Abstract
Background
Lignocellulosic biomass is one of earth’s most abundant resources, and it has great potential for biofuel production because it is renewable and has carbon-neutral characteristics. Lignocellulose is mainly composed of carbohydrate polymers (cellulose and hemicellulose), which contain approximately 75 % fermentable sugars for biofuel fermentation. However, saccharification by cellulases is always the main bottleneck for commercialization. Compared with the enzyme systems of fungi, bacteria have evolved distinct systems to directly degrade lignocellulose. However, most reported bacterial saccharification is not efficient enough without help from additional β-glucosidases. Thus, to enhance the economic feasibility of using lignocellulosic biomass for biofuel production, it will be extremely important to develop a novel bacterial saccharification system that does not require the addition of β-glucosidases.
Results
In this study, a new thermophilic bacterium named Ruminiclostridium thermocellum M3, which could directly saccharify lignocellulosic biomass, was isolated from horse manure. The results showed that R. thermocellum M3 can grow at 60 °C on a variety of carbon polymers, including microcrystalline cellulose, filter paper, and xylan. Upon utilization of these substrates, R. thermocellum M3 achieved an oligosaccharide yield of 481.5 ± 16.0 mg/g Avicel, and a cellular β-glucosidase activity of up to 0.38 U/mL, which is accompanied by a high proportion (approximately 97 %) of glucose during the saccharification. R. thermocellum M3 also showed potential in degrading natural lignocellulosic biomass, without additional pretreatment, to oligosaccharides, and the oligosaccharide yields using poplar sawdust, corn cobs, rice straw, and cornstalks were 52.7 ± 2.77, 77.8 ± 5.9, 89.4 ± 9.3, and 107.8 ± 5.88 mg/g, respectively.
Conclusions
The newly isolated strain R. thermocellum M3 degraded lignocellulose and accumulated oligosaccharides. R. thermocellum M3 saccharified lignocellulosic feedstock without the need to add β-glucosidases or control the pH, and the high proportion of glucose production distinguishes it from all other known monocultures of cellulolytic bacteria. R. thermocellum M3 is a potential candidate for lignocellulose saccharification, and it is a valuable choice for the refinement of bioproducts.
Similar content being viewed by others
Background
Lignocellulosic biomass is one of the most abundant resources on earth. However, until now, most lignocellulosic wastes are either unutilized or combusted directly [1], which causes serious environmental pollution. To reclaim wastes and mitigate the world’s dependence on fossil fuels, the bioconversion of lignocellulosic biomass to biofuel has drawn increasing attention during the past decade [2–4]. However, with respect to lignocellulosic biomass conversion, current technologies are still not suitable for large-scale applications because of the crystallinity and heterogeneity of feedstocks. Pretreatment, one of the main bottlenecks [5], is first required to separate celluloses from the lignin, and then the newly freed carbohydrate polymers should be hydrolyzed to simple monosaccharides. Lignocellulose saccharification is conventionally accomplished through physical, chemical, and biological methods [6]. Although physical and chemical saccharification can effectively hydrolyze lignocellulose, huge amounts of toxic derivatives of bran aldehydes, anthracene, and furfural are generated by these processes [7], which significantly inhibits subsequent fermentation [8]. Moreover, physical and chemical saccharification usually proceed under severe reaction conditions; thus, corrosion-resistant and high-pressure reactors are required, which will markedly increase the reaction costs [9]. Conversely, biological saccharification can be performed under mild conditions to hydrolyze lignocellulose effectively without producing downstream derivatives; thus, it is regarded as an ideal process for lignocellulose saccharification [10].
Biological saccharification of lignocellulosic biomass in nature is generally considered to be performed by a variety of microorganisms or microbial communities, including fungi and bacteria. To date, most biological processes employ cellulases secreted by wild-type fungi, such as Trichoderma reesei [11], Fusarium oxysporum [12], Piptoporus betulinus [13], Penicillium echinulatum [14], Penicillium purpurogenum [15], Aspergillus niger [16], and Aspergillus fumigatus [17], for saccharification. However, in terms of the specific cellulolytic activity, the cellulase system of the thermophilic, anaerobic bacterium Clostridium thermocellum has been reported to degrade cellulose more effectively than fungal enzyme systems [18]. Additionally, several anaerobic bacteria, such as Caldicellulosiruptor saccharolyticus [19], Caldicellulosiruptor lactoaceticus [20], Ruminococcus albus [21], and Clostridium cellulofermentans [22], which have evolved distinct enzyme systems [23], were reported to efficiently and directly degrade lignocellulose. Using multi-enzyme complexes, these bacteria can saccharify lignocellulose during cultivation, which would greatly reduce operating costs [24, 25].
