Abstract
Background
Anopheles gambiae, An. arabiensis, and An. funestus are widespread malaria vectors in Africa. Anopheles rivulorum is the next most widespread species in the An. funestus group. The role of An. rivulorum as a malaria vector has not been fully studied, although it has been found to be a minor or opportunistic transmitter of Plasmodium falciparum.
Methods
Mosquitoes were collected indoors over a 12-hour period using a light source attached to a rotating bottle collector in order to determine peak activity times and to provide DNA for meal source identification. Gravid female mosquitoes were collected indoors via an aspirator to generate F1 progeny for testing insecticidal susceptibility. Blood meal sources were identified using a multiplexed PCR assay for human and bovine cytochrome-B, and by matching sequences generated with primers targeting vertebrate and mammalian cytochrome-B segments to the Genbank database.
Results
Anopheles rivulorum fed on human blood in the early evening between 18:00 and 20:00, when insecticide-treated bed nets are not in use, and the presence of Plasmodium falciparum sporozoites in 0.70% of the An. rivulorum individuals tested was demonstrated. Susceptibility to permethrin, deltamethrin, and DDT is higher in An. rivulorum (84.8%, 91.4%, and 100%, respectively) than in An. funestus s.s. (36.8%, 36.4%, and 70%, respectively), whereas mortality rates for propoxur and fenitrothion were 100% for both species. Resistance to pyrethroids was very high in An. funestus s.s. and the potential of the development of high resistance was suspected in An. rivulorum.
Conclusion
Given the tendency for An. rivulorum to be active early in the evening, the presence of P. falciparum in the species, and the potential for the development of pyrethroid resistance, we strongly advocate reconsideration of the latent ability of this species as an epidemiologically important malaria vector.
Similar content being viewed by others
Background
Anopheles gambiae Giles, An. arabiensis Patton, and An. funestus Giles are widespread malaria vectors in Africa. Both An. gambiae s.s. and An. funestus s.s. are highly anthropophilic[1, 2], while An. arabiensis prefers to feed on humans but will feed on other hosts as well[3]. A fourth species, An. rivulorum Leeson, is the next most widespread species in the An. funestus group[4]. The role of An. rivulorum as a malaria vector has not been fully studied[5–10]. Previous studies have demonstrated that An. rivulorum has the potential to replace other major malarial vectors such as An. funestus s.s. after spraying of indoor insecticides eliminates the more abundant species[11].
The use of insecticide-treated bed nets (ITNs) is a simple and inexpensive self-protection measure against malaria, which has been shown to reduce the morbidity of children (<5 years old) by 50% and global child mortality by 20%–30%[12, 13]. The dramatic reduction in malaria transmission in Kenya[13] may be due to fewer opportunities for the major anthropophilic and endophagous vectors to feed successfully on humans. However, the increase in the use of ITNs in Kenya has led to an increase in insecticide resistance[14–16] and in the replacement of vector species[17].
This study reports the vectorial and behavioral characteristics and the insecticide susceptibility of An. rivulorum in western Kenya, and strongly recommends a reevaluation of this species as a minor vector of P. falciparum. A large portion of the paper reports on insecticide susceptibility as well.
Methods
Study area
The Gembe East area in the Mbita District of Nyanza Province in western Kenya was used as the study area. The area is drier in the eastern part of the district, and becomes progressively wetter towards the higher altitudes in the western parts of Gwassi Hills and Mfangano Island. In the highlands, the rainfall ranges between 800–1900 mm per annum, while the lowlands receive slightly less at 800–1200 mm each year. The rainfall pattern in the area is bimodal, with the long rainy season occurring from March through May, and the short rainy season occurring in November and December. Malaria infection rates rise steadily between September and February and peak briefly in June, following the long rains[18]. The Mbita and Suba districts are 2 focal points identified as high vector transmission areas in Kenya, and more than 50% of the population is exposed to malaria at a rate of ≥40% Pf PR2-10 (Pf parasite rate corrected to a standard age-range of 2 to less than 10 years old)[19]. Efforts have recently been renewed in this area to increase the use of effective preventative measures such as ITNs and combined vector control approaches. The Akado Medical Centre Project Mosquito Net, the Power of Love Foundation in partnership with World Swim Against Malaria, and the Ministry of Health distributed 6000 ITNs to children under 5 years of age and to pregnant women in the Gembe area. This increased the ITN coverage rate by at least 35%, from 17% to 52%.
