Abstract
Dendritic cells (DCs) are the most potent professional antigen-presenting cells (APCs), which play a pivotal role in inducing either inflammatory or tolerogenic response based on their subtypes and environmental signals. Emerging evidence indicates that DCs are critical for initiation and progression of autoimmune diseases, including multiple sclerosis (MS). Current disease-modifying therapies (DMT) for MS can significantly affect DCs’ functions. However, the study on the impact of DMT on DCs is rare, unlike T and B lymphocytes that are the most commonly discussed targets of these therapies. Induction of tolerogenic DCs (tolDCs) with powerful therapeutic potential has been well-established to combat autoimmune responses in laboratory models and early clinical trials. In contrast to in vitro tolDC induction, in vivo elicitation by specifically targeting multiple cell-surface receptors has shown greater promise with more advantages. Here, we summarize the role of DCs in governing immune tolerance and in the process of initiating and perpetuating MS as well as the effects of current DMT drugs on DCs. We then highlight the most promising cell-surface receptors expressed on DCs currently being explored as the viable pharmacological targets through antigen delivery to generate tolDCs in vivo.
Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system (CNS) pathologically featured by multi-spatiotemporal demyelination as well as neuronal and axonal degeneration and damage [1], affecting physical functions, cognition, quality of life, and employment. The underlying mechanisms of this disease remain to be unraveled. Immune tolerance invalidation triggered by various environmental factors (e.g., tobacco smoking, Epstein–Barr virus infection, ambient ultraviolet radiation, and vitamin D levels) in the context of genetic susceptibility [e.g., major histocompatibility complex (MHC) HLA-DRB1 locus] is thought to play a central role [2,3,4]. Under physiological conditions, most autoreactive T or B cells are deleted through central tolerance mechanisms in the primary lymphoid organs, such as thymus and bone marrow, while those that have escaped central tolerance will be counteracted through peripheral tolerance in the secondary lymphoid organs (including lymph nodes, spleen and mucosal-associated lymphoid tissues) as well as peripheral tissues [5]. As the immune tolerance has been broken in MS, CNS-directed autoreactive T and B cells can be activated in the periphery via molecular mimicry, CNS-releasing myelin protein-derived antigens, novel autoantigen or bystander activation [6]. Then, innate immune cells, cluster of differentiation (CD)8+ cytotoxic T cells, CD4+ T helper 1 (TH1) and TH17 cells, and B cells infiltrate into the CNS parenchyma and induce an inflammatory cascade, leading to demyelination, axonal loss and brain atrophy [6]. Emerging evidence has indicated that dendritic cells (DCs) act as a pivotal cell type governing the immune tolerance [7], and play a critical role in the initiation and perpetuation of MS [8, 9]. Given the unique ability of DCs to coordinate innate and adaptive immunity as well as the dual capacity to induce either inflammatory or tolerogenic responses depending on their subtypes and environmental signals, emerging therapies targeting or exploiting DCs have been being considered to combat autoimmune responses [10, 11]. In the present review, we summarized the role of DCs in governing immune tolerance and initiating and perpetuating MS as well as the effects of current disease-modifying therapies (DMT) on DCs. What is more, we focus on the potential therapeutic value of inducing tolDCs in MS therapy with emphasis on the promising surface receptors expressed on DCs currently being explored as the potential viable targets.
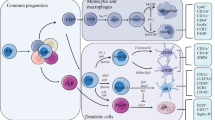
DCs as the pivotal cells governing immune tolerance
Human DC subsets and DC activation status
Human DCs constitute a phenotypically and functionally heterologous population of antigen-presenting cells (APCs) [11,12,13,14,15,16,17], which are generally divided into four major subsets based on developmental origin, specific surface markers and transcriptome profiles: type 1 conventional DC subset (cDC1), type 2 conventional DC subset (cDC2), plasmacytoid DC subset (pDC) and monocyte-derived DC subset (moDC) [18]. The features of human DC subsets with different locations, surface receptors and cytokine profiles as well as various functions are presented in Table 1. Different from the relative homogeneity of the cDC1 subset, the cDC2 subset can be further subdivided into two principal lineages defined as cDC2A and cDC2B lineages according to distinguishing developmental origins regulated by the transcription factors T-bet and RORγt, respectively [19]. The cDC2B lineage is more prone to secreting pro-inflammatory cytokines than the cDC2A lineage, while both are potent stimulators to prime CD4+ T cells [19]. In addition, several single-cell RNA-sequencing studies have reported the heterogeneity of cDC2s [18, 20]. Most recently, Ginhoux et al. proposed to define CD5−CD163+CD14+ cDC2 population as “DC3s”, a new DC subset that expands in inflammatory state independently of cDC1/cDC2 lineage [21]. However, further research is needed to better define the distinct differentiation pathways and molecular states occurring in different inflammatory environments.
During homeostatic conditions, most DCs reside throughout the body in a so-called immature phenotype, constantly surveying the surroundings by continually capturing and processing nearby environmental signals, including endogenous autoantigens and foreign microbial antigens [22]. Maturing DCs undergo various changes, including: (a) the processing of phagocytosis antigens; (b) the elevated presentation of antigens on MHC class I and II molecules; (c) the increased expression of critical co-stimulatory molecules, such as CD40, CD80, and CD86; (d) the enhanced secretion of polarizing cytokines, such as interleukin (IL)-6, IL-12, tumor necrosis factor (TNF)-α; (e) the up-regulation of chemokine receptor expression, such as CCR7 [12, 22]. DCs specifically present antigens to T cells via MHC-I or II molecules, whereas the downstream reaction of antigen recognition depends on the balance of signaling via co-receptors and cytokines which are associated with diverse stimuli received by DCs [11].
Tolerogenic DCs (tolDCs), another activated status of DCs, had been generally previously viewed to undergo incomplete maturation with limited changes in gene expression [23]; however, it has been overturned by a novel finding via genome-wide transcriptome analysis, that the transcriptome changes occurring during tolDC maturation were as complex as and largely overlapping with those occurring during immunogenic DC maturation [24]. In general, the tolDCs have the features with low levels of MHC and co-stimulatory molecules as well as pro-inflammatory cytokines, and expression of immunosuppressive molecules, such as inhibitory co-receptors (inducible co-stimulatory molecular ligand, programmed cell death ligand (PD-L)1 (B7-H1, CD274), PD-L2, B7-H3, and B7-H4), anti-inflammatory cytokines [IL-10 and transforming growth factor (TGF)-β], nitric oxide and indoleamine 2,3-dioxygenase (IDO) [25, 26].
Importantly, the tolDCs are capable of inducing regulatory T cells (Tregs), as well as quelling autoreactive CD4+ and CD8+ T cells in an antigen-specific manner [27], providing a precision therapeutic approach for autoimmune diseases. However, some researchers proposed that immunogenic and tolerogenic DCs might not be distinguished simply by the expression of pro-inflammatory genes, the presence of co-stimulatory molecules (CD80 and CD86) or TNF receptor family members [CD40, OX40 (CD134), and 4-1BB (CD137)], or by the absence of co-inhibitory molecules (e.g., PD-L1) [24]. This controversy challenges the induction and identification of tolDCs.
The role of DCs in maintaining immune tolerance
Multiple natural tolerance mechanisms including central and peripheral tolerance perform a pivotal role in restraining the inappropriate immune responses [5]. Ohnmacht et al. reported that CD11c+ DC-depleted mice showed elevated frequency of CD4+ thymocytes as well as CD4+ T cell expansion and infiltration into peripheral tissues, resulting in a spontaneous fatal autoimmune disease featured by weight loss, splenomegaly, neutrophilia, TH1 and TH17 responses and autoantibody production [28]. Therefore, DCs are involved in implementing both tolerance processes and resolving ongoing immune responses by silencing and/or eliminating autoreactive T cells or inducing the generation and expansion of Tregs besides their roles in initiating and enhancing immunogenicity.
In the primary lymphoid organs, DCs maintain central immune tolerance by participating in negative selection of the autoreactive T cells. Both cDCs and pDCs in human thymus have been demonstrated to be capable of inducing the development of Tregs [29, 30]. A recent study identified a novel thymic CD14+Sirpα+ population of moDCs effectively recruited into the medulla region of thymus by medullary thymic epithelial cells in a Toll-like receptor (TLR)-dependent manner, conducing to the genesis of Tregs and thereby the establishment of central immune tolerance [31]. Interestingly, circulating DCs encountering many peripheral innocuous antigens can be recruited to the medulla region of thymus through an adhesion cascade [32].
Peripheral tolerance occurring in the secondary lymphoid organs provides a second layer of regulation, which is mainly mediated by peripheral DCs as well as other APCs [5]. In the absence of appropriate activation signals for instance co-stimulatory receptors and inflammatory cytokines, peripheral tolDCs capture, process and present endogenous antigens, eliciting the following T cell outcomes rather than inducing naïve T cell activation: (a) Deletion: autoreactive T cells undergo apoptosis mediated by “death receptors” on DCs such as Fas (CD95) and are deleted from the immune repertoire [11]; (b) Anergy: anergized T cells enter a nondividing, hyporeactive, and functionally inactive state, which is considered irreversible [33]; (c) Conversion into Tregs [34], exerting dominant immune suppression by secreting anti-inflammatory cytokines [35]. DCs induce peripheral T cell tolerance by various mechanisms, for example: (a) elevated production of anti-inflammatory cytokines (IL-10, IL-27, and TGF-β); (b) down-regulation of extracellular levels of adenosine and adenosine triphosphate; (c) enhanced synthesis of IDO inhibiting T cell proliferation accompanied by the ligation of CD80/CD86 on DCs with cytotoxic T-lymphocyte antigen 4 (CTLA-4) expressed on T cells; (d) expression of PD-L1 promoting T cell anergy; (e) up-regulated expression of TNF-related apoptosis-inducing ligand (TRAIL), CD95L and perforin inducing T cell deletion [26, 36].
In general, DCs are involved in implementing both central and peripheral tolerance processes. It is crucial and promising to elucidate the underlying mechanisms of DCs governing central and peripheral tolerance for exploring novel therapeutic targets of autoimmune diseases.
The role of DCs in MS/experimental autoimmune encephalomyelitis (EAE)
It is recognized that DCs perform a continuous immune surveillance role in the healthy CNS, locating in vascular-rich regions, including perivascular spaces, meninges and choroid plexus [37]. During neuroinflammation, such as MS, DCs drastically accumulate in the CNS, invade the parenchyma, and prime myelin-specific T cell responses, contributing to the occurrence and development of MS [38, 39]. The inflammatory infiltration within MS lesions mainly consists of B cells, plasma cells, T cells, macrophages and DCs [40]. Once in the perivascular space, the DC-activated CD4+ T cells from the peripheral draining lymph nodes encounter CNS-derived antigen–MHC complexes presented by local APCs, followed by reactivation producing pro-inflammatory mediators, triggering infiltration of inflammatory cells [41]. Notably, cDCs, rather than other CNS-resident APCs including border-associated macrophages or microglia, have been demonstrated to be sufficient and essential for this process [37, 39, 42, 43]. A recent study reported that in cDCs, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2, also known as CYBB/NOX2, could modulate antigen processing and presentation and exert an essential role in the recruitment of encephalitogenic TH cells during neuroinflammation [44].
Indeed, disease severity has been indicated to be related with the DC invasion into the CNS parenchyma in EAE, an animal model of MS. Sagar et al. found that DC transmigration measured by near-infrared imaging correlated with the severity of inflammation in EAE [45]. Furthermore, augmenting CNS-infiltrating DCs by intracerebral microinjection of stimulatory DCs [46] or by systemic treatment of recombinant FMS-like tyrosine kinase 3 ligand (Flt-3L): IgG [39] might exacerbate the onset and clinical disease of EAE. On the other hand, depletion of CD11c+ DCs could interfere with tolerance, leading to an enhanced inflammatory response and aggravated clinical course in murine EAE [34]. Similarly, selective depletion of CD11c+CD11b+ DCs and immature DCs but not CD11c+CD8+ DCs and mature DCs by injecting clodronate-loaded liposomes could partially, yet significantly, abrogate intravenous myelin oligodendrocyte glycoprotein (MOG)-induced suppression of EAE, implying that CD11c+CD11b+ DCs exert a tolerogenic effect [47].