These anaerobic, lignocellulose-degrading microbes usually reside in the digestive systems of some cellulose-feeding animals, such as cows, horses, and termites, which can effectively utilize cellulose substrates as their main foods. A recent study showed that bacteria in the hindgut of horses could effectively convert a high-fiber, forage-based feedstock into small molecules [26]. Thus, the examination of this natural biomass utilization system may have great potential to identify mechanisms, enzymes, and organisms for further improving managed industrial processes for biomass conversion. However, it is difficult to simulate the environment of the hindgut when growing bacteria. Recently, it was reported that the dominant phyla in horse manure were mainly Firmicutes, Proteobacteria, Verrucomicrobia, Bacteroidetes, and Ruminococcus, which is similar to the microbial community structure in the hindgut of horses [27]. In this regard, characterizing some individual microorganisms isolated from horse manure may be a promising method to obtain a unique consortium of microorganisms that are capable of effectively degrading lignocellulosic biomass.
Although lignocellulose-degrading bacteria have several advantages in lignocellulosic degradation, the sources of cellulosic bacteria, especially cellulosic bacteria that can accumulate oligosaccharides or produce high-value products, are rare. Such bacteria would be valuable for pilot- or commercial-scale applications. Therefore, it is necessary to identify new sources of these bacteria and investigate their ability to saccharify lignocellulosic biomass.
Results
Enrichment of cellulose-degrading thermophilic bacteria for oligosaccharide accumulation
The results showed that a culture enriched from horse manure degraded Avicel and accumulated oligosaccharides (Additional file 1). A microbial community analysis showed that the bacterial community of the horse manure was similar throughout the enrichment process (Additional file 2). According to sequencing and BLAST analyses, Ruminiclostridium thermocellum was the predominant specie. To isolate functional organisms, a serially diluted, stable, enriched culture was plated on cellulose agar, and bacterial colonies with extensive clearing zones were screened. One isolated strain was capable of producing oligosaccharides from cellulose. An analysis of the 16S rDNA gene sequence of this strain indicated that it is a member of the genus Ruminiclostridium. A phylogenetic analysis of 16S rDNA genes revealed that the strain shared 99 % sequence identity with the 16S rDNA gene of R. thermocellum ATCC 27045 (Fig. 1; Additional file 3). Therefore, this strain was identified and named R. thermocellum M3. The results of series of physiological and biochemical experiments showed that R. thermocellum M3 is an oval-shaped (0.5–0.7 × 2.0–3.0 μm), Gram-positive bacterium with no visible tufted flagella, as shown in Additional file 4. Table 1 shows that R. thermocellum M3 utilized a wide range of carbon sources such as glucose, fructose, Avicel, and carboxymethyl cellulose. Organic nitrogen sources, such as yeast extract and beef extract, and even inorganic nitrogen sources such as (NH4)2SO4, NH4Cl, and NaNO3, served as nitrogen sources for R. thermocellum M3 (Table 1). The main metabolic products of R. thermocellum M3 were acetate, along with lower amounts of ethanol and lactate; moreover, butyrate and butanol were not observed.
Fermentation during defined cultivation conditions
Ruminiclostridium thermocellum M3 exhibited cellulose saccharification ability over a wide temperature range, from 45 to 70 °C, as shown in Fig. 2a. The oligosaccharide yield increased from 105.6 ± 14.2 to 474.4 ± 25.9 mg/g Avicel as the temperature increased from 45 to 60 °C; however, the yield decreased when the temperature was further increased to 70 °C. The profile of the cell mass concentration was similar to the oligosaccharide yield (Fig. 2a). The maximum cell mass concentration of 295.9 ± 13.7 mg/L was obtained at 60 °C. In addition, as depicted in Fig. 2b, R. thermocellum M3 saccharified Avicel at pHs ranging from 6.0 to 9.0. The optimal pH for Avicel saccharification and cell growth was 7.5, and the maximum values reached 468.8 ± 21.5 mg/g Avicel and 296.8 ± 4.7 mg/L, respectively.
Yeast extract (YE) is one of the most important nitrogen sources for bacteria [28]. Figure 2c shows that the oligosaccharide yield increased when the YE concentration increased from 0 to 2.5 g/L. A similar profile was observed for the cell mass. The cell mass increased from 229.5 ± 5.1 to 291.4 ± 4.2 mg/L as the YE concentration increased from 0 to 2.5 g/L. However, Avicel saccharification remained constant when the YE concentration exceeded 1.5 g/L. Approximately 48 % of the Avicel was saccharified at a YE concentration of 1.5 g/L.
We also investigated the optimal substrate concentration for oligosaccharide production. Figure 2d shows that R. thermocellum M3 grew on Avicel at concentrations ranging from 2.0 to 7.0 g/L, and that the cell mass concentration increased from 268.2 ± 32.2 to 317.4 ± 25.7 mg/L, respectively. At a concentration of 2.0 g/L, approximately 25 % of the Avicel could be saccharified, and this value increased to 47.8 ± 3.2 % when the Avicel concentration increased to 5.0 g/L; however, the saccharification proportion decreased to 34.80 ± 0.35 % as the Avicel concentration increased to 7.0 g/L. Unlike the cell mass and the saccharification proportion, the oligosaccharide yield gradually increased as the Avicel concentration increased from 2.0 to 5.0 g/L, and then it remained constant as the concentration increased from 5.0 to 7.0 g/L.
