Abstract
Background
Malaria transmission in Africa is facilitated by multiple species of Anopheles mosquitoes. These vectors have different behaviors and vectorial capacities and are affected differently by vector control interventions, such as insecticide-treated nets and indoor residual spraying. This review aimed to assess changes in the contribution of different vector species to malaria transmission in east and southern Africa over 20 years of widespread insecticide-based vector control.
Methods
We searched PubMed, Global Health, and Web of Science online databases for articles published between January 2000 and April 2023 that provided species-specific sporozoite rates for different malaria vectors in east and southern Africa. We extracted data on study characteristics, biting rates, sporozoite infection proportions, and entomological inoculation rates (EIR). Using EIR data, the proportional contribution of each species to malaria transmission was estimated.
Results
Studies conducted between 2000 and 2010 identified the Anopheles gambiae complex as the primary malaria vector, while studies conducted from 2011 to 2021 indicated the dominance of Anopheles funestus. From 2000 to 2010, in 57% of sites, An. gambiae demonstrated higher parasite infection prevalence than other Anopheles species. Anopheles gambiae also accounted for over 50% of EIR in 76% of the study sites. Conversely, from 2011 to 2021, An. funestus dominated with higher infection rates than other Anopheles in 58% of sites and a majority EIR contribution in 63% of sites. This trend coincided with a decline in overall EIR and the proportion of sporozoite-infected An. gambiae. The main vectors in the An. gambiae complex in the region were Anopheles arabiensis and An. gambiae sensu stricto (s.s.), while the important member of the An. funestus group was An. funestus s.s.
Conclusion
The contribution of different vector species in malaria transmission has changed over the past 20 years. As the role of An. gambiae has declined, An. funestus now appears to be dominant in most settings in east and southern Africa. Other secondary vector species may play minor roles in specific localities. To improve malaria control in the region, vector control should be optimized to match these entomological trends, considering the different ecologies and behaviors of the dominant vector species.
Graphical Abstract

Similar content being viewed by others
Background
Approximately 2 billion malaria cases and 12 million malaria deaths have been averted over the past two decades due to the scale-up of malaria interventions such as insecticide-treated nets (ITNs), indoor residual spraying (IRS), and effective case management [1]. ITNs, IRS, and case management were estimated to contribute 68%, 13%, and 19% of the decline in malaria cases, respectively, between 2000 and 2015 [2]. Unfortunately, malaria transmission persists, and in some settings there has been stagnation or even reversal of gains [3, 4]. The World Health Organization (WHO) estimates that, globally, we are 48% off the malaria control targets set in the Global Technical Strategy (GTS) (of 31 cases per 1000 population and 7.8 deaths per 1000 population by 2021) and that this situation could worsen [1, 5]. Current challenges include parasite mutations causing drug resistance and undetectability by rapid diagnostic tools [1, 6, 7], insecticide resistance in mosquitoes [8, 9], behavioral resilience or adaptation of the vectors [10,11,12,13,14], and human behaviors and occupational practices that expose people to infections [15, 16]. These challenges, coupled with the poor socioeconomic situation and weak health systems in endemic countries, mean that the ambitious targets set out in the GTS [5] will remain elusive without additional tools, efforts, and funding.
Malaria transmission in Africa is facilitated by different species of Anopheles mosquitoes, which have different behaviors and vectorial capacities. Generally, the four major vector species are Anopheles gambiae, Anopheles funestus, Anopheles coluzzii, and Anopheles arabiensis, which are the most anthropophilic Anopheles species in the world [10, 17]. In addition, several other species play an important but minor role in different localities, and in recent years, the Asian malaria vector Anopheles stephensi has also been spreading in Africa [18]. Because of their different behaviors around human dwellings, malaria vector species are affected differently by indoor insecticidal interventions, which currently dominate malaria control in Africa. For example, ITNs and IRS can effectively control populations of indoor-biting and indoor-resting species such as An. gambiae sensu stricto (s.s.) and An. funestus s.s. but are less effective against other species such as An. arabiensis, which readily bites non-human hosts and in outdoor settings [19, 20]. In fact, historical evidence from east and southern Africa suggests that An. funestus was likely the most important malaria vector prior to implementation of IRS as part of the Global Malaria Eradication Program which in some cases eliminated An. funestus from some areas and kept it at bay for several years [21,22,23,24]. More recent evidence suggests that with the wide-scale use of ITNs starting in the mid-2000s, the formerly dominant malaria vector, An. gambiae s.s., has been largely controlled in many parts of east and southern Africa [25,26,27]. Data from these areas also indicate a shift in both the composition and behavior of important malaria vector species [12, 23, 27,28,29], as well as increasing recognition of other vector species previously thought to be of secondary importance, such as Anopheles parensis, Anopheles rivulorum, and Anopheles coustani [28, 30,31,32].
These observations suggest the need to re-appraise the malaria transmission landscape and to better understand the dominant vector species in different settings across Africa. Understanding the characteristics of these vector species, their responsiveness to interventions, and their insecticide resistance profiles will be particularly important for any further progress in malaria control. This study aimed to conduct a systematic literature search and analyze the proportional contributions of different vector species to malaria transmission. Our focus was on the east and southern Africa regions, where indoor insecticidal interventions have historically been highly effective against major malaria vectors, notably An. gambiae and An. funestus [21,22,23,24]. The evidence review was limited to the period after 2000 when renewed malaria control efforts began following the formation of the Roll Back Malaria (RBM) Partnership in 1998 [33, 34] and the African leaders RBM summit in Abuja, Nigeria, in 2000 [35].
Methods
Literature search and compilation
A systematic search of published literature was conducted for articles describing malaria transmission by different vector species in Africa using three bibliographic databases, PubMed [36], Global Health [37], and Web of Science [38]. A combination of keywords and subject headings was used, including “sporozoite”, “sporozoite rate”, “entomological inoculation rate”, “EIR”, “Anopheles”, and “Africa” (Table 1). The search was limited to articles published between 1 January 2000 and 30 April 2023. The results were downloaded and imported into the EndNote reference manager [39], where duplicates were identified and removed.