Adult mosquito collection and rearing of F1 progenies
Indoor adult collections took place in Nyandago, Nyaroya, Akuot, Alala, Alero, Kirindo, Ngou, and Uwi village in the Gembe East area on the shores of Lake Victoria. The house residents were informed about the study and their written consent was obtained before mosquito collection. Collections were performed during February 5–11, 2010; April 19–June 10, 2010; September 13–October 28, 2010; and January 27–February 4, 2012. Collections were done with a battery-powered aspirator (C-cell Aspirator, BioQuip Products, Rancho Dominguez, CA, USA) between 07:00–09:00 by 3 persons. After collection, gravid females were individually confined into a 20 ml glass vial containing 2 ml of water. A strip of filter paper (ca 3 × 4 cm) was placed inside each vial to collect eggs. Hatched larvae were reared with dechlorinated tap water until adult emergence. Larvae were fed a mixture of powdered animal food (CE-2; Clea Inc., Tokyo, Japan.) and dried yeast (Ebios®; Mitsubishi Tanabe Pharma, Tokyo, Japan). Grass leaves were added to rearing water containing An. funestus s.s. and An. rivulorum larvae to provide a resting place. The containers were exposed to sunlight to facilitate phytoplankton growth as a larval food resource[20]. Unfed F1 female adults were used for insecticide susceptibility tests.
Adult mosquito collection by miniature light traps equipped with collection bottle rotators
Indoor mosquito trapping was done with 6 sets of the Center for Disease Control (CDC) miniature light trap (Model 512) equipped with a collection bottle rotator (Model 1512) (John W. Hock Co., Gainesville, FL, USA) in 3 houses in Nyaroya village and 4 houses in Nyandago village from September 16–October 5, 2010 and July 11–18, 2012. The house residents were informed about the study and their written consent was obtained before mosquito collection. The collection bottle rotator, which has 8 separate plastic collection bottles, was programmed to collect active mosquitoes at 2-hour intervals between 16:00–08:00. The traps were placed in the corner of the living room as far apart from the places where people sleep as possible. Female mosquitoes were identified and classified as unfed, blood-fed, and gravid. The abdominal contents of fed females were used for DNA extractions in order to identify the blood source.
Identification of meal sources in blood-fed mosquitoes
Mosquitoes collected in the field were individually placed in 1.5 ml plastic tubes containing silica gel desiccant and stored at −10°C until processed. Engorged abdomens were separated from the rest of the body for blood source identification.
DNA was extracted from the blood in each abdomen using the REDExtract-N-Amp™ Tissue PCR Kit (SIGMA, St. Louis, MO, USA) per the manufacturer’s instructions. Species identification of the host blood source was performed by 2 different methods; multiplex PCR as described by Kent and Norris[21], or direct sequencing as described by Sawabe et al.[22].
Multiplex PCR was carried out using published cytochrome-B primers for human (Human741F, ggcttacttctcttcattctctcct) and cow (Cow121F, catcggcacaaatttagtcg) and a universal reverse primer (UNREV1025, ggttgtcctccaattcatgtta)[21]. A 20 μl cocktail consisting of 0.5 μM of each primer and previously diluted (300 times) 1 μl of the DNA template was placed in a 0.2 mL cell containing lyophilized AccuPower™ PCR Premix (BIONEER, Daejeon, Korea). The PCR was performed under the following conditions: a hot start at 95°C for 5 min; 35 cycles of template denaturing at 95°C for 1 min, primer annealing at 54°C for 1 min, and amplicon extension at 72°C for 1 min; and a final extension at 72°C for 7 min. Human and cow blood was detected by agarose gel electrophoresis (2% TAE) as 334 bp and 561 bp bands, respectively[21].