Avicel biodegradation characteristics
To investigate the cellulose saccharification kinetics of R. thermocellum M3 under optimal conditions, R. thermocellum M3 was inoculated in optimized ATCC 1191 medium (MA medium) (Avicel 5 g/L, YE 1.5 g/L) at 60 °C, pH 7.5. As shown in Fig. 3a, during the fermentation process, the Avicel concentration decreased gradually; approximately 47 % of the Avicel was saccharified after 54 h of fermentation. The oligosaccharide yield simultaneously increased with increasing cell mass, reached its maximum at 54 h, and then remained constant. Glucose accounted for the majority (approximately 97 %) of the products (466.7 ± 34.8 mg/g Avicel), and it was accompanied by a small amount of cellobiose (<3 %). Cellobiose was barely observed before 18 h; however, the cellobiose concentration increased to 12.0 ± 0.5 mg/g Avicel at 30 h and remained at 12 mg/g Avicel until the end of the fermentation. As incubated to the fresh medium, the concentrations of the metabolites (e.g., acetate, lactate) gradually increased. After 54 h of fermentation, the acetate and lactate concentrations increased to 2300 and 700 mg/L, respectively. Meanwhile, the pH decreased to below 6.0 (Fig. 3b). Similar to the metabolic products, the cell mass concentration also peaked at 263.3 ± 18.6 mg/L at 54 h and then remained constant.
To confirm that a pH decrease causes the oligosaccharide accumulation to plateau during fermentation, glucose was also used as a substrate at an initial pH of 7.5 and 60 °C. When glucose was selected as the carbon source, the glucose concentration was consistent with the trend of pH and cell mass when the pH approached 5.5. At this pH, little cell growth was observed, and the glucose concentration remained constant (Additional file 5).
The carbon balance of R. thermocellum M3 was further evaluated based on the utilization of Avicel and the production of cell biomass (total protein), oligosaccharides, and end products, and carbon closures ranged from 99.6 to 96.8 %. Initially, carbon balance closures of 99.6 ± 0.3 % were observed at 6 h. At this point, approximately 0.6 and 8 % of the carbon flowed into the cell mass and oligosaccharides, respectively. After 24 h, the value increased to 4.3 % (cell mass) and 17.9 % (oligosaccharides). When the cultivation increased to 72 h, the weights of the cell mass and metabolites remained constant; however, the oligosaccharides increased from 17.9 to 32.4 %, and the carbon balance closure declined slightly to 96.8 ± 0.2 %. This indicated that volatile acids and oligosaccharides were the dominant components in the end stages. Approximately 46 % of the total carbon was converted to acetate, lactate, and ethanol, and approximately 33 % of the total carbon was converted to oligosaccharides; the rest of the carbon was used for the production of CO2 and cell mass (Fig. 3c).
The cell-associated cellulase activities of R. thermocellum M3 under the optimal culture conditions were also determined, and they are shown in Fig. 3d. The cell-associated cellulase activities increased gradually within 18 h of fermentation and decline rapidly thereafter. The main type of cellulase was β-glucosidase (maximum cellulase activity of approximately 0.38 U/mL), followed by exoglucanase (maximum cellulase activity of approximately 0.18 U/mL) and endoglucanase (maximum cellulase activity of approximately 0.16 U/mL). Different from the activities of the cell-associated cellulases, the activities of extracellular cellulases in the culture supernatant increased continuously until 24 h of culture (Additional file 6). The main type of extracellular cellulase was β-glucosidase (maximum cellulase activity of approximately 0.35 U/mL), followed by exoglucanase (maximum cellulase activity of approximately 0.18 U/mL) and endoglucanase (maximum cellulase activity of approximately 0.17 U/mL). The peak cellulase activities of R. thermocellum M3 were obtained before 28 h. The cell-associated cellulase specific activities were slightly higher than the extracellular cellulase activities (Additional file 7).
Biodegradation characteristics of untreated lignocellulosic biomass
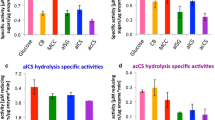
To assess the ability of R. thermocellum M3 to convert lignocelluloses into oligosaccharides, natural lignocellulosic biomass (e.g., rice straw, corn straw, corn cobs, and poplar sawdust) were selected as substrates. In general, oligosaccharides could be generated from all kinds of substrates, as shown in Fig. 4a. The lignocellulosic biomass hydrolysate mainly consisted of glucose and xylose (Fig. 4a), and the oligosaccharide yields varied with the different substrates as follows: rice straw (107.8 ± 7.7 mg/g) > corn straw (89.4 ± 9.3 mg/g) > corn cobs (77.8 ± 5.9 mg/g) > poplar sawdust (52.7 ± 2.3 mg/g) (Fig. 4b). Figure 4c shows the cellulase activities of the crude protein samples in the rice straw culture. The cellulase and hemicellulase activities increased gradually until peaking at 60 h, and then they decreased. The maximum β-glucosidase, endoglucanase, and exoglucanase activities were 0.33 ± 0.03, 0.19 ± 0.02, and 0.17 ± 0.03 U/mL, respectively (Fig. 4c). Compared to saccharification by R. thermocellum M3, the oligosaccharide components produced by a commercial cellulase were the same, and they mainly consisted of glucose, followed by xylose and trace amounts of arabinose (Fig. 4d). The results showed that the oligosaccharides produced by a commercial cellulase gradually increased before 12 h of fermentation and then remained at a constant level (Additional file 8). The compositions of the lignocellulosic feedstock are shown in Additional file 9.