Inclusion and exclusion criteria
The articles were screened to identify those describing entomological inoculation rates (EIR) and the proportion of sporozoite-infected mosquitoes (sporozoite rate, SR) from entomological surveys conducted in the east and southern Africa regions. We included studies with data collected in either east Africa (Burundi, Kenya, Rwanda, Tanzania, and Uganda) or southern Africa (Botswana, Lesotho, Madagascar, Malawi, Mozambique, Namibia, South Africa, Eswatini, Zambia, and Zimbabwe) between January 2000 and April 2023. We included full-text articles or manuscripts reporting data from field surveillance of Anopheles vectors, including control or baseline data for intervention studies that separated such data from intervention data. Only studies with mosquito collection performed in both the rainy and dry seasons and those that reported the proportion of sporozoite-infected mosquitoes or EIR separately by species were considered. Studies were included if the primary vector group and complex (An. funestus sensu lato [s.l.] and An. gambiae s.l.) were both screened for sporozoites or if only one of them was tested because the other had either been collected in insignificant numbers or was not found. In addition, the studies had to have reported positive sporozoite infections for at least one of the Anopheles species tested.
Conversely, excluded studies consisted of mathematical modeling reports with no primary data, semi-field or laboratory studies, studies not conducted in east or southern Africa, those for which no surveillance dates had been given, and all studies conducted before 2000. Also excluded were studies reporting mosquitoes collected in only one season of the year, studies reporting only the overall proportion of sporozoite-infected mosquitoes and EIR instead of indicating the infections by vector species tested, studies reporting zero proportion of sporozoite-infected mosquitoes for all species tested, and studies that focused on one species despite multiple Anopheles species being collected in significant numbers. Studies where very few mosquitoes were tested for sporozoites relative to the number of mosquitoes collected (e.g., in one site, one mosquito was tested among 195 collected mosquitoes) and studies that combined intervention data and control data such that these could not be disaggregated into the proportion of control and treatment sporozoite-infected mosquitoes were also excluded.
Data extraction
For each of the selected articles, the following data variables were extracted into a Microsoft Excel spreadsheet: study location (country, province, district, and village), latitude and longitude of the study site, the main vector control method(s) at the study site, dates of data collection, timing of rainy and dry seasons, number of collection nights, collection location (indoor/outdoor), collection method, method used to identify vectors (morphological, polymerase chain reaction [PCR]), proportion of sporozoite-infected mosquitoes, methods used to identify sporozoites (dissection, enzyme-linked immunosorbent assay [ELISA], or PCR), Plasmodium species, and EIR. Data on the proportions of female mosquitoes infected with any Plasmodium sporozoites were extracted to assess the infectivity of different malaria vectors. EIR data extracted were used to estimate the contribution of different vector species to malaria transmission.
Data on the proportion of sporozoite-infected mosquitoes and EIR data were extracted from selected articles to represent the smallest study unit presented in the articles (e.g., village or ward) for both rainy and dry seasons. For articles that had segregated data on sporozoite-infected mosquitoes or EIR indoors and outdoors, the estimates were aggregated and the parameters were estimated using the formulae below (see Eqs. 1 and 2). Where the sampling had been conducted for more than 1 year, the estimates for each year were extracted or estimated from the study data. Also, for studies that did not report EIR but provided components for its estimation, the estimation for each species was calculated as follows:
All EIR estimates were annualized, considering the number of days or months during which data collection was performed. The recalculation of EIR was only done for studies that collected host-seeking mosquitoes. However, for those that collected resting mosquitoes, such as with pyrethrum spray catches (PSC), the EIR was extracted as presented in the article. On a few occasions, EIR data was not presented per species but overall EIR and percent contribution of each species to the EIR. In such instances, the percentage contribution was extracted as presented in the article, and EIR per species was calculated by multiplying the proportion of contribution by overall EIR.
Data analysis
To estimate the contribution of different vectors to malaria transmission, the proportional contribution of species-specific EIR to the overall EIR in the study site was calculated using the formula:
Mosquitoes were categorized into three groups: (i) An. gambiae s.l., corresponding to data presented for An. gambiae s.s., An. arabiensis, or An. merus, and when members of An. gambiae s.l. were unspecified; (ii) An. funestus s.l. corresponding to data presented for An. funestus s.s. and when members of An. funestus s.l. were unspecified; and (iii) other secondary vectors corresponding to other Anopheles species. Both EIR and Plasmodium sporozoite infection data were tabulated by study date and sites. The ggplot2 package [40], implemented in R statistical software [41], was used to plot the proportions of sporozoite-infected mosquitoes over time, using scatter plots. Smooth trend lines were added using the generalized additive method. Using QGIS (Quantum Geographical Information System) software [42], maps were created to illustrate the proportional contribution of different vector species in the different study sites in east and southern Africa for the periods 2000–2010 and 2011–2021.
Results
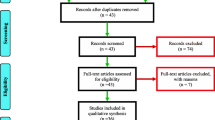
A total of 1111 articles were obtained from the literature search, of which 549 duplicates were screened out. An additional 417 articles were removed because the studies did not meet the inclusion criteria. The remaining 145 articles were subjected to full-text scrutiny, and 57 articles were included in the final analysis (Fig. 1).