In MS patients, the frequency and maturity of DCs may correlate with the disease stages and clinical patterns. Compared with myeloid DCs from healthy controls, those from relapsing–remitting (RRMS) or secondary-progressive MS (SPMS) had a higher proportion of CD40 expression. In addition, SPMS patients had an increased frequency of myeloid DCs expressing CD80 and those producing IL-12 and TNF-α, a decreased frequency of those expressing PD-L1 when compared with RRMS patients or healthy controls. In addition, the polarization effects on naïve T cells differed between myeloid DCs from patients with RRMS and SPMS [48]. Ectopic lymphoid follicles containing T cells, B cells, plasma cells and follicular DCs were detected along the meninges in SPMS patients [49]. DCs from patients with MS could secrete elevated amounts of pro-inflammatory cytokines, interferon (IFN)-γ, TNF-α, IL-6, IL-23 and IL-12p70, as well as increased levels of migratory molecules, such as CCR5 and CCR7, when compared to healthy individuals [50, 51]. However, pDCs from patients with MS expressed significantly low level of CD86 and 4-1BBL, compared with those from healthy controls and patients with a nonspecific inflammatory condition. When stimulated, pDCs from MS patients might manifest inefficient maturation features [52]. In addition, another study showed a significant reduction in the frequency of circulating pDCs in progressive MS patients [51]. These results suggest that DCs may be involved in the immunologic basis for the different clinical patterns of MS.
In conclusion, it is clear that peripherally derived DCs are essential for initiation and progression of MS/EAE (Fig. 1). The dual capacity of DCs to drive neuroinflammation and to confer antigen-specific T cell tolerance makes them a potential therapeutic target for MS by either inhibiting their immunogenicity or enhancing their tolerance. Indeed, various emerging immunotherapies targeting DCs have been developed for the treatment of MS.
Roles of dendritic cells in the pathogenesis of multiple sclerosis and impacts of disease-modifying therapies. Immune tolerance invalidation is thought to play an important role in the pathogenesis of MS. DCs and B cells capture and process CNS-releasing myelin protein-derived antigens, migrate to secondary lymphoid organs and tissues, and present the self-antigens to T cells to induce myelin-reactive TH1 and TH17 cells. The activated T cells, B cells and DCs travel across the blood brain barrier (BBB). DCs drastically accumulate in the CNS, invade the parenchyma, and prime myelin-specific T cell responses. Once in the perivascular space, the DC-activated T cells from the peripheral draining lymph nodes encounter CNS-derived antigen–MHC complexes presented by local antigen-presenting cells (APCs) especially cDCs, followed by T cell reactivation. These autoreactive immune cells as well as pro-inflammatory mediators then migrate into CNS parenchyma. Ectopic lymphoid follicles containing T cells, B cells, plasma cells and follicular DCs are detected along the meninges in SPMS patients. The dual capacity of DCs to drive immunity and to confer antigen-specific T cell tolerance makes them a potential therapeutic target for MS by either inhibiting their immunogenicity or enhancing their tolerance. Current DMT drugs exert direct or indirect effects on DCs. IFN-β, sphingosine 1-phosphate receptor (S1PR) modulators, dimethyl fumarate (DMF), teriflunomide and glatiramer acetate reduce the polarization of pro-inflammatory TH1 and/or TH17 cells by influencing the maturation, antigen presentation and/or cytokine expression profiles of DCs. DMF, natalizumab and alemtuzumab are capable to induce tolerogenic DCs (tolDCs) conducing to the genesis of regulatory T cells (Tregs), thereby promoting the restoration of tolerogenic networks. Glatiramer acetate and IFN-β can also exert immunomodulatory effects through induction of Tregs. In addition, glatiramer acetate, daclizumab and cladribine might shift the cytokine synthesis pattern of human DCs toward an anti-inflammatory TH2 profile. Mitoxantrone and cladribine have non-specific effects, but also affect antigen presentation and cytokine production of DCs. S1PR modulators, IFN-β, DMF, teriflunomide and natalizumab can block the transit of DCs and autoreactive lymphocytes across the BBB. In the CNS, S1PR modulators exert beneficial effects on microgliosis and astrogliosis, alleviating demyelination. TolDCs, Tregs and TH2 cells generated in the periphery also modulate TH1 and TH17 cell production and microglial activity in the CNS
Impact of DMT on DCs
DCs play an important role in the pathogenesis of MS; however, these cells are rarely considered as targets for treatment in MS. Current DMT for MS may also affect DCs, even if T and B cells are the most frequently discussed targets for these therapies [53, 54]. The impacts of current DMT drugs on DCs are presented in Fig. 1.
IFN‑β
The anti-inflammatory and regenerative effects of IFN-β are thought to underlie the efficacy of IFN-β in treating RRMS [55]. Diverse modes of action of IFN-β help contributing to its anti-inflammatory and immunoregulatory effects in MS, such as induction of autoreactive T cell apoptosis and inhibition of inflammatory cell migration across the blood–brain barrier (BBB), etc. [55, 56]. Notably, studies have indicated that IFN-β also performs several effects on DCs and other APCs.
First, IFN-β exerts both immunostimulatory and immunosuppressive effects on DCs. IFN-β could inhibit DC development at early stages but up-regulate the expression of co-stimulatory molecules on moDCs, enhancing the capacity of DCs to stimulate the secretion of IL-5, IL-10 and IL-13 by T cells [57]. In DCs derived from untreated MS patients and healthy controls, treatment with IFN-β-1a could up-regulate the gene expression of IL-12p35 and IL-27p28 and down-regulate the expression of IL-1β and IL-23p19 via signal transducer and activator of transcription (STAT)1 and STAT3 phosphorylation, respectively, consequently suppressing DC-mediated TH17 cell differentiation [58]. Furthermore, IFN-β−/− DCs, when stimulated by lipopolysaccharide (LPS) or MOG peptide, might exhibit up-regulated expression of MHC-II and CD80 and secrete cytokines contributing to polarization of pathological TH17 cells rather than Tregs, compared with IFN-β+/+ DCs [59]. Genome-wide expression profiling and functional experiments showed that IFN-β non-responders had increased expression of co-stimulatory molecule CD86 on myeloid DCs before initiation of IFN-β therapy, implying that the clinical response to IFN-β may be related to the activation status of myeloid DCs [60], which is needed to elucidate the exact mechanisms of the nexus between activation status of myeloid DCs and action of IFN-β in MS/EAE in the future.
In addition to its general immunomodulatory actions, IFN-β could also inhibit the migratory capacity of DCs by down-regulating the production of migration-associated molecules [e.g., CCR7, matrix metalloproteinase (MMP)-9] via STAT1 signaling [61]. This inhibitory effect could be reversed when IFN-β was knocked out, further demonstrating the immunoregulatory role of IFN-β in the migration of DCs during EAE development [59]. With regard to MS-derived pDCs, IFN-β treatment could significantly down-regulate the TLR9 agonist-specific expression of CCR7, which was generally increased on pDCs from the untreated MS patients [62].
Finally, IFN-β-treated monocytes and DCs of MS patients increased expression of PD-L1, a potent inhibitor of autologous CD4+ T cell activation contributing to the maintenance of peripheral tolerance [63]. Besides, IFN-β could induce the apoptosis of mature DCs through the induction of caspase-11 expression and caspase-3 activation, which required the activation of STAT1 and NF-κB [64].
Interestingly, DC-targeted IFNs could increase the frequency of pDCs and induce their tolerogenic potential with up-regulated expression of IDO and TGF-β as well as induction of Tregs, effectively ameliorating EAE without significant side effects [65]. Thus, DC-specific targeted treatment may provide more efficient and safer therapy in MS.
Glatiramer acetate
Glatiramer acetate is a synthetic random polymer composed of four amino acids including l-alanine, l-lysine, l-glutamic acid and l-tyrosine, structurally resembling myelin basic protein (MBP) [66]. It exerts anti-inflammatory and immunomodulatory effects and the best-documented mechanism of its effect is inducting TH2 cells and secreting anti-inflammatory cytokines, such as IL-4, IL-10 and TGF-β [53, 67], as well as inducting Tregs via activation of transcription factor FOXP3 [68]. Particularly, DCs are involved in the modes of action of glatiramer acetate in suppressing the induction of EAE and modulating the course of RRMS.
Glatiramer acetate promotes DC-mediated T cell differentiation shifting to TH2 polarization and Treg expansion [69]. In vitro studies have shown that glatiramer acetate can significantly suppress the proliferative effect of DCs on lymphocytes [70] and compete with MBP for binding to MHC molecules consequently inhibiting the antigen presentation process [71]. What is more, glatiramer acetate could perform a novel selective inhibitory role upon the production of DC-derived pro-inflammatory mediators (e.g., TNF and the major TH1 polarizing factor IL-12) and exert a facilitating role in DC-mediated induction of effector IL-4-producing TH2 cells as well as secretion of anti-inflammatory cytokines (e.g., IL-10, TGF-β), without affecting the maturation or immunostimulatory potential of DCs [72, 73]. In contrast to these findings, the inductive effect of glatiramer acetate on DC-derived IL-10 production rather than the inhibitory effect on DC-derived TNF-α production was observed upon stimulation [74]. Glatiramer acetate-mediated anti-inflammatory shift of DCs is considered to be related to the suppression of STAT1 signaling [73]. In addition, expression of co-stimulatory molecule CD40 on DCs from MS patients treated with glatiramer acetate was significantly reduced and correlated with the risk of recurrence [75]. However, the underlying mechanisms remain to be unraveled.
Sphingosine 1-phosphate receptor (S1PR) modulators
Sphingosine 1-phosphate (S1P), a bioactive metabolic product of cytomembrane sphingolipids, acts as a key regulator of various physiological and pathophysiological processes, including cellular behaviors (e.g., survival, proliferation, adhesion and migration), vascular barrier function, and homeostasis, signaling via five distinct high-affinity G protein-coupled receptor (GPCR) subtypes, S1PR1 to S1PR5 [76]. Regulation of vascular barrier integrity and immune cell trafficking by S1P–S1PR signaling system is highly relevant to inflammatory processes, autoimmune response [77,78,79,80]. Importantly, the presence of S1PR1 is critical for the egress of lymphocytes from lymph nodes into lymph, a process mediated by the gradient of S1P concentration [81]. Till now, there have been four S1PR modulators (distinct receptor subtypes targeted) approved for treatment of MS [fingolimod (S1PR1,3,4,5), siponimod (S1PR1,5), ozanimod (S1PR1,5), and ponesimod (S1PR1)], and several therapeutic candidates currently in clinical development [e.g., etrasimod (S1PR1,4,5), ceralifimod (S1PR1,5), and amiselimod (S1PR1,4,5)] [76, 81]. Currently, most of published data on the role of S1PR modulators in the CNS come from in vivo and in vitro studies of fingolimod or siponimod.
The primary mechanism of effects of S1PR modulators has been considered to be selectively sequestering autoreactive lymphocytes within the lymph nodes and affecting T cell differentiation via binding to S1PR1 on lymphocytes, preventing the infiltration of autoreactive lymphocytes into inflamed CNS via binding to S1PR1 on endothelial cells and astrocytes [81], influencing microgliosis and astrogliosis [82, 83], as well as protecting against demyelination and promoting remyelination by interacting with S1PR5 on oligodendrocytes [84].
It is noteworthy that S1PR modulators are closely related to DC functions as well. First, DCs act as metabolic gatekeepers of thymic export in an S1P lyase (SPL)-dependent manner. Deleting SPL in DCs rather than thymic epithelial cells or other stromal cells could prevent T cell egressing from the thymus by disrupt the S1P gradient, which could be rescued by adoptive transfer of wild-type DCs [85]. Moreover, S1PR1-mediated TH17 differentiation is thought to be dependent on sustained S1PR1 signaling in myeloid cells rather than an intrinsic T cell effect [86]. Lack of S1PR4 expression on DCs could profoundly affect cytokine production and migration of DCs, and reduce TH17 polarization [87]. In addition, mature DCs generated in the presence of fingolimod showed an impaired phagocytic capacity and immunostimulatory property with down-regulated IL-1β, IL-6, IL-12, IL-23 and TNF-α, but up-regulated IL-10 secretion. T cells co-cultured with fingolimod-treated DCs were inhibited differentiating into pro-inflammatory TH1 cells or TH17 cells [88, 89]. Application of fingolimod in patients with MS could rebalance the immune tolerance networks by regulating the secretion of pro-inflammatory cytokines by APCs including DCs and monocytes, without a significant reduction in the absolute number of these cells [90].
Many studies have reported that this kind of medicines have the ability to regulate chemotaxis and trafficking of DCs. Administration of fingolimod to normal mice significantly decreased DCs in lymph nodes and spleen with down-regulated expression of CD11b, platelet endothelial cell adhesion molecule 1 (PECAM-1, CD31), intercellular cell adhesion molecule 1 (ICAM-1, CD54), and CCR7 on circulating DCs [91]. In vitro, trans-endothelial migratory capacity of fingolimod-treated immature DCs in response to the CCR7 ligand CCL19 was reduced [91]. Fingolimod could down-regulate the expression of CCR6 on immature DCs, inhibiting the migration of these cells [92]. Another in vitro study reported that fingolimod could reduce chemotaxis of both immature and mature DCs [88]. However, unimpaired migration of pDCs into the CNS could exert beneficial effects in the treatment of EAE with AUY954, an S1PR1-specific agonist [93].