Discussion
The cellulosic microorganisms in horse manure
Manure has been recognized as an excellent source of cellulose-fermenting thermophiles [26]. Horse manure is a source of nitrogen, phosphorous, potassium, easily available carbon compounds, microbes, and neutralizing compounds that enable effective growth of the microbes responsible for organic matter decomposition and the consequent rise in temperature [29]. Some typical cellulose-fermenting thermophiles, such as C. thermocellum [30], Clostridium thermobutyricum [31], Clostridium thermoaceticum [32], and Moorella thermoacetica [33], have been isolated from horse manure. Erick et al. [27] obtained a cellulosic thermophilic fungus, A. fumigatus, which efficiently saccharified cellulose at 45 °C, from horse manure. Wiegel et al. [31] isolated Clostridium thermobutyricum, which was able to consume cellulose for butyrate, CO2, and hydrogen production, from cellulolytic enrichment cultures that were inoculated with horse manure. In this study, the target functional bacteria were first detected in denaturing gradient gel electrophoresis maps (Additional file 2), indicating that cellulose utilization by the microbial community in the horse manure was stable during the enrichment. In addition, the metabolites that were produced during the enrichment suggested that the horse manure had great potential as a source of cellulosic-saccharifying bacteria (Additional file 1). Eventually, we found that the isolated microorganism R. thermocellum M3 consumed cellulose and accumulated oligosaccharides simultaneously, indicating that it has great potential for directly converting cellulose to glucose. Although the strain R. thermocellum ATCC 27045 was able to degrade cellulose effectively, little glucose was obtained during the cellulose fermentation. For example, Ellis et al. investigated the metabolic characteristics of C. thermocellum ATCC27405 at 60 °C, pH 6.95, and 5 g/L Avicel, and the glucose yield was low [34]. Islam et al. [35] also employed C. thermocellum ATCC 27405 to degrade pure α-cellulose fibers of different concentrations at 60 °C, pH 7.3, but no glucose was obtained under low and high α-cellulose concentrations. Tian also found a low glucose yield during the fermentation of sugarcane bagasse (20 g/L) by C. thermocellum ATCC 27405 (55 °C, pH 6.99) [36]. However, high glucose accumulation by R. thermocellum M3 was detected during the fermentation of cellulose, and glucose was produced preferentially over other saccharification products.
The accumulation of oligosaccharides in the culture
While the simultaneous growth of microorganisms and the accumulation of oligosaccharides is unlikely, we demonstrated that R. thermocellum M3 could accumulate considerable amounts of oligosaccharides during cell growth (Fig. 3c). It was observed that approximately 32 % of the carbon flow was shunted into oligosaccharides after 54 h of fermentation, compared with 18 % at 24 h. This implies that the oligosaccharide concentration continued increasing at low pH, even though bacterial growth ceased. Therefore, we presume that more oligosaccharides were produced than could be consumed during cell growth. As a result, oligosaccharides dramatically accumulated when cell growth was inhibited at pHs below 6.0.
During the batch fermentation of R. thermocellum M3, the pH decreased from 7.5 to 6.0 as acetate and lactate accumulated (Fig. 3b). The pH decrease was accompanied by increased oligosaccharide production. It was previously shown that R. thermocellum cannot grow well at pHs below 5.8 [37]. However, its cellulolytic enzymes still hydrolyze cellulose under acidic conditions (pH = 4.0–5.5) [38]. The additional glucose utilization also illustrated that low pH limited the further utilization of glucose by microbes while cellulolytic enzymes still retained their activities (Fig. 3b, c; Additional file 6). This phenomenon could potentially explain the high accumulation of fermentable sugars by R. thermocellum M3 when cell growth was inhibited. Moreover, the accumulation of fermentable oligosaccharides by R. thermocellum M3 was also achieved when using cellulose as the substrate.
The ratio of glucose to cellobiose
Different from other species of R. thermocellum, R. thermocellum M3 consumed cellulose without the need for an added β-glucosidase [39], and its maximum β-glucosidase activity was higher than that of some R. thermocellum strains [40–42]. In the cellulosome system of R. thermocellum M3, the activity of β-glucosidase dominated endoglucanase or exoglucanase activities, which led to a higher oligosaccharide yield and a higher proportion of glucose. The results suggest that the β-glucosidase could hydrolyze most of the cellobiose into glucose.