Study characteristics
The studies included in this analysis were conducted in nine African countries: Kenya (n = 23), Madagascar (n = 3), Malawi (n = 2), Mozambique (n = 5), South Africa (n = 1), Tanzania (n = 13), Uganda (n = 5), Zambia (n = 4), and Zimbabwe (n = 1) (Table 2). Data presented in the studies were collected between 2000 and 2021, and contained a total of 113 unique data points representing different sites and times of data collection. Extraction of sporozoite data resulted in 105 data points, and extraction of EIR created 67 data points. Mosquitoes were collected using different trapping methods: in the majority of studies (n = 32, containing 63 data points), collection was only performed indoors (Table 2). Twenty-four studies collected mosquitoes both indoors and outdoors (containing 46 data points) and two studies collected mosquitoes only outdoors (containing four data points). Most studies used either Centers for Disease Control and Prevention (CDC) light traps or PSC (n = 51, containing 97 data points). These traps were used alone or together, or supplemented with other trapping methods to collect both indoor biting and resting mosquitoes. CDC light traps were also common for the collection of outdoor biting mosquitoes (used in 13 of 30 studies that collected mosquitoes outdoors). The other methods used included mechanical and mouth aspirators (n = 10), pit shelters (n = 9), human landing catches (HLC, n = 8), clay pots (n = 5), exit traps (n = 3), BG-Suna traps, (n = 2) Furvela tent traps (n = 1), and artificial resting boxes (n = 1). Between 2000 and 2010, indoor collection was typically performed using PSC (27 data points, 51.9%) or CDC light traps (20 data points, 38.5%). Between 2011 and 2022 there was greater use of CDC light traps (37 data points, 94.9%) but PSC still played a role (29 data points, 50.9%). ELISA was the most common method used to test for sporozoite infections in mosquitoes (n = 45 studies, containing 87 data points). PCR was used in 11 studies (containing 21 data points), and only two studies (containing five data points) used dissection to detect sporozoites (Table 2). The methods used to identify mosquitoes differed between the periods 2000–2010 and 2011–2022. There was an increase in the use of molecular methods for mosquito identification, from 75% (n = 39) of the data points for An. gambiae s.l. and 15% (n = 8) for An. funestus in 2000–2011 to 92% (n = 56) of the data points for An. gambiae s.l. and 74% (n = 45) for An. funestus in 2011–2021 (Additional file 1: Table S1). Only 48% of the data points in the 2000–2010 period were identified in the articles as having vector control interventions in place, which included mainly ITNs, IRS, and untreated bed nets; in the remaining data points in that period, either studies reported having no intervention or publications did not provide data on vector control interventions in place. From 2011 to 2022, all data points reported vector control interventions in the study sites, which included ITNs and IRS, and in one study larvicidal and untreated bed nets (Additional file 1: Table S1).
Proportion of mosquitoes infected with Plasmodium sporozoites
Of the 105 data points that contained data on the proportion of Plasmodium-infected mosquitoes, 46 were from studies conducted between 2000 and 2010, and 59 were from 2011 to 2021. Of the 113 data points, only 89 reported the species of Plasmodium identified in mosquitoes. The most common Plasmodium species was Plasmodium falciparum, which was found alone in 83 data points, and in a few studies it was reported to be present with other Plasmodium species such as P. malariae, P. vivax, or P. ovale (six data points). There was no trend in Plasmodium species over time.
In studies that collected data between 2000 and 2010, members of An. gambiae s.l. had the highest proportions of sporozoite infections in 56.5% (n = 26) of the sites while only in 43.5% (n = 20) of the sites An. funestus s.l. had the highest infection proportions. The proportion of infected An. gambiae s.l. ranged between zero and 17.4% (median = 1.4%), while other vectors including members of An. funestus s.l. ranged between zero and 6.3% (median = 1.5%). On the other hand, in studies conducted between 2011 and 2021, members of An. funestus had the highest proportion of sporozoite infections in 57.6% (n = 34) of the sites, while An. gambiae s.l. had the highest proportion of sporozoite infections in only 28.8% (n = 17) of the sites. In this period, there was one (1.7%) site where An. gambiae and An. funestus had equal proportions of sporozoite-infected mosquitoes and seven (11.9%) other sites where vector species other than An. gambiae or An. funestus had the highest proportion of sporozoite infections. In studies conducted between 2011 and 2021, the proportion of infected mosquitoes ranged between zero and 26.4% (median = 2.0%) among all members of An. funestus s.l., 0 and 15% (median = 0.8%) among An. gambiae s.l., and between 0 and 9.1% (median = 0.4%) among the secondary vectors (Table 3, Additional file 1: Table S1).
We detected an overall drop in the proportion of sporozoite-infected mosquitoes among An. gambiae s.l. but no discernible decline in An. funestus between 2000 and 2021. This is, however, without considering the proportion of sporozoite-infected An. funestus and An. gambiae in 2019, which were exceptionally high and most were from a single study. Due to fewer data points presented for secondary vectors from the published articles, no clear trend could be observed (Fig. 2).
In studies where the members of An. gambiae complex and An. funestus group were molecularly distinguished and their sporozoite infections reported, the most common sibling species of An. gambiae complex were An. arabiensis and An. gambiae s.s., and on fewer occasions An. merus, while the most common members of the An. funestus group were An. funestus s.s., and on fewer occasions An. rivulorum, An. leesoni, and An. longipalpis.
The relative contribution of different vector species to malaria transmission
Analysis suggests a decline in overall EIR (all Anopheles combined) in recent years relative to the early 2000s (Fig. 3). This decline has been experienced with changes in the contribution of different species in malaria transmission. Multiple Anopheles vectors have contributed to malaria transmission, with a major shift occurring between 2010 and 2012 when the dominance of An. gambiae began fading (Fig. 4). In the period from 2000 to 2010, most studies reported that the EIR contribution was primarily from members of An. gambiae s.l.. In 28 out of 37 sites, members of An. gambiae s.l. contributed more than 50% to the overall EIR (Fig. 4). Conversely, only 8 of the 37 sites had other An. funestus as the majority contributors to the overall EIR, and one site with an equal contribution between An. gambiae s.l. and An. funestus s.l. Since 2011, however, there has been a decrease in the contribution of An. gambiae s.l. to the overall EIR. In 19 out of 30 studies, An. funestus mosquitoes contributed more than 50% to the EIR. Conversely, only six and another three of the 30 sites had members of An. gambiae s.l. and other secondary vectors contribute more than 50% to the EIR, respectively. Two sites had equal contributions between An. gambiae s.l. and An. funestus s.l. Furthermore, members of An. funestus were more important than other secondary vectors in various sites in east and southern Africa (Fig. 5).