In brief, S1PR modulators perform immunomodulatory roles through intricate mechanisms with sequestering autoreactive lymphocytes within the lymph nodes and influencing T cell differentiation, regulating the immunogenicity and trafficking of DCs, as well as cross-talks between these two aspects.
Dimethyl fumarate
Dimethyl fumarate (DMF) and its active metabolite, monomethyl fumarate (MMF) function primarily by restricting the survival, proliferation, activation and cytokine secretion of specific effector and memory T cell subsets, thereby shifting the balance between TH1/TH17 and TH2 immune responses [94]. In addition, application of DMF has been shown to exert neuroprotective effects against oxidative stress for neurons, astrocytes and other CNS cells via activating the nuclear factor erythroid 2-related factor (Nrf2) signaling pathway [95].
Hydroxycarboxylic acid receptor 2 (HCAR2), a GPCR subtype, is a pleiotropically linked receptor for MMF that mediates the protective effects as well as inflammatory side effects via activating distinct pathways in different cell types [96, 97]. The effects of DMF/MMF on DCs mainly include inhibition of DC maturation, alteration of DC cytokine profile, and subsequently modulation of TH1/TH17 and TH2 cell differentiation balance, as well as induction of tolDCs [98,99,100,101]. It is well-known that antioxidant Nrf2 signaling plays a critical role in the underlying mechanism of effects of DMF/MMF on DCs [102]. Several studies indicated that alternative signaling pathways independent of Nrf2 are also involved with DMF effects on modulation of adaptive and innate immunity [98, 103, 104]. For example, the DMF-induced inhibition of NF-κB signaling pathway is thought to play an important role in the mechanism of DMF/MMF action on DCs, and can be further inhibited by DMF-mediated down-regulation of extracellular signal-regulated kinase (ERK)1/2 and its downstream kinase mitogen stress-activated kinase 1 (MSK1) [98]. Given that ERK activation has been demonstrated to be involved in CCL2-mediated DC transmigration across the BBB, DMF-mediated inhibition of ERK1/2 might contribute to the decrease of CNS recruitment of DCs which correlates with disease severity in EAE [45]. A recent study reported that DMF could modulate the functions of human pDCs through another mechanism of action independent of Nrf2. DMF could target the IL-1R-associated kinase 4 (IRAK4)–MyD88 signaling and inhibit IRAK4-mediated cytokine secretion in a cysteine 13-dependent manner, thereby disrupting human innate immune signaling [104]. Interestingly, in vitro experiments indicated that exposure of DCs to low doses of electrophilic Nrf2 activators including DMF could exert anti-inflammatory effects by inhibiting the production of pro-inflammatory cytokines (e.g., IL-1β and IL-12) in an Nrf2-dependent manner, while high doses of DMF might in contrast promote inflammatory apoptosis and concomitant IL-1β secretion independently of Nrf2 [105]. The exact mechanism by which DMF acts on DCs has not been fully elucidated, which need to be further explored as the pharmaceutical relevance in discovering novel molecular targets to optimize MS therapy.
Teriflunomide
Teriflunomide, the active metabolite of leflunomide, exerts a cytostatic effect on lymphocyte proliferation by selectively, reversibly blocking dihydro-orotate dehydrogenase, which is a key mitochondrial enzyme for pyrimidine de novo biosynthesis [106, 107]. To date, there have been few reports on the effects of teriflunomide on DCs, mainly coming from other autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, autoimmune uveitis, etc. Leflunomide, teriflunomide and the derivative (FK778) have been evidenced to inhibit DC maturation and migration, leading to an impaired potency to produce the pro-inflammatory cytokines/chemokines and to initiate proliferation and polarization of CD4+ T cells to TH1/TH17 cells [108,109,110,111,112]. These events are related to the suppression of NF-κB and activator protein-1 signaling pathways apart from the inhibition of pyrimidine synthesis [110,111,112].
Monoclonal antibodies
Several monoclonal antibodies, such as alemtuzumab (anti-CD52), natalizumab (anti-α4 integrin), daclizumab (anti-CD25), ocrelizumab (anti-CD20), and ofatumumab (anti-CD20), most of which target to T or B cells, have been approved for MS therapy. There are evidences suggesting that these antibodies can impact DCs’ function.
Alemtuzumab (anti-CD52) can induce depletion and repopulation of T and B cells, also lead to a significant decline in the subsets of circulating DCs and a relative increase in the regulatory and memory DC phenotypes, promoting the restoration of tolerogenic networks [113,114,115]. In contrast, granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-23 production by DCs remained unaltered upon alemtuzumab application [114]. Most recently, Barbour et al. reported that anti-mouse CD52 monoclonal antibody could significantly ameliorate EAE severity, with increased expression of MHC-II and co-stimulatory molecules on peripheral DCs at first day post-injection and down-regulated level at 3 week post-injection [116].
Natalizumab, a recombinant humanized monoclonal antibody targeting the α4 subunit of α4β1- and α4β7-integrins that act as adhesion molecules in a wide range of human leukocytes including DCs, interferes with the migration of peripheral immune cells to the CNS [9, 117]. A detailed histological characterization found that the number of DCs, but not macrophages, microglia or T cells, within lesions decreased with the prolonged treatment duration [40]. Another study indicated that natalizumab could remarkably reduce the expression of MHC molecules and the numbers of DCs and CD4+ T cells in cerebral perivascular spaces of the patients with MS [118]. Moreover, DCs showed dose-dependent decrease of very late activation antigen 4 (VLA-4) expression levels and impaired capacity to prime antigen-specific T cell responses [119]. Notably, natalizumab therapy could enhance the expression of molecules with tolerogenic function including HLA-G, HLA-DR, PD-L1 and that of molecules with migratory function such as CCR7 on pDCs in patients with MS [120]. These findings suggest that DCs may be the primary effector cells of natalizumab therapy. The anti-inflammatory effects of natalizumab may be largely due to the reduced CNS infiltration of DCs and functional impairment of DC-T interactions as well as shift toward the tolerogenic phenotype of DCs.
Daclizumab is a humanized monoclonal antibody targeting IL-2 receptor α (IL-2Rα) chain (CD25) [121]. Antigen-activated DCs express IL-2Rα, representing an alternative cell type susceptible to daclizumab blockade. Given that the ability to secrete IL-2 endows mature DCs with unique T cell stimulatory capacity [122], daclizumab can block IL-2 trans-presentation by activated DCs to primed T cells, consequently restricting initial steps of antigen-specific T cell activation and expansion [123]. In addition, IL-2 signaling is critically required for the secondary expansion of CD8+ memory T cells [124]. Furthermore, daclizumab might shift the cytokine synthesis pattern of human DCs toward an anti-inflammatory TH2 profile in vitro [125]. Of note, however, IL-2 signaling is also critical for the development and function of CD4+CD25+FOXP3+ Tregs and the neutralization of IL-2 leads to induction of autoimmune diseases [126, 127].
Mitoxantrone
Mitoxantrone is a chemotherapeutic agent approved for treatment of progressive and worsening MS based on its immunosuppressive properties. Mitoxantrone inserts itself within DNA strands, thereby disturbing the proliferation of various cells, particularly B cells, T cells, and macrophages. It has selective immune effects by inhibiting the production of pro-inflammatory cytokines, such as IFN-γ, TNF-α and IL-2 [128]. With regard to DCs, mitoxantrone can interfere with their antigen-presenting ability and induce programmed cell death or cell lysis [129]. Taken together, the effects of mitoxantrone on DCs are nonspecific.
Cladribine
Cladribine (2-chlorodeoxyadenosine) is a synthetic purine nucleoside analog, resulting in a selective diminution in peripheral lymphocytes by disrupting DNA replication and repair [130]. In addition to the well-characterized cytotoxic activity toward lymphocytes, cladribine also exerts immunomodulatory effects on DCs. Apart from the capacity to induce apoptosis of DCs [131], cladribine can influence the maturation and T cell priming capacity of DCs, mediating T cell differentiation shift to TH2 polarization [132]. Interestingly, in vitro experiments showed that cladribine in therapeutic relevant concentrations could not induce the apoptosis of differentiated macrophages or DCs, nor could interfere with their phenotype [133], suggesting that cladribine does not impact the innate immune system under steady state conditions which is consistent with insight from clinical trials [134].
In short, current DMT drugs exert the direct or indirect effects on DCs, primarily influencing DC maturation and migration, resulting in an impaired potency to produce pro-inflammatory molecules and to initiate proliferation and polarization of CD4+ T cells, as well as reducing infiltration of inflammatory cells into the CNS. The underlying mechanisms might imply to search alternative novel targets for MS therapy.
Inducing tolDCs for treatment in MS
Currently approved drugs for the treatment of MS have capacities to provide a remedy to alleviate symptoms via immunomodulatory role, rather than to provide a cure by directly addressing the immune tolerance invalidation. The inhibitory effects of these therapeutics on systemic inflammation may conduce to numerous adverse security risks, such as increased susceptibility to malignancies or opportunistic infections [25]. Novel immunotherapies are emerging to overcome these problems by achieving antigen-specific tolerance, which can suppress the pathogenic autoantigen induced autoimmune responses without compromising the protective immune responses.
Given the specialty of DCs to coordinate innate and adaptive immunity as well as the dual capacity to induce either inflammatory or tolerogenic responses, emerging therapies targeting or exploiting DCs may become a promising approach for MS treatment. DC-based therapy currently includes two major approaches [13]. One approach achieves global immune regulation by selectively targeting the dysregulated DC functions, such as maturation, antigen uptake, cytokine production or migration, or even by depleting DC subsets with key pathogenic roles. Another approach aims to restore the immune tolerance via DC-based autoantigen-specific therapeutic intervention by loading autoantigens to DCs [13]. Most of the current DMT drugs effect on DCs primarily via the former as described above.
Ex vivo tolDC induction strategies are typically based on reeducation of patient-derived DCs by various tolerance-inducing pharmacological agents (e.g., vitamin D3, aryl hydrocarbon receptor ligands, such as 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), and IL-27, etc.). The induced tolDCs will subsequently be reinfused into the body, presenting tolerogenic signals to T cells to suppress pathogenic autoimmune responses in a nonspecific manner [135,136,137]. Some tolDC-based vaccines for the treatment of MS have been shown to be safe and feasible in several phase I clinical trials [138, 139]. However, this kind of therapeutic strategies requires complicated handling and exorbitant expenditure. Due to the personalized nature of tolDCs generated in this way, its clinical application is limited.
In recent decades, in situ elicitation of a tolerogenic phenotype of DCs by specifically targeting varieties of cell-surface receptors has provided an alternative route to overcome these limitations and has been showing great promise in MS therapy, which will be discussed in detail in the following sections.
In vivo induction of nonspecific tolDCs
Induction of nonspecific tolDCs in vivo via several cytokines or by activating aryl hydrocarbon receptors has been well-summarized by Takenaka and Quintana [26]. The immune checkpoints, such as PD-1/PD-L1 and CTLA-4 axis, are imperative for modulating the balance between immunity and tolerance.
PD-1 blockade or PD-L1 knockout could accelerate disease onset and exacerbate disease severity of EAE due to increased lymphocyte infiltration, antigen-specific T cell activation and cytokine production in the CNS [140, 141]. In contrast, up-regulation of PD-L1 on DCs via DNA demethylation could suppress EAE progression [2]. Signals delivered by PD-L1 via PD-L1-IgG2aFc fusion protein could exhibit a significant and long-lasting effect on disease severity by selectively interfering with TH17 responses [142]. Recently, we demonstrated that tolDCs induced by 1,25-dihydroxyvitamin D3 could exert therapeutic effects on EAE mice through the enhancement of PD-1/PD-L1 signaling pathway [143]. Therefore, the PD-1/PD-L1 axis may play a vital role in autoimmune conditions including MS/EAE and might be a promising target for novel immunotherapy.
CTLA-4 is capable to bind to CD80/CD86 molecules on DCs, transmitting negative co-stimulatory signals via trans-endocytosis of the B7-family molecules or regulation of tryptophan catabolism [144, 145]. Human DCs treated with CTLA4–IgG1Fc fusion protein are in an autophagy-deficient status with reduced immunogenic potential, probably due to the activation of the PI3K/Akt/mTOR pathway and FoxO1 nuclear exclusion and consequently down-regulated autophagosome formation [146]. Tregs could ameliorate autoimmunity by restraining DC function via CTLA-4-dependent down-regulation of autophagy [146]. These data suggest that CTLA-4 could mediate reverse signaling in DCs, which is beneficial for the control of autoimmune responses.