PCR amplification to generate amplicons for sequencing was done using published primers VerU-1 (aagacgagaagacccyatgga) and VerU-2 (cctgatccaacatmgaggtcgta) designed to detect the cytochrome-B gene of vertebrates; and Mammalian-1 (tgayatgaaaaaycatcg) and Mammalian-2 (tgtagttrtcwgggtckccta) cytochrome-B designed to detect the cytochrome-B gene of mammals[22]. The PCR mixture contained 4 μl of REDExtract-N-Amp™ ReadyMix cytochrome-B (SIGMA, St. Louis, MO, USA), 0.5 μM of each primer, and 1 μl of the DNA template in a total volume of 10 μl. PCR was conducted under the following conditions: a hot start of 94°C for 2 min; 35 cycles of template denaturing at 94°C for 30 sec, primer annealing at 55°C for 30 sec, and amplicon extension at 72°C for 1 min; and a final extension at 72°C for 4 min. Direct sequencing was performed with a 3730 DNA Analyzer (Applied Biosystems, Carlsbad, CA, USA). The results were analyzed by MEGA 4.0 public domain software (http://www.megasoftware.net/). Sequences were used to perform basic local alignment searches against Genbank (National Center for Biotechnology Information websitehttp://ww.ncbi.nlm.nih.gov/BLAST/) to determine the identities of the host species[22].
Detection of P. falciparum in mosquitoes
Evidence of the presence of P. falciparum was first determined by enzyme-linked immunosorbent assay (ELISA). The head and thorax of each mosquito was homogenized in phosphate buffered saline (pH 7.4) and tested for the circumsporozoite antigen using a monoclonal antibody ELISA[23]. ELISA positive samples were confirmed by a Plasmodium specific PCR. DNA extraction was performed on the QIAcube (kit AIAamp DNA Micro Kit 56304, Qiagen, Tkyo, Japan) following the manufacturer’s instructions and the methods by Durnez et al.[24]. A 20 μl aliquot of the ELISA-lysate was added to 80 μl of ATL-buffer and extracted in the QIAcube machine, which eluted the DNA in 50 μl AE-buffer. The pestles used in the extraction had been sterilized in HCl solution to prevent contamination. The nested PCR was carried out according to the methods by Snounou et al.[25]. The first amplification was done using published primers rPLU-5 (cctgttgttgccttaaacttc) and rPLU-6 (ttaaaattgttgcagttaaaacg). The PCR mixture contained 0.4 μl of Mighty Amp DNA Polymerase (Takara Bio, Inc., Tokyo, Japan), 0.6 μM of each primer, and 1 μl of the DNA template in a total volume of 20 μl. PCR was conducted under the following conditions: a hot start of 98°C for 2 min; 35 cycles of template denaturing at 98°C for 10 sec, primer annealing at 58°C for 15 sec, and amplicon extension at 68°C for 1 min; and a final annealing at 57°C for 2 min and extension at 72°C for 4 min. The 2nd amplification was done using Plasmodium falciparum specific primers rVIV1 (cgcttctagcttaatccacataactgatac) and rVIV2 (acttccaagccgaagcaaagaaagtcctta). The PCR mixture contained 0.2 μl of Mighty Amp DNA Polymerase (Takara Bio, Inc., Tokyo, Japan), 0.3 μM of each primer, and 1 μl of the DNA template in a total volume of 10 μl. PCR was conducted under the following conditions: a hot start of 98°C for 2 min; 35 cycles of template denaturing at 98°C for 10 sec, primer annealing at 58°C for 15 sec, and amplicon extension at 68°C for 30 sec; and a final annealing at 57°C for 2 min and extension at 72°C for 4 min.
Insecticide susceptibility tests using World Health Organization test tubes for F1 progenies
Adult susceptibility tests to insecticides was done using World Health Organization (WHO) test tube kits for F1 progenies and performed according to WHO instructions (WHO/CDS/CPC/MAL/98.12). Papers impregnated with either 0.75% permethrin, 0.05% deltamethrin, 4% DDT, 1% fenitrothion, or 0.1% propoxur were used for the tests. F1 larvae from the separate egg batches oviposited by field collected females were pooled in one batch to get adult females. Ten F1 1–3-day-old female mosquitoes were released into WHO test tubes for exposure to insecticide-impregnated paper for one hour, and the time to knockdown was recorded. One hr exposure to fenitrothion was performed in our bioassay, although 2 hr is stipulated in WHO instructions. Insects were then transferred to a clean tube, fed via cotton soaked with a 5% glucose solution, and mortality was recorded after one day. KT50 (time required for 50% knockdown) was obtained and average mortality was calculated. Two to 4 replications were performed for each insecticide. Control test was performed in each replication.
Species identification
Mosquito adults were examined microscopically to distinguish An. funestus s.l. from other anophelines based on identification keys developed by Gillies and Coetzee[26]. Individual species within An. funestus s.l. were identified using the multiplex polymerase chain reaction (PCR) method described by Koekemoer et al.[27].