Relative contributions of malaria vectors from different east and southern Africa sites based on studies conducted between 2000 and 2021. EIR denotes entomological inoculation rate, which is the number of infectious bites an individual receives per unit of time. The * sign on the names in the x-axis indicates that more than the mentioned study sites were involved in the survey, while the final year of the survey is indicated in brackets. On top of the bars are abbreviations of countries: KE Kenya, MD Madagascar, MW Malawi, MZ Mozambique, SA South Africa, TZ Tanzania, UG Uganda, ZM Zambia, ZW Zimbabwe
Locations of the study sites, along with the respective contributions of different vectors to malaria transmission (a) between 2000 and 2005, (b) between 2006 and 2011, (c) between 2012 and 2017, and (d) between 2018 and 2021. The years marked on the map signify the final year of data collection as reported in the studies included
Discussion
The main Afro-tropical malaria vectors include An. gambiae, An. arabiensis, An. funestus, and An. coluzzii, which all play a major role in malaria transmission across Africa. In the past decade, the widespread use of indoor insecticidal interventions, notably ITNs and IRS, may have impacted the vector species differently due to their different behaviors, and possibly led to changes in the dominance between these vectors in malaria transmission [27, 82]. This analysis was conducted to systematically compile reports of entomological surveys conducted between 2000 and 2022 to assess the roles of different vectors in malaria transmission in east and southern African countries. The main finding was that the contribution of An. funestus to malaria transmission has become more pronounced than in previous decades, while the role of the formerly dominant malaria vector, An. gambiae, appears to have declined. The increasing importance of An. funestus may not be a new phenomenon, as An. funestus may have been the most important vector before the Global Malaria Eradication Program. Currently, An. funestus is increasingly becoming the major contributor to malaria transmission across multiple sites within the region, as its proportion of sporozoite-infected mosquitoes and proportional contribution to EIR now consistently exceeds those of An. gambiae s.l. We also observed a decrease in the EIR and the proportion of sporozoite-infected An. gambiae but no obvious decrease in the proportion of sporozoite-infected An. funestus between 2000 and 2021.
We postulate that increased coverage of insecticidal indoor vector control interventions and the differential susceptibility of An. gambiae and An. funestus to these interventions may have led to the increasing contribution of An. funestus to malaria transmission observed in this study. Increased funding in the late 2000s and early 2010s [1, 96] led to the rollout of insecticidal vector control interventions, predominantly ITNs and to a lesser extent IRS, across sub-Saharan Africa. Similar patterns are seen in the included studies with reported use of insecticidal vector control interventions in less than half the data points included pre-2011, increasing to all studies between 2011 and 2022. Studies across Africa indicate that ITNs are effective against mosquitoes such as An. gambiae s.s. that mostly prefer to bite humans inside houses [25,26,27]. Anopheles funestus largely shares these behaviors, and therefore it should be expected that the scale-up of ITNs across Africa from the early twenty-first century should have effectively controlled both An. funestus and An. gambiae. However, indicators in this study show that the importance of An. funestus in malaria transmission has now become more noticeable even in areas where An. funestus is outnumbered by other vectors [81, 82]. Anopheles funestus is strongly resistant to pyrethroid insecticides used in ITNs, and in several settings insecticide resistance developed earlier and more rapidly in An. funestus than other vector species [97,98,99,100,101], perhaps explaining why they may have been less impacted by ITNs. Also, several other traits give An. funestus an advantage in malaria transmission by increasing the risk of the vector contracting Plasmodium parasites. Among these are its high anthropophilic tendency, the ability to survive longer [17, 102, 103], and a greater tendency to take multiple blood meals to complete a single gonotrophic circle (Jumanne, unpublished). In addition, An. funestus tends to rest in areas that are out of reach of indoor interventions [104], and tends to seek blood meals in the early morning or evening when humans are unprotected [11, 14]. Changes in entomological procedures such as ELISA during the last few years may have also contributed to the reduction in EIR observed over time. In the 2010s, changes were made to ELISA procedures, where boiling of the lysate at 100 °C for 10 min was recommended [105]. This was to reduce false positives, since the ELISA method had previously been sensitive to protozoans, including non-Plasmodium parasites [105].
In addition to the primary vectors, secondary vectors play a part in malaria transmission across east and southern Africa. In this review, we aimed to assess the relative importance of vector species across the region and thus only included studies that assessed sporozoite infections in multiple species when more than one species was collected in one site. This meant that we excluded several studies indicating the involvement of different secondary vectors such as An. vaneedeni and An. rivulorum in malaria transmission [31, 32, 106], but which were silent about the importance of other collected vectors. Several studies indicated the importance of secondary vectors such as An. coustani in specific locations, including some studies reporting an unexpectedly high contribution of secondary vectors to malaria transmission, mainly contributed by mosquitoes collected outdoors [28]. This should thus be treated with caution due to the inconsistent and unexpected nature of the contribution of the secondary vector. The majority of studies did not test secondary vectors for sporozoites, so it was difficult to gauge the trend of secondary vectors in malaria transmission in this review. More recently, An. stephensi, an invasive urban malaria vector, has been identified in East Africa with the potential to increase malaria transmission [18, 107]. It will be important to expand surveillance for this species and determine its relative contribution to malaria transmission alongside native vector species.
We observed large differences in how data were reported across studies, which made it challenging to pool published data to obtain averages over time and space. We therefore call on researchers to report results in a way that discloses details of spatial and temporal variability in vectors to be able to pinpoint where and which species is important. This includes (i) indicating dates of the survey; (ii) proper description of the study sites (georeferences, ecology and economic activities, the timing of the seasonal rains, interventions used and coverage, and dates of intervention campaigns); (iii) proper mosquito identification (morphological and molecular identification to confirm and identify sibling species); (iv) full report of how different vector species were treated in the survey; (v) if more than one site (village) was involved, separating the results for each site to enable other researchers to identify the spatial variability in the estimates; and (vi) reporting mosquitoes collected by different traps separately.