In addition, CD83 represents a promising immune checkpoint, which exists in a membrane-bound state (mCD83) or a soluble isoform (sCD83) [147]. Originally mCD83 as a marker for mature DCs has been shown to be widely distributed in a great variety of cell types (e.g., activated T and B lymphocytes, Tregs, as well as thymic epithelial cells) and to play vital roles in the orchestration of immunity and tolerance [147]. Several in vitro and in vivo studies have revealed that sCD83 has immunosuppressive properties by inducing tolDCs and suppressing DC-mediated T cell activation [148,149,150,151,152,153]. Strikingly, sCD83 treatment could significantly ameliorate the symptoms of EAE, with strongly reduced cytokine production, T cell proliferation, and leukocyte infiltration into the CNS [153]. Furthermore, DC-specific mCD83 deficiency could confer an overactivated DC phenotype, characterized by an enhanced capacity to induce antigen-specific T cell proliferation and TH17 differentiation, as well as to impair the suppressive function of Tregs, leading to dramatically aggravated immune responses in the EAE model due to the dysregulation of tolerance mechanisms [154]. These data provide new insights into the roles of CD83 in DCs and its therapeutic potential in MS/EAE.
In vivo induction of autoantigen-specific tolDCs via targeting receptors on DCs
The effective therapeutic strategies designed for in vivo autoantigen-specific tolDC induction are supposed to deliver controlled amounts of disease-associated autoantigens into the antigen-processing and presentation procedures. Several strategies explored involves conjugating antigens with antibodies specific for the cell-surface receptors or with specific glycan structures that act as ligands for these receptors, as well as loading antigens into nanoparticles or liposomes [10, 11, 155]. Ideally, engineering materials should be uptaken by DCs without inducing a specific immune response, which can be achieved by co-delivery of tolerogenic agents (e.g., dexamethasone, rapamycin, IL-10, ITE and vitamin D3) together with disease antigens [156,157,158,159,160,161]. The most promising receptors currently being explored as potential feasible targets for in vivo tolDC generation will be discussed as the below (Table 2).
DC receptor for endocytosis-205 (DEC205)
One of the first and most frequently used receptors for in vivo targeting of DCs is DC receptor for endocytosis-205 (DEC205, also known as CD205). DEC205, a type I transmembrane C-type lectin receptor (CLR) homologous to the macrophage mannose receptor, is an endocytic receptor highly expressed by DC subsets [162]. DEC205-mediated endocytosis transfers captured antigens directly from the extracellular space to late endosomes or lysosomes rich in MHC molecules, entailing a greatly improved efficiency of antigen presentation [162, 163]. DEC205 provides an efficient receptor-based mechanism for DCs to process and present anti-DEC205 antibody-coupled antigens in vivo, resulting in peripheral T cell tolerance in the steady state or strong immunogenic responses in the presence of a maturation stimulus [163, 164]. Since then, DEC205 targeting has been explored in a variety of animal models of autoimmune diseases including EAE for MS. For example, several studies have demonstrated that delivery of autoantigen MOG peptide to DCs by anti-DEC205-MOG conjugates can lead to generation and expansion of IL-10-producing Tregs, deletion of MOG-specific T cells, down-regulation of TH1/TH17 cell activity, which can delay disease onset and ameliorate symptoms of EAE consequently [165,166,167], similar to the phenomena observed after anti-DEC205-mediated proteolipid protein (PLP) delivery to DCs in PLP-induced EAE [168]. Furthermore, it is interesting that migratory DCs induce MOG-specific Tregs more potently than lymphoid-resident DCs in the steady state [167]. In consequence, these data corroborate the potential of targeting DC via DEC205 to induce immune tolerance, protecting against MS and other autoimmune diseases.
However, unlike mouse DEC205 predominantly expressed by DCs, human DEC205 is expressed on more populations of leukocytes, including myeloid blood DCs and monocytes with relatively high levels of DEC205, B lymphocytes with moderate levels, and pDCs, T lymphocytes and NK cells with low levels [169]. Kato et al. reported that immature DCs expressed low levels of DEC205, which was significantly increased during activation, suggesting that DEC205 is an activation-associated molecule [170]. The broader expression pattern of human DEC205 could be an impediment for the development of clinical DEC205-mediated DC targeting strategies.
Mannose receptor (MR)
The mannose receptor (MR, also known as CD206) is a type I transmembrane CLR mainly expressed by macrophages and DCs. In human DCs, MR has been demonstrated to mediate efficient presentation to T cells with high capacity and broad specificity receptors for glycosylated antigens (e.g., mannose, glucose, maltose, fucose and GlcNAc) [171]. In mice, the MR is expressed by a variety of cell types, including endothelial cells, perivascular microglia and mesangial cells, in addition to macrophages. MR can be detected in cultured moDCs; however, in situ expression of MR on murine DCs remains unknown [172]. In vitro experiments showed that application of a specific anti-MR monoclonal antibody PAM-1 and selected natural ligands such as biglycan and mannosylated lipoarabinomannan could result in an altered profile of cytokines/chemokines in moDCs with the ability to dampen the TH1 immune response and to favor the amplification of TH2 immune response [173]. A limited number of in vivo studies have shown that mannosylated myelin peptides could inhibit the development of EAE by inducing a state of immune tolerance primarily by acting on T cells [174,175,176]. The different expression patterns of MR would be an obstacle to fully replicating the receptor function in animal models and exploring the feasibility of MR-mediated DC targeting strategies.
Langerin
Langerin (CD207), a type II membrane-associated C-type lectin, acts as an endocytic receptor on surfaces of Langerhans cells as well as DC subsets often co-expressing with DEC205 receptor, mediating efficient antigen presentation in vivo through capture and internalization of various ligands (e.g., fucose, mannose, n-acetylglucosamine, and sulfated sugars) [177]. Steady-state migratory RelB+langerin+ dermal DCs with a partially mature phenotype (MHC-IIintCD86intCD40hiCCR7+), rather than epidermal Langerhans cells or lymphoid-resident DCs, can mediate peripheral induction and expansion of the antigen-specific Tregs in draining lymph nodes of mice using endogenous TGF-β [178]. In addition, lung langerin+ migratory DCs are capable to drive Treg cell differentiation [167]. In contrast, langerin+CD103+ DCs are superior to other DC subsets in stimulating myelin-reactive T cell proliferation and TH1/TH17 cell differentiation in a GM-CSF-dependent manner, while deletion of this DC subset in vivo confers resistance to EAE [179]. Delivery of autoantigen MOG peptide to skin and lung langerin+CD103+ migratory DCs by anti-langerin–MOG coupling can result in the generation and expansion of MOG-specific Tregs, consequently lessening symptom severity of EAE [167]. However, Flacher et al. reported that langerin+ dermal DCs targeting by anti-langerin–ovalbumin coupling could trigger long-lasting cytotoxic CD8+ T responses in the presence of additional adjuvants including the TLR3 ligand poly(I:C) and anti-CD40 agonist antibody, while Langerhans cells could induce cross-tolerance in similar conditions [180]. These data mark langerin as a promising target for in vivo delivery of self-antigens to DCs to protect against EAE; however, the effects can be affected by the sub-populations and mature status of DCs as well as the intensity of the activation signals.
Especially, in humans, langerin is expressed at low levels on DCs isolated from liver, lung, dermis and tissue-draining lymph nodes, in addition to being highly expressed on epidermal Langerhans cells [181]. Importantly, the expression of langerin on DC subsets in humans distinct from that in mice. Langerin is restricted to the CD1c+ DC subset (cDC2) in humans which is homologous to CD11b+ DCs in mice, while langerin is expressed by the XCR1+ DC subset (cDC1) of mice. Interestingly, langerin has not been identified on the freshly isolated CD1c+ blood DCs, but can be rapidly induced by TGF-β or serum via an activin receptor-like kinase 3-dependent pathway [181]. Therefore, langerin targeting strategies leads to different outcomes in the experiments with humans and mice, which may be problematic in the clinical translation of these strategies.
DC-specific ICAM-3 grabbing non-integrin (DC-SIGN)
DC-specific ICAM-3 grabbing non-integrin (DC-SIGN) is a type II CLR, which is highly expressed on the surface of immature DCs but down-regulates upon maturation [182]. DC-SIGN is capable to recognize both exogenous ligands derived from various glycans-containing pathogens and endogenous glycoproteins (e.g., ICAM-3, ICAM-2, Mac-1, MOG, etc.) [183]. DC-SIGN was originally described as an adhesion molecule capable to bind ICAM-3 on naïve T cells with high affinity, facilitating initial DC-T interaction and DC-mediated T cell proliferation and activation [184]. DC-SIGN can bind to ICAM-2 on endothelial cells, regulating chemokine-induced trans-endothelial migration of DCs [185]. Its intracellular domain includes molecular motifs capable to activate Raf-1/NF-κB signaling pathway and subsequently regulates DC maturation [186]. Moreover, the internalization motifs of DC-SIGN are indicative of a role in antigen processing and presentation to T cells [187]. Hence, DC-SIGN is involved in regulating multiple aspects of immune function, including DC trafficking and maturation, antigen uptake and presentation, as well as DC-T interactions.
Interestingly, DC-SIGN contributes to pro-inflammatory (TH1/TH17) or anti-inflammatory (TH2) immune responses through the activation of distinct signaling cascades triggered by mannose or fucose, respectively. Mannose-rich antigens induce the recruitment of kinase Raf-1 via the DC-SIGN signalosome consisting of scaffold proteins LSP1, KSR1 and CNK, resulting in the acetylation of the NF-κB subunit p65 and thereby enhancing the production of pro-inflammatory cytokines to promote TH1/TH17 response [186, 188]. In contrast, fucose-rich antigens or fucosylated glycans favor adaptive TH2 immunity via activation of atypical NF-κB family member Bcl3 in an IKKε-CYLD-dependent way [189].
The relative specificity of DC-SIGN for DCs and its dynamic immunoregulatory roles endow DC-SIGN with potential as a molecular target to regulate the phenotype of immune response. Arosio et al. presented a potent DC-SIGN targeting antigen delivery device that developed using gold nanoparticles functionalized with α-fucosyl-β-alanyl amide, and with neutral effects toward DC maturation and IL-10 production [190]. When loaded with rapamycin, porous silicon nanoparticles targeting DC-SIGN could be taken up by splenic and peripheral blood DCs and enhance the generation of Tregs [191]. In consequence, DC-SIGN-mediated DC targeting strategy may be a feasible approach to induce immune tolerance in vivo.
Currently, however, DC-SIGN has rarely been explored as a DC target in MS/EAE, probably due to the difficulty of fully replicating its function in animal models. Further studies should focus on the homology of DC-SIGN receptor and its feasibility in DC-targeted immunotherapy.
DC immunoreceptor (DCIR, CLEC4A)
DC immunoreceptor (DCIR), another member of type II CLRs, contains a carbohydrate recognition domain in its extracellular portion and an immunoreceptor tyrosine-based inhibition motif (ITIM) in the cytoplasmic portion. DCIR is expressed on various cell types including myeloid DCs, pDCs, immature and mature moDCs, macrophages, monocytes, B lymphocytes and neutrophils, and functions as an inhibitory receptor [192]. DCIR is considered as an APC receptor that is efficiently internalized into human moDCs in a clathrin-dependent manner upon triggering with anti-DCIR monoclonal antibodies. DCIR triggering can down-regulate TLR8-induced production of IL-12 and TNF-α, while do affect neither TLR4-/TLR8-mediated CD80 and CD86 up-regulation nor TLR2-/TLR3-/TLR4-mediated cytokine production [193]. Similarly, DCIR can be readily endocytosed into human pDCs in a clathrin-dependent manner upon receptor triggering. DCIR triggering can negatively affect TLR9-mediated IFN-α production while do not up-regulate the expression of co-stimulatory molecules. In turn, TLR9 triggering down-regulate the levels of DCIR on pDC maturation [194]. Furthermore, Dcir−/− mice could develop exacerbated EAE with severe demyelination due to excess infiltration of DCs and T cells, indicating DCIR is a vital negative regulator of DC expansion [195]. Collectively, these data well elucidate the potential importance of DCIR in modulating DC function and DCIR/TLR cross-talk in maintaining the immune homeostasis. In addition, antigens targeted to DCs via DCIR are presented to T cells [194]. Therefore, delivery of autoantigen to DCs by targeting DCIR is a novel potential strategy to induce immunological tolerance.
Several studies have been conducted to investigate the potentials of DCIR targeting strategy in EAE treatment. Targeting DCs with anti-DCIR2-PLP139–151 fusion antibody could ameliorate EAE by deleting pathogenic TH1/TH17 cells as well as inducing expansion and activation of pre-formed Treg cells rather than inducing de novo generation of Tregs from naïve CD4+ T cell precursors [196]. However, antigen delivery with anti-DCIR2 was less efficient than anti-DEC205 or anti-langerin antibodies for the generation of MOG-specific Tregs, which was independent of administration route, time course, antibody dose, or antigen type [167]. Thus, DCIR2 may be a promising candidate for in vivo antigen delivery in mice, which needs more studies to validate the effectiveness and feasibility of DCIR2-mediated DC targeting in human settings.