Data analysis
KT50 was calculated by Bliss' probit method[28]. Mosquito densities were calculated as the mean number of female mosquitoes collected per collection time (2 hours) per house. The data for each replication were excluded from analysis when the total number of mosquitoes collected during a night was less than 5.
Results
Species abundance, P. falciparum sporozoite rate and blood meal composition
The total number of An. funestus s.l. female mosquitoes collected in the Gembe East area is shown in Table1. The number of An. funestus s.s. was higher than An. rivulorum throughout the year in 2010. In contrast, the species composition was reversed in 2012.
Of the 250 An. funestus s.s. and 284 An. rivulorum female mosquitoes used, 5 (2.0%) and 5 (1.76%) females were found to be P. falciparum positive by CSP-ELISA and 2 (0.80%) and 2 (0.70%) females were P. falciparum positive by PCR detection, respectively (Table2).
Blood meal compositions for the 2 species are shown in Table3. Proportion of human blood was higher in An. funestus s.s. (48.0%–50.7%) as compared to that in An. rivulorum (6.7%–16.0%). An. rivulorum was found to feed on various animals such as cows, bats, birds, apes, mice, bushbuck, and hippopotami; in comparison, An. funestus s.s. fed on cows, bats, and dogs.
Indoor flight activity pattern of An. rivulorum females trapped by CDC miniature traps equipped with a collection bottle rotator
The houses used for indoor mosquito collections were of standard construction for the area, with mud walls and eaves, traditional opening structures between the roof and the walls, and divided into 1–2 bedrooms and 1 living room. The number of ITNs used in each household ranged from 0–2 (average 0.8 net/house), with 0–3 people sleeping in each bedroom (average 2.0 persons/bed room), and 0–4 people (mainly children > 5 years old) sleeping in each living room without ITNs (average 1.8 persons/living room). The number of cows, sheep, and goats reared around each house ranged from 0–17 (average 12.2), 0–8 (average 4.4), and 0–9 (average 6.0), respectively.
Among the 31 collections conducted, 13 were not used due to <5 mosquitoes collected on that night, and 4 collections had to be discarded due to equipment malfunction. The remaining 14 collections were used for analysis.
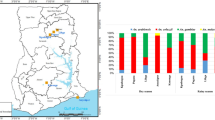
The number of trapped An. rivulorum females rose sharply during early evening (18:00–20:00), falling drastically after this period (Figure1). The number of blood-fed females also peaked in this period. Among the 249 females collected, 104 were unfed (41.8%), 20 contained blood from a human source (8.1%), and 125 had fed from an animal source (50.2%). Among the 20 females engorged with human blood, 13 (65%) were trapped in the 18:00–20:00 period.
Insecticidal susceptibility of An. funestus s.l. female adults
Insecticide susceptibility of adult F1 An. funestus s.l. female mosquitoes is shown in Figure2.
An. funestus s.s. showed high resistance to both permethrin and deltamethrin (36.8% and 36.4% mortality, both >60 min KT50, respectively), while the resistance against DDT was lower (70.0% mortality and 29 min KT50). KT50 and mortality for propoxur and fenitrothion were 23.2 min - 100%, and >60 min - 100%, indicating that An. funestus s.s. is susceptible to these insecticides. Susceptibility of An. rivulorum to pyrethroids was higher compared to An. funestus s.s. but resistance was suspected in this species according to WHO criteria, since mortalities to permethrin and deltamethrin were less than 100% (84.8% and 91.4%, respectively), while KT50s were smaller than those in An. funestus s.s. (30.5 min and 25.5 min, respectively). Susceptibility of An. rivulorum against DDT, propoxur, and fenitrothion was high (25 min KT50 - 100% mortality, 17 min KT50 - 100% mortality, and >60 min KT50 - 100% mortality, respectively).