This study had several limitations. Firstly, there were several sites in the east and southern Africa region where malaria is endemic but there was either a very small number of studies or no studies at all with entomological data on malaria transmission. Most of the studies included in this review were conducted in Kenya and Tanzania, implying that while this systematic review may be strongly indicative of the trends, it does not fully represent the overall picture of the role of different vectors in the region. Secondly, in most of the studies, the EIR or sporozoite-infected proportions of mosquitoes were estimated from only An. gambiae s.l. and An. funestus s.l. Thus, it is likely that the importance of other secondary vectors remains less well understood and may have been underrepresented. Third, the studies considered involved the use of a diverse set of methods for trapping, trapping locations (indoors, outdoors, or both), and detection of sporozoites (ELISA, PCR, dissection). Several studies tested individual mosquitoes for sporozoites while others used subsamples or tested mosquitoes in pools. All these methods have different sensitivity and may introduce biases in estimating the importance of vector species. However, it was difficult to segregate the reviewed articles by method; thus, the analysis was conducted for all articles. Fourth, in the studies included in the review, we noticed a move away from morphological identification and a rise in the use of molecular approaches for mosquito identification over time. This may contribute to bias, since morphological identification may have misclassified vectors. We were unable to extract data for specific species within complexes or groups due to discrepancies in identification procedures. As a result, the results of this study are mostly represented as An. gambiae s.l. (for which the most dominant members were An. gambiae s.s. and An. arabiensis) and An. funestus group (for which the dominant is An. funestus s.s.). Lastly, this review did not assess how the importance of different vectors may vary across different ecological conditions. There may have been ecological changes over time which may for example have increased habitat suitability for An. funestus or decreased habitat suitability for An. gambiae s.l.
Given the apparent rising importance of An. funestus in east and southern Africa, new vector control interventions will be required in addition to ITNs and IRS. This may include sterile insect techniques [108], genetic modification of mosquitoes [109], attractive targeted sugar baits [110,111,112], space spraying of mosquito swarms [113, 114], and spatial repellents [115, 116]. However, in the meantime, as the majority of these interventions are still under development, the available methods should be deployed innovatively and judiciously, including IRS with effective insecticides such as organophosphate and neonicotinoids (to which most vectors including An. funestus remain susceptible [18, 97, 101]), new ITNs with dual active ingredients, expanded use of larval source management (LSM), or combining ITNs or IRS with LSM.
Conclusions
In this review, we compiled reports of entomological surveys assessing malaria transmission. The proportional contribution of different vector species has changed significantly over the past 20 years. As the role of An. gambiae has declined, An. funestus now appears to be dominating most settings in east and southern Africa. Other secondary vector species may be playing minor roles in specific localities. To achieve greater improvements in malaria control in these areas, vector control should be optimized to match these entomological trends, taking into account the different ecology and behaviors of the dominant vector species. While innovative methods are being developed, currently available tools should be enhanced, including next-generation ITNs and IRS, and LSM.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CDC:
-
Centers for Disease Control and Prevention
- EIR:
-
Entomological inoculation rate
- ELISA:
-
Enzyme-linked immunosorbent assay
- GTS:
-
Global Technical Strategy
- HLC:
-
Human landing catches
- IRS:
-
Indoor residual spraying
- ITNs:
-
Insecticide-treated nets
- LSM:
-
Larval source management
- PCR:
-
Polymerase chain reaction
- PSC:
-
Pyrethrum spray catch
- QGIS:
-
Quantum geographical information system
- RBM:
-
Roll Back Malaria
- WHO:
-
World Health Organization
References
WHO. World malaria report. Geneva: World Health Organization; 2022.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
WHO. World Malaria Report. World health. Geneva: World Health Organization; 2020. p. 2020.
WHO. World malaria report. Geneva: World Health Organization; 2021. p. 2021.
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
Agaba BB, Yeka A, Nsobya S, Arinaitwe E, Nankabirwa J, Opigo J, et al. Systematic review of the status of pfhrp2 and pfhrp3 gene deletion, approaches and methods used for its estimation and reporting in Plasmodium falciparum populations in Africa review of published studies 2010–2019. Malar J BioMed. 2019. https://doi.org/10.1186/s12936-019-2987-4.
Menard D, Dondorp A. Antimalarial drug resistance: a threat to malaria elimination. Cold Spring Harb Perspect Med. 2017;7:1–24.
Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet. 2016;387:1785–8.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J BioMed. 2014. https://doi.org/10.1186/1475-2875-13-S1-P53.
Sangbakembi-Ngounou C, Costantini C, Longo-Pendy NM, Ngoagouni C, Akone-Ella O, Rahola N, et al. Diurnal biting of malaria mosquitoes in the Central African Republic indicates residual transmission may be “out of control. Proc Natl Acad Sci USA. 2022. https://doi.org/10.1073/pnas.2104282119.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011. https://doi.org/10.1186/1475-2875-10-80.
Sougoufara S, Doucouré S, Sembéne PMB, Harry M, Sokhna C. Challenges for malaria vector control in sub-Saharan Africa: Resistance and behavioral adaptations in Anopheles populations. J Vector Borne Dis. 2017;54:4–15.
Sougoufara S, Diédhiou SM, Doucouré S, Diagne N, Sembène PM, Harry M et al. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: A new challenge to malaria elimination Malar J. 2014;13,125
Monroe A, Moore S, Koenker H, Lynch M, Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: a review of the published literature. Malar J. 2019;18:6.
Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni P, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14:e0217414.
Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. A global index representing the stability of malaria transmission. Am J Trop Med Hygiene. 2004;5:486.
Durnez L, Coosemans M. Residual Transmission of Malaria: An Old Issue for New Approaches. Anopheles mosquitoes—New insights into malaria vectors. 2013.
Okumu F, Finda M. Key Characteristics of residual malaria transmission in Two Districts in South-Eastern Tanzania— Implications for Improved Control. Oxford: Oxford University Press; 2021.
Smith A, GP. Malaria in the Taveta area of Kenya and Tanzania. Part V. Transmission eight years after the spraying period. East Afr Med J. 1967;44:469–74.
Coetzee M, Kruger P, Hunt RH, Durrheim DN, Urbach J, Hansford CF. Malaria in South Africa: 110 years of learning to control the disease. S Afr Med J. 2013;103:770–8.
Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Tropical Med Int Health. 2004;9:846–56.
Curtis CF, Mnzava AEP. Comparison of house spraying and insecticide-treated nets for malaria control. Bull World Health Organ. 2000;78:1389.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province Kenya. Malar J. 2010. https://doi.org/10.1186/1475-2875-9-62.
Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187.
Lwetoijera DW, Harris C, Kiware SS, Dongus S, Devine GJ, McCall PJ, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley. Tanzania Malar J. 2014;13:331.
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District. Kenya Parasit Vectors. 2013. https://doi.org/10.1186/1756-3305-6-114.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island. Equ Guinea Malar J. 2011;10:184.