DC NK lectin group receptor-1 (DNGR-1, CLEC9A)
DC NK lectin group receptor-1 (DNGR-1, also known as CLEC9A), a type II transmembrane CLR, has attracted special attention due to its restricted expression pattern in human and mice DCs. DNGR-1 is selectively expressed by BDCA3+ myeloid DCs (cDC1s) in humans and by homologous CD8α+ DCs in mice, mediating endocytosis after sensing necrotic cells [197]. This receptor contains a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM)-like motif, which can recruit Syk kinase and induce the expression of pro-inflammatory cytokines [197, 198]. DNGR-1 triggering via anti-DNGR-1-antigen coupling can efficiently lead to receptor internalization and (cross-)presentation by human BDCA3+ myeloid DCs or murine CD8α+ DC subset to antigen-specific T cells, while anti-DNGR-1 antibody alone did not affect the maturation of DCs [199].
The restricted expression and endocytic property of DNGR-1 endow it potential as a promising DC target for in vivo antigen delivery. In addition, DNGR-1 activation can alleviate tissue damage-related immunopathology by dampening neutrophil recruitment [200]. In the steady state, administration of anti-DNGR-1-antigen conjugates could promote the differentiation into Tregs from naïve CD4+ T cells induced by CD8α+ DCs. However, co-administration with distinct adjuvants such as poly(I:C) and curdlan could prevent induced tolerance and drive TH1 and TH17 immune response, respectively [201]. Besides, targeting antigen to mouse DCs via DNGR-1 could induce strong CD4+ T cell responses biased toward a follicular helper phenotype [202]. Taken together, DNGR-1-mediated DC targeting strategies can induce tolerance or immunity depending on the type of antigen and adjuvant used. A novel protein-based DNGR-1 binding agent with a single domain nanobody has been shown to have DC targeting properties, exerting therapeutic effects on EAE when conjugated with IFNQ124R [203]. However, the sample size of this experiment was limited. The optimal conditions for tolerance induction via DNGR-1 targeting remain to be extensively studied.
Myeloid inhibitory C-type lectin receptor (MICL, CLEC12A)
The myeloid inhibitory C-type lectin receptor (MICL, also known as CLEC12A, CD371) is another candidate DC-specific antigen targeting. Both murine and human MICL can selectively sense uric acid crystals, potentiating cell death-induced immunity under sterile conditions [204]. In mice, MICL is expressed mainly by CD8+ DCs and pDCs and by a small subset of CD8− DCs as well as macrophages, monocytes and B lymphocytes. Similarly, MICL is expressed primarily by BDCA3+ DCs and pDCs as well as monocytes, macrophages and granulocytes in humans [205]. Its endocytic property has endowed this receptor with potential as an alternative DC target for antigen delivery. Delivery of ovalbumin to cDCs by conjugating to anti-MICL monoclonal antibodies could enhance antibody responses, while antibodies alone induced only moderate responses [205]. Interestingly, blockade of MICL with antibodies or MICL knockout might delay the onset and attenuate the severity of EAE by impairing DC binding and transmigration across the BBB [206]. However, the MICL-mediated targeting DC-induced immune tolerance strategies remains in its infancy.
Sialic-acid binding immunoglobulin-type lectins (Siglecs)
Sialic-acid binding immunoglobulin-type lectins (Siglecs) are a family of immunomodulatory receptors expressed on a wide range of immune cells (NK cells, B and T lymphocytes, macrophages, microglia, monocytes, cDCs and pDCs, etc.) with different expressing patterns [207], enabling them to participate in diverse immune responses. In addition, the conserved Siglec-4, also known as myelin-associated glycoprotein, is expressed on oligodendrocytes and Schwann cells, playing a role in adhesion and signaling in glia–glia and/or axon-glia interactions and protecting neurons from acute toxic insults via a ganglioside-dependent mechanism [208]. The Siglec family functions via binding to sialic acids, which are present on the membrane of all living cells and act as a self-associated molecular pattern (SAMP) [207]. In the cytoplasmic domains, most Siglecs, such as Siglec-3 (CD33)-related Siglecs (e.g., Siglec-3, -5 till -12, -17, -E, -F and -G) and the conserved Siglec-2/4 carry regulatory motifs (e.g., ITIM, immunoreceptor tyrosine-based switch motif (ITSM), etc.), inducing inhibitory signaling and functioning as immune checkpoints to suppress unwanted immune responses through recruiting and activating Src homology 2 domain containing protein tyrosine phosphatase (SHP)-1 and SHP-2 [209]. Several other Siglecs (Siglec-14, -15, -16 and -H) possess amino acid residues in transmembrane domain which can correlate with activating the adaptor proteins, such as DNAX activation protein of 12 kDa (DAP12), an ITAM-bearing adaptor involved in cell activation [210]. Therefore, the Siglec family has the ability to suppress or augment immune responses.
The immunomodulatory capacity and endocytic property of Siglecs endows them with potential as targets in treatment of autoimmune diseases. In vivo experiments found that antigen (MOG peptides) delivery to murine pDCs by targeting Siglec-H could induce a decrease in CD4+ T cell expansion and TH1/TH17 cell polarization, without conversion to Tregs or deviation to TH2 cells, which could subsequently delay the onset and reduce the severity in EAE [211]. Likewise, both in vivo in EAE and in vitro, sialic acid-modified antigens targeting Siglec-E on DCs could induce an antigen-specific tolerogenic programming in DCs, dampening TH1/TH17 cell expansion and enhancing Tregs [212]. Taken together, targeting DCs through Siglecs might be a promising strategy to induce tolerogenic immune responses for treatment of MS. However, translation of research outcomes from animals to humans might be hampered by the ethnic heterogeneity of this receptor. Therefore, more preclinical studies are imperative to elucidate the feasibility.
TLRs
TLRs are a collection of transmembrane proteins that induce overlapping yet distinct gene expression patterns, contributing to pro-inflammatory responses to fight infection/cancer [213, 214]. Notably, many TLRs, such as TLR2, TLR4, and TLR9, have been demonstrated to act as pivotal modulators of autoimmune process in MS/EAE [215,216,217].
TLR2 was reported to be expressed by oligodendrocytes and up-regulated in MS lesions, participating in the hyaluronan-mediated inhibition of oligodendrocyte precursor cell maturation and remyelination in a MyD88-dependent manner [215]. Interestingly, helminth antigens could exert strong regulatory effects on DCs and B cells via TLR2 in a MyD88-dependent or -independent manner [218]. A recent research reported that cell surface β-glucan polysaccharides of yeast could induce generation of Tregs from naïve T cells via a Dectin1–Cox2 signaling pathway in DCs and restrain TH1 polarization of effector T cells in a TLR2-dependent manner, thereby exerting powerful anti-inflammatory effects [219]. Thus, TLR2 may be a possible alternative DC target to modulate immune response. However, Shaw et al. proposed that receptor interacting protein 2 (RIP2), rather than TLR2, played a key role in the activation of CNS-infiltrating DCs [220]. In addition, the wide expression of TLR2 in other innate immune cells, such as macrophages, monocytes and microglia, also hampers the exploration of TLR2 targeting strategies.
With regard to TLR4, its triggering can induce different effects in different cell types. In mice deficiency of TLR4 solely in CD4+ T cells, the symptoms of EAE were almost completely abrogated, primarily due to the blunted TH1 and TH17 responses [216]. However, TLR-4 stimulation could activate DCs sufficiently to drive pathogenic T cell function in EAE [221]. Therefore, TLR4 has rarely been explored as a target for DCs.
TLR9, primarily expressed on B cells and pDCs and typically responsible for recognizing microbial cytidine–phosphate–guanosine (CpG) DNA, is up-regulated during EAE course [217]. It was reported that TLR9−/− mice developed EAE with delayed severity compared with wild-type mice [217]. Excitingly, treatment with type A CpG oligodeoxynucleotides (ODNs) could effectively up-regulate Treg percentage in the spleen and inhibit TH1/TH17 immune response in the CNS, resulting in delayed onset of EAE, alleviated demyelination of the spinal cord and reduced severity of the disease. Those events were indicated to be mediated by type A CpG-induced tolerance phenotype of pDCs. Adoptive transfer of pDCs isolated from type A CpG-treated mice could also suppress CNS inflammation and attenuate EAE development [222]. Application of GpG ODNs, a TLR9 antagonistic ligand, could significantly suppress the activation of TH1 cells and induce a shift toward a protective TH2 immune response, alleviating the disease severity of EAE [223, 224]. Considering the intrinsic inflammation induced by delivery vehicles may exacerbate disease, several researchers have designed GpG ODNs self-assembled or co-assembled with MOG peptide into nanostructured polyelectrolyte multilayers/polyplexes to blunt TLR9 signaling to promote immunological tolerance [225,226,227]. Collectively, these data highlight the important role of TLR9 in driving MS/EAE and the potential of TLR9 targeting by specific ligands to induce immune tolerance and consequently alleviate the severity and progression of this disease. Future studies are needed to elucidate the uptake, trafficking and the underlying mechanisms of tolerance induction.
A most recent study reported that nanobody–autoantigen (MOG35–55) conjugates targeting MHC-II complexes on DCs could confer long-lasting protection against autoimmune conditions of EAE through tolerance induction, especially when co-administrated with the glucocorticoid dexamethasone via a cleavable linker [228]. These findings further support the therapeutic value of targeting DC-induced tolerance in MS treatment as well as the superiority of nanobodies for targeted delivery of small-molecule drugs or antigenic peptides. Collectively, the specific antigen delivery via DC targeting strategies mediated by various surface receptors may be promising to induce immune tolerance, which needs to be further explored.
In vivo induction of autoantigen-specific tolDCs via non-inflammatory mRNA vaccines
The ideal strategy for treating autoimmune disorders is to generate autoantigen-specific tolerance in a non-inflammatory setting without suppressing the normal immune response. It is worth noting that in vivo DC-modifying strategies via targeting surface receptors have limitations due to the complexity of receptor expression profiles and overlapping signaling pathways. It is exciting that Ugur Sahin and colleagues has formulated an mRNA vaccine consisting of a lipid nanoparticle packed with a modified mRNA encoding MOG35–55, representing a novel tolDC-inducing approach [229]. The modification, replacing uracil with 1-methylpseudouridine (m1Ψ), aimed to abrogate the activation of TLR signaling and subsequent strong TH1 responses [230, 231]. In mice with MOG35–55-induced EAE, the m1Ψ mRNA vaccine could allow in vivo delivery of MOG peptides into DCs in a non-inflammatory context and induce MOG-specific tolerance, thereby alleviating the demyelination of the spinal cord as well as the clinical symptoms [229]. Thus, the m1Ψ mRNA vaccine encoding specific autoantigens is a promising approach to confer bystander tolerance and enable control of multiple autoimmune disorders including MS/EAE.
Conclusions
Currently approved drugs for the treatment of MS provide a remedy to alleviate symptoms via immunomodulatory effects. In recent decades, reshaping immune balance achieved through inducing immune tolerance has proven to be a promising strategy for MS therapy. The unique ability of DCs to coordinate innate and adaptive immunity as well as the dual capacity to induce either immunity or tolerance makes DCs as an attractive target. Several surface receptors on DCs have been explored as promising targets to induce DC-mediated immune tolerance via delivery of specific autoantigens. Nanobodies or nanostructured polyelectrolyte multilayers have shown great advantages and prospects as delivery vehicles. However, the complexity of receptor expression profiles on various immune cells and overlapping signaling pathways have probably limited the development of this therapeutic strategy. Micro-environmental factors are also involved in modulating the immune effects of receptor-mediated DC-targeting strategies. In addition, incompatibilities between DCs in mice and human beings still hamper the clinical translation of research findings. Strikingly, selective delivery of autoantigens into DCs via non-inflammatory mRNA vaccines is an effective way to induce and maintain natural immune tolerance, promising for the treatment of a variety of autoimmune diseases. Further studies are supposed to identify the molecular mechanisms responsible for the tolerogenic phenotype of DCs to guide feasible immunotherapeutic strategies.
Availability of data and materials
Not applicable.