Discussion
The importance of An. rivulorum as a vector of human malaria in Africa was first advocated by Wilkes et al. in 1996[29]. Most of the mosquito sampling in that study was done at resting sites close to cattle corrals and pit shelters. All-night landing catches on human bait were done outdoors, since this species was considered to be exophilic and zoophilic[4]. Some reports, however, presented evidence that this species was neither completely exophilic nor zoophilic. Oyewole and Awolola reported that 42% of An. rivulorum in Lagos, Nigeria was collected indoors[8]. All mosquito collections were performed indoors in the current study. The increase in the proportion of An. rivulorum from 2010 to 2012 possibly seemed to be due to a rise in the availability of larval habitats. Larvae of An. rivulorum are closely associated with aquatic vegetation, such as the water hyacinths in Lake Victoria, and the fluctuation of the lake water levels may have affected habitat availability[30].
Plasmodium falciparum in An. rivulorum was absent or low in previous reports: 0% (0/80)[7], 0% (0/90)[8], 0% (0/264)[10], and 0.5% (1/203)[29]. Wilkes et al. reported that 0.5% of An. rivulorum (5/1022) was positive via observation of sporozoites using a compound microscope, and that 1 of the 2 samples examined by ELISA was P. falciparum positive[29]. Temu et al. detected 2 Pf positive individuals in 10 An. rivulorum when examined by nested polymerase chain reaction (PCR), although the number of samples was not enough to be statistically evaluated[6]. The P. falciparum positive rate for An. rivulorum in the present study (0.70%) seemed to be relatively high compared to previous reports.
Anopheles rivulorum is more zoophilic than An. funestus s.s. and a broad range of animals were found to be serving as hosts. Host preference, however, appears to be affected by the human/animal ratio, as mosquitoes are more zoophilic in an area where animals are more abundant than humans[31]. A high level of anthropophily in An. rivulorum was reported by Kweka et al.[7], indicating that 64.5% of An. rivulorum sampled in north-eastern Tanzania had taken a blood meal from a human host. Awolola et al. reported that 39.6% of An. rivulorum were found to have ingested human blood in a forest area of southern Nigeria[10]. Likewise, Wilkes et al. collected a number of An. rivulorum using human bait (>14 females/h)[29]. The evidence above indicates that this species has the potential to be a transmitter of human malaria. In our study, a remarkable flight activity peak of An. rivulorum was observed in the early evening (18:00–20:00), indicating that this species prefers to feed during a period before humans are likely to be in bed and under an ITN. These data correlate with the results shown by Wilkes et al. using human bait[29].
The difference in pyrethroid susceptibilities between An. funestus s.s. and An. rivulorum may be explained by the different probabilities of each species coming into contact with the pyrethroids thought to be used exclusively in ITNs. An. funestus s.s. and 2 other major vectors in the study area, An. gambiae s.s. and An. arabiensis, have developed high pyrethroid resistance via unique mechanisms[14, 15]. Pyrethroid resistance in An. gambiae s.s. has developed by the dramatic increase of the L1014S mutation in the voltage gated sodium channel (kdr), and mixed function oxydase-related metabolic resistance has developed in both An. arabiensis and An. funestus s.s.[14]. These resistances were thought to be caused solely by wide-spread distribution of ITNs containing pyrethroids in the study area during the past decade. Indoor spraying with residual insecticides has not been done in the study area[14]; therefore, neither carbamates nor organophosphates are supposed to have been used to control malaria vectors. However, the chance of An. rivulorum being exposed to the pyrethroids impregnated in most of the ITNs seems to be low compared to other vector species, as most of the feeding in this species takes place in early evening. Although most people may not be under the ITNs early in evening, the nets are still in houses hung or folded, making a chance of exposure for mosquitoes. The mortalities on permethrin and deltamethrin (<100%) indicate that the selection pressure of pyrethroids on this species is not negligible. The monitoring of pyrethroid resistance is therefore necessary in An. rivulorum as in the other vector species.
Conclusion
In conclusion, the present report clarifies that An. rivulorum feeds on human blood, especially in early evening when ITNs are not in use. Plasmodium falciparum sporozoites were detected in 0.70% of mosquitoes tested, and presents data indicating a possibility that this species has developed pyrethroid resistance according to the latest WHO criteria (Global Plan for Insecticide Resistance Management in Malaria Vectors;http://www.who.int/malaria/vector_control/ivm/gpirm/en/index.html). Therefore, we strongly recommend that the latent ability of this species as a minor vector should be reconsidered and that the urgent need for implementation of a resistance management strategy for such species. The development of new technologies to control such malaria vectors will also be essential[32].