The extracellular β-glucosidase activity is usually low when R. thermocellum degrades cellulose to cellobiose as the main product. It has been reported that the multi-enzymatic system of the cellulolytic bacterium R. thermocellum is strongly inhibited by the major-end product cellobiose [43]. Therefore, an additional β-glucosidase usually must be added to R. thermocellum cultures if cellulose is to be hydrolyzed efficiently. Fortunately, R. thermocellum M3 efficiently hydrolyzed cellobiose to glucose, as was demonstrated by the high specific cellulase activities from both cells and the culture supernatant. Therefore, the high β-glucosidase activity/specific activities could lead to the high proportion of glucose (Fig. 3d; Additional file 7), which was significantly higher than those previously reported for other thermophilic and cellulosic saccharification strains (P = 0.009; Table 2).
Conclusions
In this study, the novel thermophilic bacterium R. thermocellum M3 was isolated from horse manure and characterized. R. thermocellum M3 effectively utilized cellulose and raw lignocellulose feedstocks and accumulated oligosaccharides. Different from other strains, R. thermocellum M3 exhibited excellent thermophilic and cellulolytic characteristics, as well as great saccharification activity, without the need for pH control or external β-glucosidase addition. In addition, the high proportion of glucose to cellobiose suggests that R. thermocellum M3 has great economic potential for the biofuel fermentation industry.
Methods
DGGE-PCR
The DNA of the enriched cultures were extracted and purified using the bacterial DNA mini kit (Sango Biotech Co. Ltd., Shanghai, China) according to the manufacturer’s instructions. Denaturing gradient gel electrophoresis (DGGE) was performed according to the methods described by Xing et al. [44]. The variable V3 region of 16S rDNA was enzymatically amplified in the PCR with primers (BSF338 and BSR534) to conserved regions of the 16S rDNA genes [45]. The separation and purification of the PCR products were conducted according to the methods described by Cao et al. [45].
Enrichment and isolation of the bacteria
The horse manure used as the seed in this research was collected from Mudanjiang farm, Heilongjiang province, China. Enrichment cultivation was conducted in 100-mL top-sealed bottles at 60 °C under static condition, as described by Cao et al. [45]. The cultivation medium was the modified ATCC 1191 (MA) medium mainly composed of 5.0 g/L Avicel, 3 g/L KH2PO4, 1.5 g/L K2HPO4 ·12H2O, 0.5 g/L (NH4)2SO4, 0.5 g/L NaCl, 0.2 g/L MgSO4 ·6H2O, 2.0 g/L YE, 0.5 g/L l-cysteine, and 1 mL resazurin (0.2 %). After repeating the enrichment process for five times, tenfold serial dilutions were placed on the solid MA medium (2 %, w/v, agar) prepared in a tube and incubated at 60 °C for 7 days. Agar samples containing well-formed cellulose-clearing colonies were transferred to a fresh MA liquid medium under N2 gas flow. The colonies were mashed with a sterilized painting stick to release the cells from the agar. Plating was performed repeatedly to ensure the purity of the isolated colonies. Further verification of purity was ensured by inspecting the microscopy images, colony morphology, and by 16S rDNA gene sequencing.
Genomic DNA of the isolated bacterium was extracted using a bacterial DNA kit (TianGen Biotechnologies Co., Ltd., Beijing, China) and amplified in accordance with the method by Wang [46]. Sequencing was performed at the Sangon Biotechnologies Co., Ltd. (http://www.sangon.com.cn). The nucleotide sequences were compared with those in the GenBank/EMBL/DDBJ nucleotide sequence databases by using the BLAST program (http://www.ncbi.nlm.nih.gov/BLAST/) and the Sequence Match program at the Ribosomal Database Project (RDP). Alignment was conducted using MEGA program (version 6.0). Phylogenetic dendrograms were reconstructed using the MEGA program (version 6.0) [47] with the neighbor-joining (NJ) algorithm and bootstrap analysis of 1000 replicates [48]. The raw sequences data of the strain R. thermocellum M3 have been submitted to the NCBI (Accession number: KU695569).
Fermentation tests
The isolated strain was cultivated anaerobically in MA medium. The inoculum acquired after 48 h incubation was added to 10 % v/v to the fresh MA medium. The cultivation was conducted in 100-mL serum bottles, each bottle containing 50-mL of the MA medium (without nitrogen and carbon source) as described above. The initial pH was adjust by 1 M HCl and 1 M NaOH ranged form 6.0 to 9.0 at intervals of 0.5. The cultivation temperature was increased stepwise from 45 to 70 °C at 5 °C intervals. YE was used as nitrogen source in this research, with 0–2.5 g/L concentration at 0.5 g intervals. Carbon source Avicel was added in 6 different concentrations: 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 g/L.
Saccharification of raw lignocellulosic materials under optimal culture conditions
Four different lignocellulosic substrates without pretreatment (except autoclaving at 121 °C for 15 min)—rice straw, corn stalks, corncob, poplar sawdust—were chosen to test R. thermocellum M3 saccharification efficiency. The lignocellulosic biomasses were all obtained from Harbin Farm (Harbin, Heilongjiang Province, China). The feedstock was chipped with a Szegvari Attritor System type: B (Union Process Inc.) through a 100-mesh sieve. The experiments were conducted with 5 g/L non-pretreated lignocellulosic materials separately using MA medium as described before. in the cultivation tests. Medium amounting to 100 mL was mixed with 10 mL inocula (the concentration of cell protein was about 280 mg/L), and kept at 60 °C for 72 h. Samples were taken every 6 h to determine oligosaccharide concentration, cell biomass, pH change, as well as cellulose-degraded and liquid-end products.