Abbreviations
- APCs:
-
Antigen-presenting cells
- BBB:
-
Blood–brain barrier
- CD:
-
Cluster of differentiation
- cDC1:
-
Type 1 conventional DC subset
- cDC2:
-
Type 2 conventional DC subset
- CLR:
-
C-type lectin receptor
- CNS:
-
Central nervous system
- CpG:
-
Cytidine–phosphate–guanosine
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen 4
- DAP12:
-
DNAX activation protein of 12 kDa
- DCIR:
-
DC immunoreceptor
- DC-SIGN:
-
DC-specific ICAM-3 grabbing non-integrin
- DCs:
-
Dendritic cells
- DEC205:
-
DC receptor for endocytosis-205
- DMF:
-
Dimethyl fumarate
- DMT:
-
Disease-modifying therapies
- DNGR-1:
-
DC NK lectin group receptor-1
- EAE:
-
Experimental autoimmune encephalomyelitis
- ERK:
-
Extracellular signal-regulated kinase
- Flt-3L:
-
FMS-like tyrosine kinase 3 ligand
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GPCR:
-
G protein-coupled receptor
- HCAR2 :
-
Hydroxycarboxylic acid receptor 2
- ICAM:
-
Intercellular cell adhesion molecule
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- IRAK4:
-
IL-1R-associated kinase 4
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
- ITE:
-
2-(1′H-Indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester
- ITIM:
-
Immunoreceptor tyrosine-based inhibition motif
- ITSM:
-
Immunoreceptor tyrosine-based switch motif
- LPS:
-
Lipopolysaccharide
- MBP:
-
Myelin basic protein
- mCD83:
-
Membrane-bound CD83
- MHC:
-
Major histocompatibility complex
- MICL:
-
Myeloid inhibitory C-type lectin receptor
- MMF:
-
Monomethyl fumarate
- MMP:
-
Matrix metalloproteinase
- MR:
-
Mannose receptor
- MSK1:
-
Mitogen stress-activated kinase 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor
- moDC:
-
Monocyte-derived DC subset
- MOG:
-
Myelin oligodendrocyte glycoprotein
- MS:
-
Multiple sclerosis
- m1Ψ:
-
1-Methylpseudouridine
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear transcription factor-κB
- NK:
-
Natural killer
- ODNs:
-
Oligodeoxynucleotides
- pDC:
-
Plasmacytoid DC subset
- PD-L:
-
Programmed cell death ligand
- PECAM-1:
-
Platelet endothelial cell adhesion molecule 1
- PLP:
-
Proteolipid protein
- RIP2:
-
Receptor interacting protein 2
- RRMS:
-
Relapsing–remitting MS
- SAMP:
-
Self-associated molecular pattern
- sCD83:
-
Soluble CD83
- SHP:
-
Src homology 2 domain containing protein tyrosine phosphatase
- Siglecs:
-
Sialic-acid binding immunoglobulin-type lectins
- SPMS:
-
Secondary-progressive MS
- STAT:
-
Signal transducer and activator of transcription
- S1P:
-
Sphingosine 1-phosphate
- S1PR:
-
Sphingosine 1-phosphate receptor
- SPL:
-
S1P lyase
- TCRs:
-
T cell antigen receptors
- TGF:
-
Transforming growth factor
- TH :
-
T helper cells
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- tolDCs:
-
Tolerogenic DCs
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- Tregs:
-
Regulatory T cells
- VLA-4:
-
Very late activation antigen 4
References
Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400.
Chang CB, Lee SP, Chen WM, Wang CM, Song YC, Chan MW, et al. Dendritic cell upregulation of programmed death ligand-1 via DNA demethylation inhibits experimental autoimmune encephalomyelitis. J Autoimmun. 2020;107: 102362.
IMSG Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365: eaav7188.
Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. 2011;76:540–8.
Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012;4: a006957.
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–58.
Devi KS, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. 2017;39:137–52.
Giles DA, Duncker PC, Wilkinson NM, Washnock-Schmid JM, Segal BM. CNS-resident classical DCs play a critical role in CNS autoimmune disease. J Clin Invest. 2018;128:5322–34.
Manouchehri N, Hussain RZ, Cravens PD, Esaulova E, Artyomov MN, Edelson BT, et al. CD11c(+)CD88(+)CD317(+) myeloid cells are critical mediators of persistent CNS autoimmunity. Proc Natl Acad Sci USA. 2021;118: e2014492118.
Castenmiller C, Keumatio-Doungtsop BC, van Ree R, de Jong EC, van Kooyk Y. Tolerogenic immunotherapy: targeting DC surface receptors to induce antigen-specific tolerance. Front Immunol. 2021;12: 643240.
Cifuentes-Rius A, Desai A, Yuen D, Johnston APR, Voelcker NH. Inducing immune tolerance with dendritic cell-targeting nanomedicines. Nat Nanotechnol. 2021;16:37–46.
Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604.
Coutant F, Miossec P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol. 2016;12:703–15.
Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3–20.
O’Keeffe M, Mok WH, Radford KJ. Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci. 2015;72:4309–25.
Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89–103.
Haniffa M, Collin M, Ginhoux F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv Immunol. 2013;120:1–49.
Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356: eaah4573.
Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallée VP, Mendoza A, et al. Transcriptional basis of mouse and human dendritic cell heterogeneity. Cell. 2019;179:846-863.e24.
Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, et al. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity. 2019;51:573-89.e8.
Ginhoux F, Guilliams M, Merad M. Expanding dendritic cell nomenclature in the single-cell era. Nat Rev Immunol. 2022;22:67–8.
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52.
Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9.
Ardouin L, Luche H, Chelbi R, Carpentier S, Shawket A, Montanana Sanchis F, et al. Broad and largely concordant molecular changes characterize tolerogenic and immunogenic dendritic cell maturation in thymus and periphery. Immunity. 2016;45:305–18.
Moorman CD, Sohn SJ, Phee H. Emerging therapeutics for immune tolerance: tolerogenic vaccines, T cell therapy, and IL-2 therapy. Front Immunol. 2021;12: 657768.
Takenaka MC, Quintana FJ. Tolerogenic dendritic cells. Semin Immunopathol. 2017;39:113–20.
Funes SC, Manrique de Lara A, Altamirano-Lagos MJ, Mackern-Oberti JP, Escobar-Vera J, Kalergis AM. Immune checkpoints and the regulation of tolerogenicity in dendritic cells: implications for autoimmunity and immunotherapy. Autoimmun Rev. 2019;18:359–68.
Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–59.
Martín-Gayo E, Sierra-Filardi E, Corbí AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–75.
Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5.
Vobořil M, Brabec T, Dobeš J, Šplíchalová I, Březina J, Čepková A, et al. Toll-like receptor signaling in thymic epithelium controls monocyte-derived dendritic cell recruitment and Treg generation. Nat Commun. 2020;11:2361.
Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–100.
Torres-Aguilar H, Aguilar-Ruiz SR, González-Pérez G, Munguía R, Bajaña S, Meraz-Ríos MA, et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010;184:1765–75.
Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–75.
Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50:302–16.
Zlotnikov-Klionsky Y, Nathansohn-Levi B, Shezen E, Rosen C, Kagan S, Bar-On L, et al. Perforin-positive dendritic cells exhibit an immuno-regulatory role in metabolic syndrome and autoimmunity. Immunity. 2015;43:776–87.
Comabella M, Montalban X, Munz C, Lunemann JD. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurol. 2010;6:499–507.
Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80.
Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–34.
Häusler D, Akgün K, Stork L, Lassmann H, Ziemssen T, Brück W, et al. CNS inflammation after natalizumab therapy for multiple sclerosis: a retrospective histopathological and CSF cohort study. Brain Pathol. 2021. https://doi.org/10.1111/bpa.12969e12969.
Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–8.
Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci Immunol. 2019;4: eaau8380.
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363: eaat7554.
Keller CW, Kotur MB, Mundt S, Dokalis N, Ligeon LA, Shah AM, et al. CYBB/NOX2 in conventional DCs controls T cell encephalitogenicity during neuroinflammation. Autophagy. 2021;17:1244–58.
Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood–brain barrier through paracellular transmigration and ERK activation. J Neuroinflamm. 2012;9:245.
Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, et al. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci. 2009;29:140–52.
Wang L, Li Z, Ciric B, Safavi F, Zhang GX, Rostami A. Selective depletion of CD11c(+) CD11b(+) dendritic cells partially abrogates tolerogenic effects of intravenous MOG in murine EAE. Eur J Immunol. 2016;46:2454–66.
Karni A, Abraham M, Monsonego A, Cai G, Freeman GJ, Hafler D, et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J Immunol. 2006;177:4196–202.
Sabatino JJ Jr, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20:728–45.
Huang YM, Xiao BG, Ozenci V, Kouwenhoven M, Teleshova N, Fredrikson S, et al. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J Neuroimmunol. 1999;99:82–90.
Thewissen K, Nuyts AH, Deckx N, Van Wijmeersch B, Nagels G, D’Hooghe M, et al. Circulating dendritic cells of multiple sclerosis patients are proinflammatory and their frequency is correlated with MS-associated genetic risk factors. Mult Scler. 2014;20:548–57.
Stasiolek M, Bayas A, Kruse N, Wieczarkowiecz A, Toyka KV, Gold R, et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain. 2006;129:1293–305.
Mishra MK, Yong VW. Myeloid cells—targets of medication in multiple sclerosis. Nat Rev Neurol. 2016;12:539–51.
McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765–79.
Dhib-Jalbut S, Marks S. Interferon-beta mechanisms of action in multiple sclerosis. Neurology. 2010;74(Suppl 1):S17-24.
Martín-Saavedra FM, González-García C, Bravo B, Ballester S. Beta interferon restricts the inflammatory potential of CD4+ cells through the boost of the Th2 phenotype, the inhibition of Th17 response and the prevalence of naturally occurring T regulatory cells. Mol Immunol. 2008;45:4008–19.
Wiesemann E, Sönmez D, Heidenreich F, Windhagen A. Interferon-beta increases the stimulatory capacity of monocyte-derived dendritic cells to induce IL-13, IL-5 and IL-10 in autologous T-cells. J Neuroimmunol. 2002;123:160–9.
Ramgolam VS, Sha Y, Jin J, Zhang X, Markovic-Plese S. IFN-beta inhibits human Th17 cell differentiation. J Immunol. 2009;183:5418–27.
Pennell LM, Fish EN. Interferon-β regulates dendritic cell activation and migration in experimental autoimmune encephalomyelitis. Immunology. 2017;152:439–50.
Comabella M, Lünemann JD, Río J, Sánchez A, López C, Julià E, et al. A type I interferon signature in monocytes is associated with poor response to interferon-beta in multiple sclerosis. Brain. 2009;132:3353–65.
Yen JH, Kong W, Ganea D. IFN-beta inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J Immunol. 2010;184:3478–86.
Aung LL, Fitzgerald-Bocarsly P, Dhib-Jalbut S, Balashov K. Plasmacytoid dendritic cells in multiple sclerosis: chemokine and chemokine receptor modulation by interferon-beta. J Neuroimmunol. 2010;226:158–64.
Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, et al. Interferon-beta enhances monocyte and dendritic cell expression of B7–H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–82.
Yen JH, Ganea D. Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood. 2009;114:1344–54.
Cauwels A, Van Lint S, Catteeuw D, Pang S, Paul F, Rogge E, et al. Targeting interferon activity to dendritic cells enables in vivo tolerization and protection against EAE in mice. J Autoimmun. 2019;97:70–6.
Schrempf W, Ziemssen T. Glatiramer acetate: mechanisms of action in multiple sclerosis. Autoimmun Rev. 2007;6:469–75.
Neuhaus O, Farina C, Yassouridis A, Wiendl H, Then Bergh F, Dose T, et al. Multiple sclerosis: comparison of copolymer-1-reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci U S A. 2000;97:7452–7.
Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA. 2005;102:6449–54.
Racke MK, Lovett-Racke AE, Karandikar NJ. The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology. 2010;74(Suppl 1):S25-30.
Sanna A, Fois ML, Arru G, Huang YM, Link H, Pugliatti M, et al. Glatiramer acetate reduces lymphocyte proliferation and enhances IL-5 and IL-13 production through modulation of monocyte-derived dendritic cells in multiple sclerosis. Clin Exp Immunol. 2006;143:357–62.
Aharoni R, Teitelbaum D, Arnon R, Sela M. Copolymer 1 acts against the immunodominant epitope 82–100 of myelin basic protein by T cell receptor antagonism in addition to major histocompatibility complex blocking. Proc Natl Acad Sci USA. 1999;96:634–9.
Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–8.
Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–43.
Jung S, Siglienti I, Grauer O, Magnus T, Scarlato G, Toyka K. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate. J Neuroimmunol. 2004;148:63–73.
Sellebjerg F, Hesse D, Limborg S, Lund H, Søndergaard HB, Krakauer M, et al. Dendritic cell, monocyte and T cell activation and response to glatiramer acetate in multiple sclerosis. Mult Scler. 2013;19:179–87.
Cartier A, Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 2019;366: eaar5551.
Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, et al. Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc Natl Acad Sci USA. 2017;114:4531–6.
Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8.
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60.
