Abstract
Reproductive aging is characterized by a decline in oocyte quantity and quality, which is directly associated with a decline in reproductive potential, as well as poorer reproductive success and obstetrical outcomes. As women delay childbearing, understanding the mechanisms of ovarian aging and follicular depletion have become increasingly more relevant. Age-related meiotic errors in oocytes are well established. In addition, it is also important to understand how intraovarian regulators change with aging and how certain treatments can mitigate the impact of aging. Individual studies have demonstrated that reproductive pathways involving antimullerian hormone (AMH), vascular endothelial growth factor (VEGF), neurotropins, insulin-like growth factor 1 (IGF1), and mitochondrial function are pivotal for healthy oocyte and cumulus cell development and are altered with increasing age. We provide a comprehensive review of these individual studies and explain how these factors change in oocytes, cumulus cells, and follicular fluid. We also summarize how modifiers of folliculogenesis, such as vitamin D, coenzyme Q, and dehydroepiandrosterone (DHEA) may be used to potentially overcome age-related changes and enhance fertility outcomes of aged follicles, as evidenced by human and rodent studies.
Similar content being viewed by others
Introduction
Female fertility rates increase after puberty and peak in the early 20s [1]. This is followed by a decline in fertility thereafter, which is salient after age of 35 years [1]. The process of reproductive aging is characterized by a quantitative and qualitative deterioration in ovarian reserve, associated with increases in aneuploidy and higher rates of miscarriage [2, 3]. Therefore, biological age is a critical factor in reproductive success.
Increased participation of women in education and the work force has been paralleled by a significant delay in childbearing [2]. Consistent with later attempts at pregnancy, female fertility rates have significantly declined, and prevalence of diminished ovarian reserve (DOR) and use of donor eggs among the population undergoing assisted reproductive technologies (ART) have increased [2]. Ovarian aging is accompanied by a quantitative deterioration and qualitative decline in ovarian reserve with an increase in oocyte aneuploidy, reduced embryo quality and increased miscarriage rates [2, 3]. In addition, among those with DOR, the granulosa cell (GC) layer of the growing follicles secrete less estradiol and anti-mullerian hormone (AMH), they require higher doses of gonadotropins, leading to a poorer response to ovarian stimulation thus necessitating use of donor eggs among the population [4]. Given these demographic and socioeconomic changes and their impact on fecundity and response rate to ART, there is increasing interest in better understanding the biological mechanisms of ovarian aging and methods to potentially overcome or delay aging of the finite pool of follicles that women are born with.
For this review, we searched various combinations of: follicle, oocyte, follicular growth, cumulus cells (CC), follicular fluid (FF), aging, fertility in PUBMED through June 2022. We included relevant full text, English language articles in this manuscript. We aim to briefly introduce folliculogenesis and to explore the mechanisms related to ovarian aging. We then discuss the role of specific small molecules in ovarian aging and how such molecules can be used to detect DOR and/or to increase fertility outcomes of aging follicles. We aim to provide a comprehensive review of changes in folliculogenesis with aging, as well as the various molecules and pathways involved in regulation of aging, including AMH, vascular endothelial growth factor (VEGF), neurotropins, insulin-like growth factor 1 (IGF1), and mitochondrial function/dysfunction. We also review how the usage of vitamin D, coenzyme Q, and dehydroepiandrosterone (DHEA) may reduce the effect of aging in oocytes and lead to enhanced fertility outcomes.
Background
Women are born with a finite pool of primordial follicles and this number declines from approximately 2 million to 400,000 by the time women reach menarche [5]. During female reproductive years, a cohort of primordial follicles is recruited monthly in order to grow a single dominant follicle and ovulate its oocyte competent for fertilization and embryo formation. In primordial follicles, the dormant oocyte is arrested at prophase I and is surrounded by a single layer of flattened GCs [6]. Primordial follicle recruitment leads to activation and maturation of the oocyte, as well as proliferation and differentiation of surrounding GCs, leading to the formation of primary follicles, which are characterized by a single layer of cuboidal GCs surrounding the oocyte. GC layers continue to proliferate in secondary follicles. They differentiate into CCs, which are cells contiguous with the maturing oocyte [7, 8] and are also important in recruitment of androgen producing theca cells (later segregated into theca interna and externa layers) from progenitor pool at ovarian mesenchyme and mesonephros [9]. Until the formation of the preantral follicle, folliculogenesis is gonadotropin independent and is a paracrine signaling unit comprised of multiple locally active growth factors and small molecules secreted by GCs of the early follicle [10] (Fig. 1).
Folliculogenesis: Primordial follicles are surrounded with a single layer of squamous granulosa cells. With primordial activation, primordial follicles are recruited into folliculogenesis, initially becoming primary follicles with a single layer of cuboidal granulosa cells. With replication, granulosa cells form layers surrounding the oocyte, thus forming the secondary follicle. The early/small antral follicle is comprised of multiple layers of granulosa cells, which are differentiated into cumulus cells that immediately surround the oocyte as well as small pockets of antrum. The antral follicle is characterized by a large antrum containing follicular fluid, and is ready to be ovulated out of the ovarian cortex. Follicles can undergo atresia at any stage of folliculogenesis. AMH is secreted by preantral follicles and control primordial follicle recruitment as well as follicular atresia. In comparison, VEGF stimulates recruitment of primordial follicles to become primary
With growth and differentiation of GCs, maturing follicles eventually express gonadotropin receptors and acquire responsiveness to gonadotropins, first to follicle-stimulating hormone (FSH) then to luteinizing hormone (LH). In this gonadotropin dependent phase of folliculogenesis, follicles become hormone secreting units. FSH enables GC growth, promotes estradiol production, and enables dormant follicle selection [11], while LH is responsible for androgen secretion from cholesterol, completion of meiosis I in the oocyte, germinal vesicle breakdown [12,13,14] and subsequent progression to metaphase II [15]. Ovulation marks the end of folliculogenesis with production of a mature oocyte capable of fertilization [16,17,18].