References
Mbogo CN, Kabiru EW, Muiruri SK, Nzovu JM, Ouma JH, Githure JI, Beier JC: Bloodfeeding behavior of Anopheles gambiae s.l. and Anopheles funestus in Kilifi District, Kenya. J Am Mosq Control Assoc. 1993, 9: 225-227.
Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO: Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994, 58: 307-316. 10.1016/0001-706X(94)90024-8.
Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI, Novak RJ: Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme. Kenya. Malaria J. 2008, 7: 43-
Gillies MT, De Meillon B, Gillies MT, De Meillon B: The Anophelinae of Africa South of the Sahara. Publications of the South African Institute for Medical Research. 1968, Johannesburg, Volume 54
Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M: Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000, 14: 181-189. 10.1046/j.1365-2915.2000.00234.x.
Temu EA, Minjas JN, Tuno N, Kawada H, Takagi M: Identification of four members of the Anopheles funestus (Diptera: Culicidae) group and their role in Plasmodium falciparum transmission in Bagamoyo coastal Tanzania. Acta Trop. 2007, 102: 119-125. 10.1016/j.actatropica.2007.04.009.
Kweka EJ, Mahande AM, Nkya WM, Assenga C, Lyatuu EE, Nyale E, Mosha FW, Mwakalinga SB, Temu EA: Vector species composition and malaria infectivity rates in Mkuzi, Muheza District, north-eastern Tanzania. Tanzan J Health Res. 2008, 10: 46-49.
Oyewole IO, Awolola TS: Impact of urbanization on bionomics and distribution of malaria vectors in Lagos, southern Nigeria. J Vect Borne Dis. 2006, 43: 173-178.
Awolola TS, Ibrahim K, Okorie T, Koekemoer LL, Hunt RH, Coetzee M: Species composition and biting activities of anthropophilic Anopheles mosquitoes and their role in malaria transmission in a holoendemic area of southern Nigeria. African Entomol. 2003, 11: 227-232.
Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M: Identification of three members of the Anopheles funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. Trans R Soc Trop Med Hyg. 2005, 99: 525-531. 10.1016/j.trstmh.2004.12.003.
Gillies MT, Smith A: The effect of a residual house spraying campaign in East Africa on species balance in the Anopheles funestus group: the replacement of Anopheles funestus Giles by Anopheles rivulorum Leeson. Bull Entomol Res. 1960, 51: 243-252. 10.1017/S0007485300057953.
Binka FN, Kubaje A, Adjuik M, Williams LA, Lengeler C, Maude GH, Armah GE, Kajihara B, Adiamah JH, Smith PG: Impact of permethrin treated bednets in child mortality in Kassena-Nankana district, Ghana: a randomized controlled trial. Trop Med Int Health. 1996, 1: 147-154.
Nevill CG, Some ES, Mung'ala VO, Mutemi W, New L, Marsh K, Lengeler C, Snow RW: Insecticide-treated bed nets reduce mortality and severe morbidity from among children on the Kenyan Coast. Trop Med Int Health. 1996, 1: 139-146.
Kawada H, Dida GO, Ohashi K, Komagata O, Kasai S, Tomita T, Sonye G, Maekawa Y, Mwatele C, Njenga SM, Mwandawiro C, Takagi M: Multimodal pyrethroid resistance in malaria vectors, Anopheles gambiae s.s., Anopheles arabiensis, and Anopheles funestus s.s. in western Kenya. PLoS One. 2011, 6: e22574-10.1371/journal.pone.0022574.
Kawada H, Futami K, Komagata O, Kasai S, Tomita T, Sonye G, Mwatele C, Njenga SM, Mwandawiro C, Minakawa N, Takagi M: Distribution of a knockdown resistance mutation (L1014S) in Anopheles gambiae s.s. and Anopheles arabiensis in Western and Southern Kenya. PLoS One. 2011, 6: e24323-10.1371/journal.pone.0024323.
Mathias D, Ochomo EO, Atieli F, Ombok M, Bayoh MN, Olang G, Muhia D, Kamau L, Vulule JM, Hamel MJ, Hawley WA, Gimnig JE: Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in Western Kenya. Malaria J. 2011, 10: 10-10.1186/1475-2875-10-10.
Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED: Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province Kenya. Malaria J. 2010, 9: 62-10.1186/1475-2875-9-62.
Gouagna LC, Okech BA, Kabiru EW, Killeen GF, Obare P, Ombonya S, Bier JC, Knols BG, Githure JI, Yan G: Infectivity of Plasmodium falciparum gametocytes in patients attending rural health centers in western Kenya. East Afr Med J. 2003, 80: 627-634.