Czeloth N, Bernhardt G, Hofmann F, Genth H, Förster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–7.
Chun J, Giovannoni G, Hunter SF. Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects. Drugs. 2021;81:207–31.
Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, et al. Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci USA. 2017;114:2012–7.
Musella A, Gentile A, Guadalupi L, Rizzo FR, De Vito F, Fresegna D, et al. Central modulation of selective sphingosine-1-phosphate receptor 1 ameliorates experimental multiple sclerosis. Cells. 2020;9:1290.
Tiwari-Woodruff S, Yamate-Morgan H, Sekyi M, Lauderdale K, Hasselmann J, Schubart A. The sphingosine 1-phosphate (S1P) receptor modulator, siponimod decreases oligodendrocyte cell death and axon demyelination in a mouse model of multiple sclerosis (I10.011). Neurology. 2016;86:I10.011.
Zamora-Pineda J, Kumar A, Suh JH, Zhang M, Saba JD. Dendritic cell sphingosine-1-phosphate lyase regulates thymic egress. J Exp Med. 2016;213:2773–91.
Tsai HC, Nguyen K, Hashemi E, Engleman E, Hla T, Han MH. Myeloid sphingosine-1-phosphate receptor 1 is important for CNS autoimmunity and neuroinflammation. J Autoimmun. 2019;105: 102290.
Schulze T, Golfier S, Tabeling C, Räbel K, Gräler MH, Witzenrath M, et al. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25:4024–36.
Müller H, Hofer S, Kaneider N, Neuwirt H, Mosheimer B, Mayer G, et al. The immunomodulator FTY720 interferes with effector functions of human monocyte-derived dendritic cells. Eur J Immunol. 2005;35:533–45.
Thomas K, Sehr T, Proschmann U, Rodriguez-Leal FA, Haase R, Ziemssen T. Fingolimod additionally acts as immunomodulator focused on the innate immune system beyond its prominent effects on lymphocyte recirculation. J Neuroinflamm. 2017;14:41.
Luessi F, Kraus S, Trinschek B, Lerch S, Ploen R, Paterka M, et al. FTY720 (fingolimod) treatment tips the balance towards less immunogenic antigen-presenting cells in patients with multiple sclerosis. Mult Scler. 2015;21:1811–22.
Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–59.
Al-Jaderi Z, Maghazachi AA. Effects of vitamin D3, calcipotriol and FTY720 on the expression of surface molecules and cytolytic activities of human natural killer cells and dendritic cells. Toxins (Basel). 2013;5:1932–47.
Galicia-Rosas G, Pikor N, Schwartz JA, Rojas O, Jian A, Summers-Deluca L, et al. A sphingosine-1-phosphate receptor 1-directed agonist reduces central nervous system inflammation in a plasmacytoid dendritic cell-dependent manner. J Immunol. 2012;189:3700–6.
Wu Q, Wang Q, Mao G, Dowling CA, Lundy SK, Mao-Draayer Y. Dimethyl fumarate selectively reduces memory t cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol. 2017;198:3069–80.
Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92.
Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest. 2014;124:2188–92.
Parodi B, Sanna A, Cedola A, Uccelli A, Kerlero de Rosbo N. Hydroxycarboxylic acid receptor 2, a pleiotropically linked receptor for the multiple sclerosis drug, monomethyl fumarate possible implications for the inflammatory response. Front Immunol. 2021;12: 655212.
Peng H, Guerau-de-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J Biol Chem. 2012;287:28017–26.
Ghoreschi K, Brück J, Kellerer C, Deng C, Peng H, Rothfuss O, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208:2291–303.
Litjens NH, Rademaker M, Ravensbergen B, Rea D, van der Plas MJ, Thio B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004;34:565–75.
Mazzola MA, Raheja R, Regev K, Beynon V, von Glehn F, Paul A, et al. Monomethyl fumarate treatment impairs maturation of human myeloid dendritic cells and their ability to activate T cells. Mult Scler. 2019;25:63–71.
Hammer A, Waschbisch A, Knippertz I, Zinser E, Berg J, Jörg S, et al. Role of nuclear factor (erythroid-derived 2)-like 2 signaling for effects of fumaric acid esters on dendritic cells. Front Immunol. 2017;8:1922.
Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci USA. 2016;113:4777–82.
Zaro BW, Vinogradova EV, Lazar DC, Blewett MM, Suciu RM, Takaya J, et al. Dimethyl fumarate disrupts human innate immune signaling by targeting the IRAK4-MyD88 complex. J Immunol. 2019;202:2737–46.
Muri J, Wolleb H, Broz P, Carreira EM, Kopf M. Electrophilic Nrf2 activators and itaconate inhibit inflammation at low dose and promote IL-1β production and inflammatory apoptosis at high dose. Redox Biol. 2020;36: 101647.
Rückemann K, Fairbanks LD, Carrey EA, Hawrylowicz CM, Richards DF, Kirschbaum B, et al. Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem. 1998;273:21682–91.
Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74:659–74.
Hou J, Wei JM, Yu ZY, Xu Y, Li J, Tang DN. The effect of malononitrilamides (FK778) on phenotypic properties of human peripheral dendritic cells. Transplant Proc. 2009;41:1859–61.
Li Z, Chen X, Chen Y, Li H, Yu J, Li Y, et al. Teriflunomide suppresses T helper cells and dendritic cells to alleviate experimental autoimmune uveitis. Biochem Pharmacol. 2019;170: 113645.
Kirsch BM, Zeyda M, Stuhlmeier K, Grisar J, Smolen JS, Watschinger B, et al. The active metabolite of leflunomide, A77 1726, interferes with dendritic cell function. Arthritis Res Ther. 2005;7:R694-703.
Zeyda M, Kirsch BM, Geyeregger R, Stuhlmeier KM, Zlabinger GJ, Hörl WH, et al. Inhibition of human dendritic cell maturation and function by the novel immunosuppressant FK778. Transplantation. 2005;80:1105–11.
Wu WL, Ho LJ, Chen PC, Tsai YT, Hsu ST, Chang DM, et al. Immunosuppressive effects and mechanisms of leflunomide in dengue virus infection of human dendritic cells. J Clin Immunol. 2011;31:1065–78.
Havari E, Turner MJ, Campos-Rivera J, Shankara S, Nguyen TH, Roberts B, et al. Impact of alemtuzumab treatment on the survival and function of human regulatory T cells in vitro. Immunology. 2014;141:123–31.
Gross CC, Ahmetspahic D, Ruck T, Schulte-Mecklenbeck A, Schwarte K, Jörgens S, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2016;3: e289.
Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: an update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord. 2017;10:343–59.
Barbour M, Wood R, Harte T, Bushell TJ, Jiang HR. Anti-CD52 antibody treatment in murine experimental autoimmune encephalomyelitis induces dynamic and differential modulation of innate immune cells in peripheral immune and central nervous systems. Immunology. 2022;165:312–27.
Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6.
del Pilar MM, Cravens PD, Winger R, Frohman EM, Racke MK, Eagar TN, et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol. 2008;65:1596–603.
de Andrés C, Teijeiro R, Alonso B, Sánchez-Madrid F, Martínez ML, Guzmán de Villoria J, et al. Long-term decrease in VLA-4 expression and functional impairment of dendritic cells during natalizumab therapy in patients with multiple sclerosis. PLoS ONE. 2012;7: e34103.
Moraes AS, Boldrini VO, Dionete AC, Andrade MD, Longhini ALF, Santos I, et al. Decreased neurofilament L chain levels in cerebrospinal fluid and tolerogenic plasmacytoid dendritic cells in natalizumab-treated multiple sclerosis patients—brief research report. Front Cell Neurosci. 2021;15: 705618.
Wiendl H, Gross CC. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol. 2013;9:394–404.
Granucci F, Vizzardelli C, Pavelka N, Feau S, Persico M, Virzi E, et al. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat Immunol. 2001;2:882–8.
Wuest SC, Edwan JH, Martin JF, Han S, Perry JS, Cartagena CM, et al. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat Med. 2011;17:604–9.
Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–3.
Mnasria K, Lagaraine C, Velge-Roussel F, Oueslati R, Lebranchu Y, Baron C. Anti-CD25 antibodies affect cytokine synthesis pattern of human dendritic cells and decrease their ability to prime allogeneic CD4+ T cells. J Leukoc Biol. 2008;84:460–7.
Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–51.
Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35.
Edan G, Morrissey S, Le Page E. Rationale for the use of mitoxantrone in multiple sclerosis. J Neurol Sci. 2004;223:35–9.
Neuhaus O, Wiendl H, Kieseier BC, Archelos JJ, Hemmer B, Stüve O, et al. Multiple sclerosis: mitoxantrone promotes differential effects on immunocompetent cells in vitro. J Neuroimmunol. 2005;168:128–37.
Wiendl H. Cladribine—an old newcomer for pulsed immune reconstitution in MS. Nat Rev Neurol. 2017;13:573–4.
Singh V, Prajeeth CK, Gudi V, Bénardais K, Voss EV, Stangel M. 2-Chlorodeoxyadenosine (cladribine) induces apoptosis in human monocyte-derived dendritic cells. Clin Exp Immunol. 2013;173:288–97.
Kraus SH, Luessi F, Trinschek B, Lerch S, Hubo M, Poisa-Beiro L, et al. Cladribine exerts an immunomodulatory effect on human and murine dendritic cells. Int Immunopharmacol. 2014;18:347–57.
Mathiesen CBK, Rudjord-Levann AM, Gad M, Larsen J, Sellebjerg F, Pedersen AE. Cladribine inhibits secretion of pro-inflammatory cytokines and phagocytosis in human monocyte-derived M1 macrophages in-vitro. Int Immunopharmacol. 2021;91: 107270.
Stuve O, Soelberg Soerensen P, Leist T, Giovannoni G, Hyvert Y, Damian D, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986.
Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:20768–73.
Xie Z, Chen J, Zheng C, Wu J, Cheng Y, Zhu S, et al. 1,25-Dihydroxyvitamin D(3)-induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology. 2017;152:414–24.
Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol. 2013;14:1054–63.
Zubizarreta I, Flórez-Grau G, Vila G, Cabezón R, España C, Andorra M, et al. Immune tolerance in multiple sclerosis and neuromyelitis optica with peptide-loaded tolerogenic dendritic cells in a phase 1b trial. Proc Natl Acad Sci USA. 2019;116:8463–70.
Willekens B, Presas-Rodríguez S, Mansilla MJ, Derdelinckx J, Lee WP, Nijs G, et al. Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): a harmonised study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open. 2019;9: e030309.
Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J Exp Med. 2003;198:71–8.
Ortler S, Leder C, Mittelbronn M, Zozulya AL, Knolle PA, Chen L, et al. B7–H1 restricts neuroantigen-specific T cell responses and confines inflammatory CNS damage: implications for the lesion pathogenesis of multiple sclerosis. Eur J Immunol. 2008;38:1734–44.
Herold M, Posevitz V, Chudyka D, Hucke S, Groß C, Kurth F, et al. B7–H1 selectively controls TH17 differentiation and central nervous system autoimmunity via a novel non-PD-1-mediated pathway. J Immunol. 2015;195:3584–95.
Cao Q, Zheng C, Xie Z, Liu L, Zhu J, Jin T. The change of PD1, PDL1 in experimental autoimmune encephalomyelitis treated by 1,25(OH)(2)D(3). J Neuroimmunol. 2020;338: 577079.
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3.
Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101.
Alissafi T, Banos A, Boon L, Sparwasser T, Ghigo A, Wing K, et al. Tregs restrain dendritic cell autophagy to ameliorate autoimmunity. J Clin Invest. 2017;127:2789–804.
Grosche L, Knippertz I, König C, Royzman D, Wild AB, Zinser E, et al. The CD83 molecule—an important immune checkpoint. Front Immunol. 2020;11:721.
Lechmann M, Krooshoop DJ, Dudziak D, Kremmer E, Kuhnt C, Figdor CG, et al. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J Exp Med. 2001;194:1813–21.
Lan Z, Ge W, Arp J, Jiang J, Liu W, Gordon D, et al. Induction of kidney allograft tolerance by soluble CD83 associated with prevalence of tolerogenic dendritic cells and indoleamine 2,3-dioxygenase. Transplantation. 2010;90:1286–93.
Peckert-Maier K, Schönberg A, Wild AB, Royzman D, Braun G, Stich L, et al. Pre-incubation of corneal donor tissue with sCD83 improves graft survival via the induction of alternatively activated macrophages and tolerogenic dendritic cells. Am J Transplant. 2022;22:438–54.
Lin W, Buscher K, Wang B, Fan Z, Song N, Li P, et al. Soluble CD83 alleviates experimental autoimmune uveitis by inhibiting filamentous actin-dependent calcium release in dendritic cells. Front Immunol. 2018;9:1567.