Temu EA, Minjas JN, Tuno N, Kawada H, Takagi M. Identification of four members of the Anopheles funestus (Diptera: Culicidae) group and their role in Plasmodium falciparum transmission in Bagamoyo coastal Tanzania. Acta Trop. 2007;102:119–25.
Burke A, Dahan-Moss Y, Duncan F, Qwabe B, Coetzee M, Koekemoer L, et al. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar J. 2019;18:257.
Burke A, Dandalo L, Munhenga G, Dahan-Moss Y, Mbokazi F, Ngxongo S, et al. A new malaria vector mosquito in South Africa. Sci Rep. 2017;7:43779.
WHO. WHO commends the Roll Back Malaria Partnership’s contribution to global progress as governing board disbands secretariat. Geneva: World Health Organization; 2015.
Nabarro DN, Tayler EM. The “roll back malaria” campaign. Science (1979). 1998. p. 2067–8.
WHO. The Abuja Declaration and the Plan of Action An extract from The African Summit on Roll Back Malaria, Abuja, 25 April 2000 (WHO/CDS/RBM/2000.17). 2003.
National Center for Biotechnology Information. PubMed. https://pubmed.ncbi.nlm.nih.gov/
Global health. https://web.s.ebscohost.com/ehost/search/advanced?vid=2&sid=1b095369-f0ff-44e0-af24-2d7bc764090c%40redis
Web of Science 2023. https://www-webofscience-com.lstmed.idm.oclc.org/wos/woscc/basic-search
The EndNote Team. EndNote. Philadelphia, PA: Clarivate. 2013.
Wickham H. ggplot2. WIREs Comput Stat. 2011;3:180–5.
R Core Team. R: A language and environment for statistical computing. Vienna: R Core Team; 2019.
QGIS Development Team. QGIS geographic information system. Beaverton: Open Source Geospatial Foundation; 2023.
Wamae PM, Githeko AK, Otieno GO, Kabiru EW, Duombia SO. Early biting of the Anopheles gambiae s.s. and its challenges to vector control using insecticide treated nets in western Kenya highlands. Acta Trop. 2015;150:136–42.
Abong B, Gimnig JE, Torr SJ, Longman B, Omoke D, Muchoki M, et al. Impact of indoor residual spraying with pirimiphos-methyl (Actellic 300CS) on entomological indicators of transmission and malaria case burden in Migori County, western Kenya. Sci Rep. 2020;10:4518.
Zhong D, Hemming-Schroeder E, Wang X, Kibret S, Zhou G, Atieli H, et al. Extensive new Anopheles cryptic species involved in human malaria transmission in western Kenya. Sci Rep. 2020;10:16139.
Ototo EN, Githeko AK, Wanjala CL, Scott TW. Surveillance of vector populations and malaria transmission during the 2009/10 El Niño event in the western Kenya highlands: opportunities for early detection of malaria hyper-transmission. Parasit Vectors. 2011;4:144.
Bayoh MN, Akhwale W, Ombok M, Sang D, Engoki SC, Koros D, et al. Malaria in Kakuma refugee camp, Turkana, Kenya: facilitation of Anopheles arabiensis vector populations by installed water distribution and catchment systems. Malar J. 2011;10:149.
Muturi EJ, Muriu S, Shililu J, Mwangangi J, Jacob BG, Mbogo C, et al. Effect of rice cultivation on malaria transmission in central Kenya. Am J Trop Med Hyg. 2008;78:270–5.
KA Lindblade JE Gimnig L Kamau WA Hawley F Odhiambo G Olang et al. Impact of sustained use of insecticide-treated bednets on malaria vector species distribution and culicine mosquitoes. 2006 43 428 432
Muturi EJ, Mbogo CM, Mwangangi JM, et al. Concomitant infections of Plasmodium falciparum and Wuchereria bancroftion the Kenyan coast. Filaria J. 2006;5:8.
Mwangangi JM, Mbogo CM, Nzovu JG, Kabiru EW, Mwambi H, Githure JI, et al. Relationships between body size of Anopheles mosquitoes and Plasmodium falciparum sporozoite rates along the Kenya coast. J Am Mosq Control Assoc. 2004;20:390–4.
Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, et al. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol. 2006;43:200–6.
Iwashita H, Dida GO, Sonye GO, Sunahara T, Futami K, Njenga SM, et al. Push by a net, pull by a cow: can zooprophylaxis enhance the impact of insecticide treated bed nets on malaria control? Parasit Vectors. 2014;7:52.
Ondeto BM, Wang X, Atieli H, Zhong D, Zhou G, Lee MC, et al. A prospective cohort study of Plasmodium falciparum malaria in three sites of Western Kenya. Parasit Vectors. 2022;15:416.
Otambo WO, Onyango PO, Wang C, Olumeh J, Ondeto BM, Lee MC, et al. Influence of landscape heterogeneity on entomological and parasitological indices of malaria in Kisumu Western Kenya. Parasit Vectors. 2022;15:340.
Debrah I, Afrane YA, Amoah LE, Ochwedo KO, Mukabana WR, Zhong D, et al. Larval ecology and bionomics of Anopheles funestus in highland and lowland sites in western Kenya. PLoS One. 2021;16:0255321.
Kipyab PC, Khaemba BM, Mwangangi JM, Mbogo CM. The bionomics of Anopheles merus (Diptera: Culicidae) along the Kenyan coast. Parasit Vectors. 2013;6:37.
Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:443.
Karisa J, Ominde K, Muriu S, Munyao V, Mwikali K, Babu L, et al. Malaria vector bionomics in Taita-Taveta County, coastal Kenya. Parasit Vectors. 2022;15:430.
Ondeto BM, Wang XM, Atieli H, Orondo PW, Ochwedo KO, Omondi CJ, et al. Malaria vector bionomics and transmission in irrigated and non-irrigated sites in western Kenya. Parasitol Res. 2022. https://doi.org/10.1007/s00436-022-07678-2.
Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356.
Machani MG, Ochomo E, Amimo F, Kosgei J, Munga S, Zhou G, et al. Resting behaviour of malaria vectors in highland and lowland sites of western Kenya: Implication on malaria vector control measures. PLoS One. 2020;15:0224718.
Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14:244.
Kinya F, Mutero CM, Sang R, Owino EA, Rotich G, Ogola EO, et al. Outdoor malaria vector species profile in dryland ecosystems of Kenya. Sci Rep. 2022;12:7131.
Finney M, McKenzie BA, Rabaovola B, Sutcliffe A, Dotson E, Zohdy S. Widespread zoophagy and detection of Plasmodium spp. in Anopheles mosquitoes in southeastern Madagascar. Malar J. 2021;20:25.
Robert V, Le Goff G, Andrianaivolambo L, Randimby FM, Domarle O, Randrianarivelojosia M, et al. Moderate transmission but high prevalence of malaria in Madagascar. Int J Parasitol. 2006;36:1273–81.
Andrianaivolambo L, Domarle O, Randrianarivelojosia M, Ratovonjato J, Le Goff G, Talman A, et al. Anthropophilic mosquitoes and malaria transmission in the eastern foothills of the central highlands of Madagascar. Acta Trop. 2010;116:240–5.
Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa district. Southern Malawi Malar J. 2012;11:380.
Kabaghe AN, Chipeta MG, Gowelo S, Mburu M, Truwah Z, McCann RS, et al. Fine-scale spatial and temporal variation of clinical malaria incidence and associated factors in children in rural Malawi: a longitudinal study. Parasit Vectors. 2018;11:129.
Charlwood JD, Cuamba N, Tomás EV, Briët OJ. Living on the edge: a longitudinal study of Anopheles funestus in an isolated area of Mozambique. Malar J. 2013;12:208.
Cuamba N, Mendis C. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J Vector Borne Dis. 2009;46:157–9.
Abilio AP, Kleinschmidt I, Rehman AM, Cuamba N, Ramdeen V, Mthembu DS, et al. The emergence of insecticide resistance in central Mozambique and potential threat to the successful indoor residual spraying malaria control programme. Malar J. 2011;10:110.
Charlwood JD, Macia GA, Manhaca M, Sousa B, Cuamba N, Bragança M. Population dynamics and spatial structure of human-biting mosquitoes, inside and outside of houses, in the Chockwe irrigation scheme, southern Mozambique. Geospat Health. 2013;7:309–20.
Salomé G, Riddin M, Braack L. Species composition, seasonal abundance, and biting behavior of malaria vectors in rural Conhane village, Southern Mozambique. Int J Environ Res Public Health. 2023. https://doi.org/10.3390/ijerph20043597.
Mboera LE, Senkoro KP, Mayala BK, Rumisha SF, Rwegoshora RT, Mlozi MR, et al. Spatio-temporal variation in malaria transmission intensity in five agro-ecosystems in Mvomero district. Tanzania Geospat Health. 2010;4:167–78.
Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone Tanzania. Sci Rep. 2021;11:1–13.
Huho BJ, Killeen GF, Ferguson HM, Tami A, Lengeler C, Charlwood JD, et al. Artemisinin-based combination therapy does not measurably reduce human infectiousness to vectors in a setting of intense malaria transmission. Malar J. 2012. https://doi.org/10.1186/1475-2875-11-118.
Kigadye ES, Nkwengulila G, Magesa SM, Abdulla S. Spatial variability in the density, distribution and vectorial capacity of anopheline species in Rufiji district, south-eastern Tanzania. Tanzan J Health Res. 2011;13:112–8.
Mwanziva CE, Kitau J, Tungu PK, Mweya CN, Mkali H, Ndege CM, et al. Transmission intensity and malaria vector population structure in Magugu, Babati District in northern Tanzania. Tanzan J Health Res. 2011;13:54–61.
Kulkarni MA, Kweka E, Nyale E, Lyatuu E, Mosha FW, Chandramohan D, et al. Entomological evaluation of malaria vectors at different altitudes in Hai district, northeastern Tanzania. J Med Entomol. 2006;43:580–8.
Mapua SA, Hape EE, Kihonda J, Bwanary H, Kifungo K, Kilalangongono M, et al. Persistently high proportions of Plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley. South-Eastern Tanzania. 2022;18:e00264.
Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12:e0177807.
Kakilla C, Manjurano A, Nelwin K, Martin J, MashauriKinung’hi FSM, et al. Malaria vector species composition and entomological indices following indoor residual spraying in regions bordering Lake Victoria. Tanzania Malar J. 2020;19:383.
Finda MF, Limwagu AJ, Ngowo HS, Matowo NS, Swai JK, Kaindoa E, et al. Dramatic decreases of malaria transmission intensities in Ifakara, south-eastern Tanzania since early 2000s. Malar J. 2018;17:362.
Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H, Ngonyani H, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17.
Nankabirwa JI, Arinaitwe E, Rek J, Kilama M, Kizza T, Staedke SG, et al. Malaria transmission, infection, and disease following sustained indoor residual spraying of insecticide in Tororo. Uganda Am J Trop Med Hyg. 2020;103:1525–33.
Lynd A, Gonahasa S, Staedke SG, Oruni A, Maiteki-Sebuguzi C, Dorsey G, et al. LLIN Evaluation in Uganda Project (LLINEUP): a cross-sectional survey of species diversity and insecticide resistance in 48 districts of Uganda. Parasit Vectors. 2019;12:94.
Mawejje HD, Asiimwe JR, Kyagamba P, Kamya MR, Rosenthal PJ, Lines J, et al. Impact of different mosquito collection methods on indicators of Anopheles malaria vectors in Uganda. Malar J. 2022;21:388.
Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219.
Ojuka P, Boum Y 2nd, Denoeud-Ndam L, Nabasumba C, Muller Y, Okia M, et al. Early biting and insecticide resistance in the malaria vector Anopheles might compromise the effectiveness of vector control intervention in Southwestern Uganda. Malar J. 2015;14:148.
Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: implications for control. Parasites Vector. 2016. https://doi.org/10.1186/s13071-016-1786-9.
Das S, Muleba M, Stevenson JC, Norris DE. Habitat partitioning of malaria vectors in Nchelenge District Zambia. Am J Trop Med Hyg. 2016;94:1234–44.