Noor AM, Patil AP, Hay SI, Muchiri E, Patil AP, Hay SI, Muchiri E, Muchiri E, Muchiri E, Alegana1 VA: The risks of malaria infection in Kenya in 2009. BMC Infect Dis. 2009, 9: 180-10.1186/1471-2334-9-180.
Tuno N, Githeko AK, Nakayama T, Minakawa N, Takagi M, Yan G: The association between the phytoplankton, Rhopalosolen species (Chlorophyta; Chlorophyceae), and Anopheles gambiae sensu lato (Diptera: Culicidae) larval abundance in western Kenya. Ecol Res. 2006, 21: 476-482. 10.1007/s11284-005-0131-0.
Kent BJ, Norris DE: Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. Am J Trop Med Hyg. 2005, 73: 336-342.
Sawabe K, Isawa H, Hoshino K, Sasaki T, Roychoudhury S, Higa Y, Kasai S, Tsuda Y, Nishiumi I, Hisai N, Hamao S, Kobayashi M: Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J Med Entomol. 2010, 47: 442-450. 10.1603/ME09256.
Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, Esser KM, Beaudoin RL, Andre RG: Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull WHO. 1987, 65: 39-45.
Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, Sochantha T, Coosemans M: False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malaria J. 2011, 10: 195-10.1186/1475-2875-10-195.
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, Do Rosario VE, Thaithong S, Brown KN: High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993, 61: 315-320. 10.1016/0166-6851(93)90077-B.
Gillies MT, Coetzee M: A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical region). South African Institute for Medical Research. 1987, Volume 55
Koekemoer LL, Kamau L, Hunt RH, Coetzee M: A cocktail polymerase chain reaction (PCR) assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002, 66: 804-811.
Bliss CI: The method of probits - A correction. Science. 1934, 79: 409-410. 10.1126/science.79.2053.409.
Wilkes TJ, Matola YG, Charlwood JD: Anopheles rivulorum, a vector of human malaria in Africa. Med Vet Entomol. 1996, 10: 108-110. 10.1111/j.1365-2915.1996.tb00092.x.
Minakawa N, Dida GO, Sonye GO, Futami K, Njenga SM: Malaria vectors in Lake Victoria and adjacent habitats in western Kenya. PLoS One. 2012, 7: e32725-10.1371/journal.pone.0032725.
Tirados I, Costantini C, Gibson G, Torr SJ: Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol. 2006, 20: 425-437. 10.1111/j.1365-2915.2006.652.x.
Kawada H, Dida GO, Ohashi K, Sonye G, Njenga SM, Mwandawiro C, Minakawa N, Takagi M: Preliminary evaluation of the insecticide-impregnated ceiling nets with coarse mesh size as a barrier against the invasion of malaria vectors. Jpn J Infect Dis. 2012, 65: 243-246.
Acknowledgements
This study was funded by a joint research effort between Nagasaki University and Sumitomo Chemical Co. Ltd to create field and semi-field bioassay systems for delivering new insecticide technology. The protocol for the study (case No. 1775) was reviewed and approved by the Scientific Steering Committee (SSC) and the National Ethics Review Committee (ERC) of the Kenya Medical Research Institute (KEMRI). We are grateful to the staff at the Thomas Odhiambo campus of the International Center of Insect Physiology and Ecology (ICIPE) for providing facilities and experimental insects. We also thank P. Lutiali, M. Syombua, J. Kongere, S. Wagalla, J. Lumumba, NUITM-KEMRI Project, Kenya Research Station, Nairobi, Kenya, F. Sonye, A. Oreng’ Obielo, G. Juma, G. Opiyo, Springs of Hope, Mbita, Kenya, and E. Kawashima, H. Iwashita, K. Minagawa, C. Kimura, Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan, for providing technical support and assisting with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HK designed the study, carried out the experiments, and drafted the manuscript. GOD and GS arranged the fields and informed consents of the participants for the study and organized the staffs for the experiments. SMN, CM, and NM critically revised the protocol for the study. All authors read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kawada, H., Dida, G.O., Sonye, G. et al. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasites Vectors 5, 230 (2012). https://doi.org/10.1186/1756-3305-5-230
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1756-3305-5-230