Eckhardt J, Kreiser S, Döbbeler M, Nicolette C, DeBenedette MA, Tcherepanova IY, et al. Soluble CD83 ameliorates experimental colitis in mice. Mucosal Immunol. 2014;7:1006–18.
Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345–51.
Wild AB, Krzyzak L, Peckert K, Stich L, Kuhnt C, Butterhof A, et al. CD83 orchestrates immunity toward self and non-self in dendritic cells. JCI Insight. 2019;4: e126246.
Lehmann CH, Heger L, Heidkamp GF, Baranska A, Lühr JJ, Hoffmann A, et al. Direct delivery of antigens to dendritic cells via antibodies specific for endocytic receptors as a promising strategy for future therapies. Vaccines. 2016;4:8.
Maldonado RA, LaMothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci USA. 2015;112:E156–65.
Cappellano G, Woldetsadik AD, Orilieri E, Shivakumar Y, Rizzi M, Carniato F, et al. Subcutaneous inverse vaccination with PLGA particles loaded with a MOG peptide and IL-10 decreases the severity of experimental autoimmune encephalomyelitis. Vaccine. 2014;32:5681–9.
Jung HH, Kim SH, Moon JH, Jeong SU, Jang S, Park CS, et al. Polymeric nanoparticles containing both antigen and vitamin D(3) induce antigen-specific immune suppression. Immune Netw. 2019;19: e19.
LaMothe RA, Kolte PN, Vo T, Ferrari JD, Gelsinger TC, Wong J, et al. Tolerogenic nanoparticles induce antigen-specific regulatory T cells and provide therapeutic efficacy and transferrable tolerance against experimental autoimmune encephalomyelitis. Front Immunol. 2018;9:281.
Yeste A, Nadeau M, Burns EJ, Weiner HL, Quintana FJ. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2012;109:11270–5.
Kim SH, Moon JH, Jeong SU, Jung HH, Park CS, Hwang BY, et al. Induction of antigen-specific immune tolerance using biodegradable nanoparticles containing antigen and dexamethasone. Int J Nanomedicine. 2019;14:5229–42.
Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5.
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38.
Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–9.
Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity. 2004;20:695–705.
Ring S, Maas M, Nettelbeck DM, Enk AH, Mahnke K. Targeting of autoantigens to DEC205+ dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol. 2013;191:2938–47.
Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–54.
Stern JN, Keskin DB, Kato Z, Waldner H, Schallenberg S, Anderson A, et al. Promoting tolerance to proteolipid protein-induced experimental autoimmune encephalomyelitis through targeting dendritic cells. Proc Natl Acad Sci USA. 2010;107:17280–5.
Kato M, McDonald KJ, Khan S, Ross IL, Vuckovic S, Chen K, et al. Expression of human DEC-205 (CD205) multilectin receptor on leukocytes. Int Immunol. 2006;18:857–69.
Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–9.
Engering AJ, Cella M, Fluitsma D, Brockhaus M, Hoefsmit EC, Lanzavecchia A, et al. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur J Immunol. 1997;27:2417–25.
Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189:1961–72.
Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, et al. Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol. 2003;171:4552–60.
Luca ME, Kel JM, van Rijs W, Wouter Drijfhout J, Koning F, Nagelkerken L. Mannosylated PLP(139–151) induces peptide-specific tolerance to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;160:178–87.
Kel J, Oldenampsen J, Luca M, Drijfhout JW, Koning F, Nagelkerken L. Soluble mannosylated myelin peptide inhibits the encephalitogenicity of autoreactive T cells during experimental autoimmune encephalomyelitis. Am J Pathol. 2007;170:272–80.
Kel JM, Slütter B, Drijfhout JW, Koning F, Nagelkerken L. Mannosylated self-peptide inhibits the development of experimental autoimmune encephalomyelitis via expansion of nonencephalitogenic T cells. J Leukoc Biol. 2008;84:182–90.
Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, et al. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–50.
Azukizawa H, Döhler A, Kanazawa N, Nayak A, Lipp M, Malissen B, et al. Steady state migratory RelB+ langerin+ dermal dendritic cells mediate peripheral induction of antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells. Eur J Immunol. 2011;41:1420–34.
King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207:953–61.
Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, et al. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med. 2014;6:1191–204.
Bigley V, McGovern N, Milne P, Dickinson R, Pagan S, Cookson S, et al. Langerin-expressing dendritic cells in human tissues are related to CD1c+ dendritic cells and distinct from Langerhans cells and CD141high XCR1+ dendritic cells. J Leukoc Biol. 2015;97:627–34.
Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–57.
Feinberg H, Mitchell DA, Drickamer K, Weis WI. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science. 2001;294:2163–6.
Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–85.
Geijtenbeek TB, Krooshoop DJ, Bleijs DA, van Vliet SJ, van Duijnhoven GC, Grabovsky V, et al. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat Immunol. 2000;1:353–7.
Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–8.
Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, et al. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–26.
Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–16.
Gringhuis SI, Kaptein TM, Wevers BA, Mesman AW, Geijtenbeek TB. Fucose-specific DC-SIGN signalling directs T helper cell type-2 responses via IKKε- and CYLD-dependent Bcl3 activation. Nat Commun. 2014;5:3898.
Arosio D, Chiodo F, Reina JJ, Marelli M, Penadés S, van Kooyk Y, et al. Effective targeting of DC-SIGN by α-fucosylamide functionalized gold nanoparticles. Bioconjug Chem. 2014;25:2244–51.
Stead SO, Kireta S, McInnes SJP, Kette FD, Sivanathan KN, Kim J, et al. Murine and non-human primate dendritic cell targeting nanoparticles for in vivo generation of regulatory T-cells. ACS Nano. 2018;12:6637–47.
Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, Pin JJ, et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol. 1999;163:1973–83.
Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. 2009;85:518–25.
Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ, et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood. 2008;111:4245–53.
Seno A, Maruhashi T, Kaifu T, Yabe R, Fujikado N, Ma G, et al. Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp Anim. 2015;64:109–19.
Tabansky I, Keskin DB, Watts D, Petzold C, Funaro M, Sands W, et al. Targeting DEC-205(−)DCIR2(+) dendritic cells promotes immunological tolerance in proteolipid protein-induced experimental autoimmune encephalomyelitis. Mol Med. 2018;24:17.
Huysamen C, Willment JA, Dennehy KM, Brown GD. CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem. 2008;283:16693–701.
Sancho D, Joffre OP, Keller AM, Rogers NC, Martínez D, Hernanz-Falcón P, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903.
Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–92.
Del Fresno C, Saz-Leal P, Enamorado M, Wculek SK, Martínez-Cano S, Blanco-Menéndez N, et al. DNGR-1 in dendritic cells limits tissue damage by dampening neutrophil recruitment. Science. 2018;362:351–6.
Joffre OP, Sancho D, Zelenay S, Keller AM, Reis e Sousa C. Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol. 2010;40:1255–65.
Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. 2011;187:842–50.
Jan Tavernier NK, Cauwels A, Gerlo S. CLEC9A binding agents. https://patents.justia.com/patent/20190352406.
Li K, Neumann K, Duhan V, Namineni S, Hansen AL, Wartewig T, et al. The uric acid crystal receptor Clec12A potentiates type I interferon responses. Proc Natl Acad Sci USA. 2019;116:18544–9.
Lahoud MH, Proietto AI, Ahmet F, Kitsoulis S, Eidsmo L, Wu L, et al. The C-type lectin Clec12A present on mouse and human dendritic cells can serve as a target for antigen delivery and enhancement of antibody responses. J Immunol. 2009;182:7587–94.
Sagar D, Singh NP, Ginwala R, Huang X, Philip R, Nagarkatti M, et al. Antibody blockade of CLEC12A delays EAE onset and attenuates disease severity by impairing myeloid cell CNS infiltration and restoring positive immunity. Sci Rep. 2017;7:2707.
Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365–95.
Mehta NR, Nguyen T, Bullen JW Jr, Griffin JW, Schnaar RL. Myelin-associated glycoprotein (MAG) protects neurons from acute toxicity using a ganglioside-dependent mechanism. ACS Chem Neurosci. 2010;1:215–22.
Avril T, Attrill H, Zhang J, Raper A, Crocker PR. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem Soc Trans. 2006;34:1024–7.
Lin CH, Yeh YC, Yang KD. Functions and therapeutic targets of Siglec-mediated infections, inflammations and cancers. J Formos Med Assoc. 2021;120:5–24.
Loschko J, Heink S, Hackl D, Dudziak D, Reindl W, Korn T, et al. Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol. 2011;187:6346–56.
Perdicchio M, Ilarregui JM, Verstege MI, Cornelissen LA, Schetters ST, Engels S, et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc Natl Acad Sci USA. 2016;113:3329–34.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–82.
Sloane JA, Batt C, Ma Y, Harris ZM, Trapp B, Vartanian T. Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc Natl Acad Sci USA. 2010;107:11555–60.
Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109:13064–9.
Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456–64.
Correale J, Farez M. Helminth antigens modulate immune responses in cells from multiple sclerosis patients through TLR2-dependent mechanisms. J Immunol. 2009;183:5999–6012.
Lee C, Verma R, Byun S, Jeun EJ, Kim GC, Lee S, et al. Structural specificities of cell surface β-glucan polysaccharides determine commensal yeast mediated immuno-modulatory activities. Nat Commun. 2021;12:3611.
Shaw PJ, Barr MJ, Lukens JR, McGargill MA, Chi H, Mak TW, et al. Signaling via the RIP2 adaptor protein in central nervous system-infiltrating dendritic cells promotes inflammation and autoimmunity. Immunity. 2011;34:75–84.
Mellanby RJ, Cambrook H, Turner DG, O’Connor RA, Leech MD, Kurschus FC, et al. TLR-4 ligation of dendritic cells is sufficient to drive pathogenic T cell function in experimental autoimmune encephalomyelitis. J Neuroinflamm. 2012;9:248.
Crooks J, Gargaro M, Vacca C, Volpi C, Pirro M, Scalisi G, et al. CpG type A induction of an early protective environment in experimental multiple sclerosis. Mediat Inflamm. 2017;2017:1380615.
Ho PP, Fontoura P, Platten M, Sobel RA, DeVoss JJ, Lee LY, et al. A suppressive oligodeoxynucleotide enhances the efficacy of myelin cocktail/IL-4-tolerizing DNA vaccination and treats autoimmune disease. J Immunol. 2005;175:6226–34.
Ho PP, Fontoura P, Ruiz PJ, Steinman L, Garren H. An immunomodulatory GpG oligonucleotide for the treatment of autoimmunity via the innate and adaptive immune systems. J Immunol. 2003;171:4920–6.
Tostanoski LH, Eppler HB, Xia B, Zeng X, Jewell CM. Engineering release kinetics with polyelectrolyte multilayers to modulate TLR signaling and promote immune tolerance. Biomater Sci. 2019;7:798–808.
Tostanoski LH, Chiu YC, Andorko JI, Guo M, Zeng X, Zhang P, et al. Design of polyelectrolyte multilayers to promote immunological tolerance. ACS Nano. 2016;10:9334–45.
Hess KL, Andorko JI, Tostanoski LH, Jewell CM. Polyplexes assembled from self-peptides and regulatory nucleic acids blunt toll-like receptor signaling to combat autoimmunity. Biomaterials. 2017;118:51–62.
Pishesha N, Harmand T, Smeding LY, Ma W, Ludwig LS, Janssen R, et al. Induction of antigen-specific tolerance by nanobody-antigen adducts that target class-II major histocompatibility complexes. Nat Biomed Eng. 2021. https://doi.org/10.1038/s41551-021-00738-5.
Krienke C, Kolb L, Diken E, Streuber M, Kirchhoff S, Bukur T, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–53.
Karikó K, Muramatsu H, Ludwig J, Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39: e142.
Karikó K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–75.
Acknowledgements
Figure 1 was modified from Servier Medical Art (http://smart.servier.com/), licensed under a Creative Common Attribution 3.0 Generic License. (https://creativecommons.org/licenses/by/3.0/).
Funding
This work was supported by grants from the General Program of the National Natural Science Foundation of China (No. 82171337), Natural Science Foundation of Jilin Province Science and Technology Development Plan Project (20190201043JC), Key Research and Development Project of Social Development Division of Jilin Science and Technology Department (20200403109SF), Special Project for Health Professionals of Jilin Provincial Finance Department (JLSWSRCZX2020-0056), Science and Technology Research Project of Jilin Education Department (JJKH20211204KJ).
Author information
Authors and Affiliations
Contributions
CL prepared the manuscript and the figure. JZ and YM helped to conceive and reviewed the manuscript. TJ conceived, wrote, and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, C., Zhu, J., Mi, Y. et al. Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis. J Neuroinflammation 19, 298 (2022). https://doi.org/10.1186/s12974-022-02663-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-022-02663-z