Saccharification of raw lignocellulosic materials by commercial cellulase
The commercial cellulase used in this study was purchased from Biotop (Biotopped, China). The saccharification of each raw lignocellulosic materials was performed in the citrate buffer (0.05 mol/L, pH 4.5) with 5.0 g/L lignocellulosic biomass and 2.4 mg/L commercial cellulase under 55 °C. Saccharification samples were taken every 2 h for each 18-h saccharification period to determine the oligosaccharide type and concentration.
Analytical methods
Oligosaccharides were determined by HPLC (Agilent HP1090) with an Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA). Given that the lignocellulosic biomass was insoluble, cell biomass was estimated with the cell protein and determined using the Bradford method [49]. The liquid metabolites were determined by HPLC (4800, Agilent Technologies, USA), as described by Wang et al. [46]. The biogas composition was measured using a gas chromatograph (Agilent Technologies; model 6890N) equipped with a thermal conductivity detector using argon as the carrier gas (capable of detecting concentrations between 200 and 500 ppm). The cellulose concentration was determined in accordance with the method by Huang et al. [50]. The saccharification was calculated as the amount of oligosaccharides produced (mg) per gram of Avicel added. The lignocellulose composition was determined using the automatic cellulose analyzer (ANKOM A200i, USA) in accordance with the manufacturer’s instructions (https://www.ankom.com/product-catalog/ankom-200-fiber-analyzer).
Carbon mass balance was calculated as output carbon mass divided by input carbon mass [46]:
where ∑C out is total carbon recovery in grams and ∑C in is initial carbon in grams. Evaluation of the carbon mass balance of cellulosic substrates requires information on initial and final carbon contributions, including cellulose concentrations, cell mass concentrations, soluble protein concentrations, concentrations of sugar, total CO2, and organic acids. The whole parameters were measured immediately when inoculation was almost completed and every 6 h thereafter until the end of cultivation (72 h). The carbon contribution from the medium components (primarily YE) was measured with a CHN analyzer (CHNS/O elemental analyzer 2400; Perkin-Elmer, Norwalk, CT). The carbon content of the soluble proteins was estimated to be 50 % (wt/wt) of total protein mass [51]. The concentration of CO2 was measured as described by Wang et al. [46].
Cellulase used for saccharification in this research were prepared as the method described by Morag et al. [52]. The cellulase activities were determined by measuring the reducing sugars released from an appropriate substrate in accordance with the method described by Rattanachomsri et al. [53]. The xylanase activities were measured in accordance with the method described by Kohring et al. [54]. One unit of enzyme activity (IU) was defined as the amount of enzyme that produced 1 μmol of reducing sugar per min. All assays were performed in triplicate, and the mean was reported along with the standard deviation.
Abbreviations
- Avicel:
-
microcrystalline cellulose
- CMC:
-
carboxymethyl cellulose
- HPLC:
-
high performance liquid chromatography
- MA medium:
-
modified ATCC 1191 medium
- RS:
-
rice straw
- CS:
-
corn straw
- CC:
-
corn cob
- PS:
-
poplar sawdust
References
Chandra R, Takeuchi H, Hasegawa T. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production. Renew Sust Energ Rev. 2012;16:1462–76.
Taha M, Shahsavari E, Al-Hothaly K, Mouradov A, Smith AT, Ball AS, et al. Enhanced biological straw saccharification through coculturing of lignocellulose-degrading microorganisms. Appl Biochem Biotechnol. 2015;175:3709–28.
Börjesson J, Peterson R, Tjerneld F. Enhanced enzymatic conversion of softwood lignocellulose by poly (ethylene glycol) addition. Enzyme Microb Technol. 2007;40:754–62.
Lange JP. Lignocellulose conversion: an introduction to chemistry, process and economics. Biofuel Bioprod Bior. 2007;1:39–48.
Chen H, Qiu W. Key technologies for bioethanol production from lignocellulose. Biotechnol Adv. 2010;28:556–62.
Ali SS, Thakur PP, Pathan JY, Zakiruddin M. Saccharification of okara by physical, chemical and biological methods. Bioinfolet. 2013;10:510–2.
Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci. 2008;9:1621–51.
Gregg D, Saddler JN. Bioconversion of lignocellulosic residue to ethanol: process flowsheet development. Biomass Bioenerg. 1995;9:287–302.
Taherdazeh MJ, Karimi K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Bioresources. 2007;2:472–99.
Reese ET. Enzymatic hydrolysis of cellulose. ApMic. 1956;4:39.
Holtzapple M, Cognata M, Shu Y, Hendrickson C. Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol Bioeng. 1990;36:275–87.
Christakopoulos P, Macris B, Kekos D. Direct fermentation of cellulose to ethanol by Fusarium oxysporum. Enzyme Microb Technol. 1989;11:236–9.
Valaškova V, Baldrian P. Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus—production of extracellular enzymes and characterization of the major cellulases. Microbiology. 2006;152:3613–22.