Chanda J, Wagman J, Chanda B, Kaniki T, Ng’andu M, Muyabe R, et al. Feeding rates of malaria vectors from a prototype attractive sugar bait station in Western Province Zambia: results of an entomological validation study. Malar J. 2023;22:70.
Saili K, de Jager C, Sangoro OP, Nkya TE, Masaninga F, Mwenya M, et al. Anopheles rufipes implicated in malaria transmission both indoors and outdoors alongside Anopheles funestus and Anopheles arabiensis in rural south-east Zambia. Malar J. 2023;22:95.
Sande S, Zimba M, Chinwada P, Masendu HT, Makuwaza A. Biting behaviour of Anopheles funestus populations in Mutare and Mutasa districts, Manicaland province, Zimbabwe: Implications for the malaria control programme. J Vector Borne Dis. 2016;53:118–26.
Bertozzi-Villa A, Bever CA, Koenker H, Weiss DJ, Vargas-Ruiz C, Nandi AK, et al. Maps and metrics of insecticide-treated net access, use, and nets-per-capita in Africa from 2000–2020. Nature Commun. 2021;12:1–12.
Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020. https://doi.org/10.1186/s12936-020-03483-3.
Choi KS, Christian R, Nardini L, Wood OR, Agubuzo E, Muleba M, et al. Insecticide resistance and role in malaria transmission of Anopheles funestus populations from Zambia and Zimbabwe. Parasit Vectors. 2014;7:1–8.
Djouaka R, Riveron JM, Yessoufou A, Tchigossou G, Akoton R, Irving H, et al. Multiple insecticide resistance in an infected population of the malaria vector Anopheles funestus in Benin. Parasit Vectors. 2016;9:453.
Menze BD, Riveron JM, Ibrahim SS, Irving H, Antonio-nkondjio C, Awono-ambene PH, et al. Multiple insecticide resistance in the malaria vector Anopheles funestus from northern Cameroon is mediated by metabolic resistance alongside potential target site insensitivity mutations. PLoS ONE. 2016;11:e0163261.
Mzilahowa T, Chiumia M, Mbewe RB, Uzalili VT, Luka-Banda M, Kutengule A, et al. Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar J. 2016;15:1–15.
Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53.
Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Johannesburg: South African Institute for Medical Research. ohannesburg; 1968.
Msugupakulya BJ, Kaindoa EW, Ngowo HS, Kihonda JM, Kahamba NF, Msaky DS, et al. Preferred resting surfaces of dominant malaria vectors inside different house types in rural south-eastern Tanzania. Malar J. 2020;19:1–15.
Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, et al. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar J. 2011;10:195.
Kawada H, Dida GO, Sonye G, Njenga SM, Mwandawiro C, Minakawa N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference sporozoite positive rate, and pyrethroid resistance. Parasit Vectors. 2012;5:230.
Ochomo EO, Muchoki M, Otieno JD, Kamau L, Rafferty C, Wacira D, et al. 2023. Molecular surveillance leads to the first detection of Anopheles stephensi in Kenya.
Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, et al. 2010. Sterile-Insect Methods for Control of Mosquito-Borne Diseases an analysis. Vector Borne and Zoonotic Diseases. 10 295
Christophides GK. Transgenic mosquitoes and malaria transmission. Cell Microbiol. 2005;7:325–33.
Stewart ZP, Oxborough RM, Tungu PK, Kirby MJ, Rowland MW, Irish SR. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS ONE. 2013;8:e84168.
Müller GC, Beier JC, Traore SF, Toure MB, Traore MM, Bah S, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali. West Africa Malar J. 2010;9:1–7.
Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali. West Africa Malar J. 2020;19:1–16.
Sawadogo SP, Niang A, Bilgo E, Millogo A, Maïga H, Dabire RK, et al. Targeting male mosquito swarms to control malaria vector density. PLoS ONE. 2017;12:1–11.
Diabate A, Tripet F. Targeting male mosquito mating behaviour for malaria control. Parasit Vectors. 2015;8:1.
Mmbando AS, Ngowo H, Limwagu A, Kilalangongono M, Kifungo K, Okumu FO. Eave ribbons treated with the spatial repellent, transfluthrin, can effectively protect against indoor-biting and outdoor-biting malaria mosquitoes. Malar J. 2018. https://doi.org/10.1186/s12936-018-2520-1.
Kaindoa EW, Mmbando AS, Shirima R, Hape EE, Okumu FO. Insecticide-treated eave ribbons for malaria vector control in low-income communities. Malar J BioMed. 2021;20:1–12.
Acknowledgements
We direct our gratitude to research team members of the outdoor mosquito control group (OMC) at Ifakara Health Institute for their input and support during the preparation of this manuscript.
Funding
Financial support was received from the Bill and Melinda Gates Foundation (BMGF) through the Pan-African Mosquito Control Association (PAMCA) (Grant Number OPP1214408 to FOO, Ifakara Health Institute) to strengthening local capacity for malaria surveillance and elimination in Africa. ALW receives funding from the National Institute for Health Research (NIHR) (Grant Ref: 133144) using UK aid from the UK Government to support global health research and the NIHR (using the UK’s Official Development Assistance (ODA) Funding) and Wellcome [Grant Ref: 220870/Z/20/Z] under the NIHR-Wellcome Partnership for Global Health Research. The funders had no role in the design, data collection, analysis, interpretation of the results, preparation of the manuscript, or decision to publish. The views expressed are those of the authors and not necessarily those of BMGF, PAMCA, Wellcome, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
BJM, FOO, and ALW conceptualized the idea for the review. BJM searched for literature, and screened (together with NHU, MJ), and compiled literature included in the review. BJM extracted data from articles, analyzed the data, and wrote the first and subsequent drafts of the manuscript with input from all other authors (NHU, MJ, HSN, PS, FOO, and ALW). All authors contributed to the decision on the direction and framework of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Permission to publish was granted by the National Institute for Medical Research (NIMR, Ref. No. BD242/437/01/2).
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The contribution of different vectors in malaria transmission for each datapoint, along with data collection dates, ecology, interventions, and identification methods used.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Msugupakulya, B.J., Urio, N.H., Jumanne, M. et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasites Vectors 16, 408 (2023). https://doi.org/10.1186/s13071-023-06019-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-06019-1