation in bioreactor systems. This study shows that the sequenced Pseudomonas aeruginosa strain PAO1 is able to produce high quantities of rhamnolipid during 30 L batch bioreactor cultivations with sunflower oil as sole carbon source and nitrogen limiting conditions. Thus PAO1 could be an appropriate model for rhamnolipid production in pilot plant bioreactor systems. In contrast to well-established production strains, PAO1 allows knowledge-based systems biotechnological process development combined with the frequently used heuristic bioengineering approach. The maximum rhamnolipid concentration obtained was 39 g/L after 90 h of cultivation. The volumetric productivity of 0.43 g/Lh was comparable with previous described production strains. The specific rhamnolipid productivity showed a maximum between 40 and 70 h of process time of 0.088 gRL/gBDMh. At the same time interval, a shift of the molar di- to mono-rhamnolipid ratio from 1:1 to about 2:1 was observed. PAO1 not only seems to be an appropriate model, but surprisingly has the potential as a strain of choice for actual biotechnological rhamnolipid production.





Similar content being viewed by others
References
Andrä J, Rademann J, Howe J, Koch M, Heine H, Zähringer U, Brandenburg K (2006) Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: immune cell stimulation and biophysical characterization. Biol Chem 387:301–310
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Bertani G (1951) Studies on lysogenisis. 1. The mode of phage II liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Chandrasekaran EV, Bemiller JN (1980) Constituent analysis of glycosaminoglycans. In: Whistler RL, Bemiller JN (eds) Methods in carbohydrate chemistry. Academic, New York
Chen F, Chen C, Riadi L, Ju L (2004) Modeling rhl quorum sensing regulation on rhamnolipid production by Pseudomonas aeruginosa. Biotechnol Prog 20:1325–1331
Dockery JD, Keener JP (2001) A mathematical model for quorum sensing in Pseudomonas aeruginosa. Bull Math Biol 63:95–116
Erkmen O, Alben E (2002) Mathematical modelling of citric acid production and biomass formation by Aspergillus niger in undersized semolina. J Food Eng 52:161–166
Giani C, Wullbrandt D, Rothert R, Meiwes J (1997) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of l-rhamnose. German Patent, US005658793A
Gunther NW (2007) Processes for the production of rhamnolipids. US Patent, 7,202,063 B1
Häußler S, Nimtz M, Domke T, Wray V, Steinmetz I (1998) Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect Immun 66:1588–1593
Hembach T (1994) Untersuchungen zur mikrobiellen Umsetzung von Maiskeimöl zu Rhamnolipid. University Press Hohenheim, Stuttgart, Germany, PhD Thesis
Holloway BW, Krishnapillai V, Morgan AF (1979) Chromosomal genetics of Pseudomonas. Microbiol Rev 43:73–102
Jarvis FG, Johnson MJ (1949) A glyco-lipide produced by Pseudomonas aeruginosa. J Am Chem Soc 71:4124–4126
Lang S, Trowitzsch-Kienast W (2002) Biotenside. B. G. Teubner, Stuttgart
Lee SY, Lee DY, Kim TY (2005) Systems biotechnology for strain improvement. Trends Biotechnol 23:349–358
Linhardt RJ, Bakhit R, Daniels L, Mayerl F, Pickenhagen W (1989) Microbially produced rhamnolipid as a source of rhamnose. Biotechnol Bioeng 33:365–368
Manso Pajarron A, De Koster CG, Heerma W, Schmidt M, Haverkamp J (1993) Structure identification of natural rhamnolipid mixtures by fast atom bombardment tandem mass spectrometry. Glycoconj J 10:219–226
Marsudi S, Unno H, Hori K (2008) Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78:955–961
Matulovic U (1987) Verfahrensentwicklung zur Herstellung grenzflächenaktiver Rhamnolipide mit immobilisierten Zellen von Pseudomonas spec. DSM 2874. Thesis: Technische Universität Braunschweig, Germany
Medina G, Juarez K, Diaz R, Sobéron-Chávez G (2003) Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology (UK) 149:3073–3081
Nguyen TT, Youssef NH, Mcinerney MJ, Sabatini DA (2008) Rhamnolipid biosurfactant mixtures for environmental remediation. Water Res 42:1735–1743
Nouwens AS, Beatson SA, Whitchurch CB, Walsh BJ, Schweizer HP, Mattick JS, Cordwell SJ (2003) Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology (UK) 149:1311–1322
Ochsner UA, Reiser J (1995) Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92:6424–6428
Ochsner UA, Koch A, Fiechter A, Reiser J (1994) Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176:2044–2054
Ochsner UA, Reiser J, Fiechter A, Witholt B (1995) Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous hosts. Appl Environ Microbiol 61:3503–3506
Pinzon NM, Ju LK (2009) Analysis of rhamnolipid biosurfactants by methylene blue complexation. Appl Microbiol Biotechnol 82:975–981
Potvin E, Sanschagrin F, Levesque R (2008) Sigma factors in Pseudomonas aeruginosa. Fems Microbiol Rev 32:38–55
Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, Lam JS, Sobéron-Chávez G (2001) Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyltransferase 2, an enzyme responsible for di-rhamnolipid biosynthesis. Mol Microbiol 40:708–718
Rahman KSM, Rahman TJ, Mcclean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Schenk T, Schuphan I, Schmidt B (1995) High-performance liquid-chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J Chromatogr 693:7–13
Soberón-Chávez G, Lépine F, Déziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68:718–725
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Sullivan ER (1998) Molecular genetics of biosurfactant production. Environ Microbiol 9:263–269
Syldatk C, Lang S, Matulovic U, Wagner F (1985a) Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Z Naturforsch [C] 40:61–67
Syldatk C, Lang S, Wagner F, Wray V, Witte L (1985b) Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z Naturforsch [C] 40:51–60
Trummler K, Effenberger F, Syldatk C (2003) An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp DSM 2874. Eur J Lipid Sci Technol 105:563–571
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. Fems Microbiol Rev 30:274–291
Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095
Wagner VE, Gillis RJ, Iglewski BH (2004) Transcriptome analysis of quorum sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 22(Suppl 1):S15–S20
Wagner VE, Frelinger JG, Barth RK, Iglewski BH (2006) Quorum sensing: dynamic response of Pseudomonas aeruginosa to external signals. Trends Microbiol 14:55–58
Wang QZ, Wu CY, Chen T, Chen X, Zhao XM (2006) Integrating metabolomics into a systems biology framework to exploit metabolic complexity: strategies and applications in microorganisms. Appl Microbiol Biotechnol 70:151–161
Wang QH, Fang XD, Bai BJ, Liang XL, Shuler PJ, Goddard WA, Tang YC (2007) Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng 98:842–853
Wei YH, Chou CL, Chang JS (2005) Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem Eng J 27:146
Williams P, Camara M (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191
Zhang GL, Wu YT, Qian XP, Meng Q (2005) Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ SCI 6B:725–730
Acknowledgment
We want to thank the Fachagentur für nachwachsende Rohstoffe e.V. (FNR) for funding the project. Thanks go to Dr. Frank Rosenau for kindly providing the strain P. aeruginosa PAO1 and Siegfried Almstedt for construction and installation of a new non-commercially available mechanical foam breaker.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, M.M., Hörmann, B., Syldatk, C. et al. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87, 167–174 (2010). https://doi.org/10.1007/s00253-010-2513-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2513-7