Camassola M, Dillon A. Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. J Appl Microbiol. 2007;103:2196–204.
Steiner J, Socha C, Eyzaguirre J. Culture conditions for enhanced cellulase production by a native strain of Penicillium purpurogenum. World J Microbiol Biotechnol. 1994;10:280–4.
Narasimha G, Sridevi A, Viswanath B, Chandra S, Reddy R. Nutrient effects on production of cellulolytic enzymes by Aspergillus niger. Afr J Biotechnol. 2006;5:472.
Soni SK, Soni R. Regulation of cellulase synthesis in Chaetomium erraticum. Bioresources. 2010;5:81–98.
Johnson EA, Sakajoh M, Halliwell G, Madia A, Demain AL. Saccharification of complex cellulosic substrates by the cellulase system from Clostridium thermocellum. Appl Environ Microbiol. 1982;43:1125–32.
Rainey F, Donnison A, Janssen P, Saul D, Rodrigo A, Bergquist P, et al. Description of Caldicellulosiruptor saccharolyticus gen. nov., sp. nov: an obligately anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol Lett. 1994;120:263–6.
Mladenovska Z, Mathrani IM, Ahring BK. Isolation and characterization of Caldicellulosiruptor lactoaceticus sp. nov., an extremely thermophilic, cellulolytic, anaerobic bacterium. Arch Microbiol. 1995;163:223–30.
Ohara H, Karita S, Kimura T, Sakka K, Ohmiya K. Characterization of the cellulolytic complex (cellulosome) from Ruminococcus albus. Biosci Biotechnol Biochem. 2000;64:254–60.
Yanling H, Youfang D, Yanquan L. Two cellulolytic Clostridium species: Clostridium cellulosi sp. nov. and Clostridium cellulofermentans sp. nov. Int J Syst Evol Microbiol. 1991;41:306–9.
Waeonukul R, Kosugi A, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Prawitwong P, et al. Efficient saccharification of ammonia soaked rice straw by combination of Clostridium thermocellum cellulosome and Thermoanaerobacter brockii β-glucosidase. Bioresour Technol. 2012;107:352–7.
Bayer EA, Belaich JP, Shoham Y, Lamed R. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu Rev Microbiol. 2004;58:521–54.
Bayer EA, Setter E, Lamed R. Organization and distribution of the cellulosome in Clostridium thermocellum. J Bacteriol. 1985;163:552–9.
Sadet-Bourgeteau S, Julliand V. Equine microbial gastro-intestinal health. The impact of Nutrition on the Health and Welfare of Horses. Amsterdam: EAAP Publication; 2010. p. 161–82.
Shepherd ML, Swecker WS, Jensen RV, Ponder MA. Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol Lett. 2012;326:62–8.
Hakobyan L, Gabrielyan L, Trchounian A. Yeast extract as an effective nitrogen source stimulating cell growth and enhancing hydrogen photoproduction by Rhodobacter sphaeroides strains from mineral springs. Int J Hydrogen Energy. 2012;37:6519–26.
Veijalainen AM, Juntunen ML, Lilja A, Heinonen-Tanski H, Tervo L. Forest nursery waste composting in windrows with or without horse manure or urea—the composting process and nutrient leaching. Silva Fenn. 2007;41:13–27.
Hiroshi Y, Wagner ID, Juergen W. Caldicoprobacter oshimai gen. nov., sp. nov., an anaerobic, xylanolytic, extremely thermophilic bacterium isolated from sheep faeces, and proposal of Caldicoprobacteraceae fam. nov. Int J Syst Evol Microbiol. 2010;60:67–71.
Wiegel J, Kuk SU, Kohring GW. Clostridium thermobutyricum sp. nov., a moderate thermophile isolated from a cellulolytic culture, that produces butyrate as the major product. Int J Syst Evol Microbiol. 1989;39:199–204.
Fontaine F, Peterson W, McCoy E, Johnson MJ, Ritter GJ. A new type of glucose fermentation by Clostridium thermoaceticum. J Bacteriol. 1942;43:701.
Wagner ID, Wiegel J. Diversity of thermophilic anaerobes. Ann N Y Acad Sci. 2008;1125:1–43.
Ellis LD, Holwerda EK, Hogsett D, Rogers S, Shao X, Tschaplinski T, et al. Closing the carbon balance for fermentation by Clostridium thermocellum (ATCC 27405). Bioresour Technol. 2012;103:293–9.
Islam R, Cicek N, Sparling R, Levin D. Influence of initial cellulose concentration on the carbon flow distribution during batch fermentation by Clostridium thermocellum ATCC 27405. Appl Microbiol Biotechnol. 2009;82:141–8.
Tian Q-Q, Liang LZhu M-J. Enhanced biohydrogen production from sugarcane bagasse by Clostridium thermocellum supplemented with CaCO3. Bioresour Technol. 2015;197:422–8.
Freier D, Mothershed CP, Wiegel J. Characterization of Clostridium thermocellum JW20. Appl Environ Microbiol. 1988;54:204–11.
Farinas CS, Loyo MM, Baraldo A, Tardioli PW, Neto VB, Couri S. Finding stable cellulase and xylanase: evaluation of the synergistic effect of pH and temperature. New Biotechnol. 2010;27:810–5.
Sundstrom D, Klei H, Coughlin R, Biederman G, Brouwer C. Enzymatic hydrolysis of cellulose to glucose lung immobilized β-glucosidase. Biotechnol Bioeng. 1981;23:473–85.
Chimtong S, Tachaapaikoon C, Sornyotha S, Pason P, Waeonukul R, Kosugi A, et al. Symbiotic behavior during co-culturing of Clostridium thermocellum NKP-2 and Thermoanaerobacterium thermosaccharolyticum NOI-1 on Corn Hull. Bioresources. 2014;9:2471–83.
Kiyoshi K, Furukawa M, Seyama T, Kadokura T, Nakazato A, Nakayama S. Butanol production from alkali-pretreated rice straw by co-culture of Clostridium thermocellum and Clostridium saccharoperbutylacetonicum. Bioresour Technol. 2015;186:325–8.
Halliwell G. Microcrystalline forms of cellulose as substrates for strains of Clostridium thermocellum and cellulase formation. Process Biochem. 1995;30:243–50.
Morag E, Halevy I, Bayer EA, Lamed R. Isolation and properties of a major cellobiohydrolase from the cellulosome of Clostridium thermocellum. J Bacteriol. 1991;173:4155–62.
Xing D, Ren N, Gong M, Li J, Li Q. Monitoring of microbial community structure and succession in the biohydrogen production reactor by denaturing gradient gel electrophoresis (DGGE). Sci China Ser C. 2005;48:155–62.
Cao GL, Zhao L, Wang AJ, Wang ZY, Ren NQ. Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnol Biofuels. 2014;7:82.
Wang AJ, Gao LF, Ren NQ, Xu JF, Liu C, Cao GL, et al. Isolation and characterization of Shigella flexneri G3, capable of effective cellulosic saccharification under mesophilic conditions. Appl Environ Microbiol. 2011;77:517–23.
Kumar R, Singh S, Singh OV. Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol. 2008;35:377–91.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(248–54):47.
Huang L, Gibbins L, Forsberg CW. Transmembrane pH gradient and membrane potential in Clostridium acetobutylicum during growth under acetogenic and solventogenic conditions. Appl Environ Microbiol. 1985;50:1043–7.
Lynd LR, Weimer PJ, Van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–77.
Morag E, Bayer EA, Lamed R. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol. 1990;172:6098–105.
Rattanachomsri U, Tanapongpipat S, Eurwilaichitr L, Champreda V. Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis (enzymology, protein engineering, and enzyme technology). J Biosci Bioeng. 2009;107:488–93.
Kohring S, Wiegel J, Mayer F. Subunit composition and glycosidic activities of the cellulase complex from Clostridium thermocellum JW20. Appl Environ Microbiol. 1990;56:3798–804.
Senthilkumar V, Gunasekaran P. Bioethanol production from cellulosic substrates: engineered bacteria and process integration challenges. J Sci Ind Res India. 2005;64:845–53.
Liu Y, Yu P, Song X, Qu Y. Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrogen Energy. 2008;33:2927–33.
Deswal D, Khasa YP, Kuhad RC. Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour Technol. 2011;102:6065–72.
Prawitwong P, Waeonukul R, Tachaapaikoon C, Pason P, Ratanakhanokchai K, Deng L, et al. Direct glucose production from lignocellulose using Clostridium thermocellum cultures supplemented with a thermostable β-glucosidase. Biotechnol Biofuels. 2013;6:184.
Levin DB, Islam R, Cicek N, Sparling R. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int J Hydrogen Energy. 2006;31:1496–503.
Authors’ contributions
TS designed the experiments and draft the manuscript. LZ carried out the experiments of molecular biology, physiology and biochemistry. LFG carried out the single factor experiment. WZL participated in the design of the study and revised the manuscript. MHC carried out the experiments of enzyme. ZCG carried out the experiments of Avicel fermentation. XDM carried out the experiments of lignocellulose feed-stock fermentation. SHH helped calculate the data of lignocellulose saccharification, carried out the experiments of DGGE, reorganized the structure of discussion and revised the response to major revision. AJW provided experiment platform and gave a final approval of the manuscript to be published. All authors read and approved the final manuscript.
Acknowledgements
This research was supported by the National Science Foundation for Distinguished Young Scholars (Grant No. 51225802), “Hundred Talents Program” of the Chinese Academy of Sciences, Project 135 of Chinese Academy of Sciences (No. YSW2013B06), the International S&T cooperation program (No. S2015GR1012) and the National Key Technology Support Program (2014BAD02B03).
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Sheng, T., Zhao, L., Gao, LF. et al. Lignocellulosic saccharification by a newly isolated bacterium, Ruminiclostridium thermocellum M3 and cellular cellulase activities for high ratio of glucose to cellobiose. Biotechnol Biofuels 9, 172 (2016). https://doi.org/10.1186/s13068-016-0585-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-016-0585-z