Autocrine and paracrine intraovarian factors are influential in both gonadotropin-independent and -dependent phases of folliculogenesis [19]. Within each follicle crosstalk between CCs and the oocyte occurs through gap junctions, formed with oocyte-provided connexin 37 and CC-expressed connexin 43 [20]. This process enables nutrient exchange between two compartments of the cumulus-oophorus complex [20] and is crucial for healthy follicle formation [21,22,23]. Quality of oocytes are therefore paralleled by proper CC functioning [24]. Through these junctions, oocyte receives pyruvate, amino acids, and nucleotides as well as provides CCs with growth factors, such as bone morphogenetic protein 15 (BMP15) and growth differentiating factor 9 (GDF9), which are obligatory for proper folliculogenesis [25,26,27,28,29]. GDF9 is responsible for GC proliferation and differentiation to CCs [30]; its levels continuously rise during folliculogenesis until ovulation [27] and absence of GDF9 leads to arrest at the primary follicular stage with subsequent follicular atresia and infertile mice [25]. Knock out of Bmp15 also disrupts folliculogenesis leading to subfertility in mice [28] and infertility in sheep [29].
Throughout the highly regulated and well-orchestrated folliculogenesis process, multiple follicles are arrested at different stages and go through irreversible atretic degeneration with increasing pace after the mid-30s [5, 31]. With aging, in addition to quantitative decline, follicles go through progressive deterioration in their function and in their capacity to form an oocyte capable of fertilization.
Aging related disruption in folliculogenesis is attributed to changes that most likely occur simultaneously and include bioenergetics dysfunction leading to increased oxidative stress and shortened telomere length in aged oocytes obtained from antral follicles, and decreased DNA repair capacity with increased mutations through loss of chromosome cohesion and spindle aberration leading to meiotic errors during folliculogenesis [32, 33].
Such age-related disruptions are not limited to the oocyte but also affect the GCs and CCs [34, 35]. In addition to the number of follicles, the amount of GCs surrounding the oocyte also diminish with age due to increased GC apoptosis [36], which is accompanied by altered production of locally active growth factors [37], attenuated proliferation [38] and disrupted steroidogenesis [39].
Role of intraovarian paracrine and endocrine molecules in folliculogenesis and the aging follicle
AMH is a critical intra-ovarian regulator of the gonadotropin independent phase of folliculogenesis and an indirect marker of the primordial follicular pool which decreases with age
AMH also known as Mullerian inhibiting substance, is a member of the TGFβ superfamily of peptides that acts through its specific receptor, AMHR2. It was first described for its role in fetal sexual differentiation in 1950s by Professor Alfred Jost. In the male fetus, around 8 weeks of gestation AMH is secreted by Sertoli cells of testis and binds to AMHR2 located at the surface of Mullerian duct leading to its regression by apoptosis [40, 41]. A mutation of the AMH gene located on chromosome 19q13 or a mutation of the AMHR2 gene located on chromosome 12q3 leads to Persistent Mullerian Duct Syndrome, an autosomal recessive disorder characterized by the presence of a uterus and fallopian tubes in an otherwise male appearing individual [42, 43]. In female fetus, AMH expression in the ovary starts at approximately 36 weeks of gestation, peaks in the mid-20s, and continues to decrease until menopause [44,45,46]. In the absence of AMH in early female fetal development, the Mullerian duct persists and form the fallopian tubes, uterus, cervix, and upper one-third of vagina. In the ovary, Amh and its receptor Amhr2 are mainly expressed in GCs of maturing follicles with altering expression levels in different stages [47]. AMH expression starts in GCs of primary follicles, gradually increasing and reaching the highest level in small antral follicles less than 6 mm in size [44, 48]. In antral follicles, the majority of AMH is secreted by CCs [47]. In atretic follicles and the corpus luteum (CL), AMH levels are undetectable [47]. AMHR2 expression mirrors the AMH expression pattern and is also secreted in smaller amounts by theca cells of preantral and antral follicles [47].
In rodents, AMH has been shown to act as a gatekeeper of finite primordial follicular reserve by inhibiting the initial gonadotropin independent recruitment of the follicles and sustaining their dormant state [45, 49] (Fig. 1). Amh knockout mice models demonstrated increased number of maturing follicles and smaller pools of primordial follicles [50, 51]. Human studies on primordial reserve using ovarian cortical biopsies are still controversial regarding AMH’s role in inhibiting primordial follicle assembly [47]. Conversely, overexpression of AMH during fetal gonadal development negatively impacts normal ovarian and follicular pool development [52]. AMH is also crucial in cyclic antral follicle selection. During gonadotropin-independent and in early gonadotropin–dependent phases of follicular development, AMH has an inhibitory role on the growing follicle [53]. For example, Amh knockout mice models have been shown to recruit significantly more growing follicles with ovarian stimulation compared to the control group [54]. This is suggested to be achieved through halting sensitivity to FSH and disrupting steroidogenesis through AMH’s down-regulatory effect on the expression of aromatase (Cyp19a1) and the LH receptor (Lhcgr) as demonstrated in rodent and porcine follicles [47] (Fig. 1). This is further supported by Hayes et al. who established that in vivo AMH treatment of mouse, decreases Cyp19a1, Lhcgr and steroidogenic acute regulatory protein (Star) expression [55]. Decreased estrogen production through inhibited aromatase, contributes to AMH’s inhibitory effect on follicular recruitment [49].
The regulation of AMH and expression of its receptors are complex and not yet fully understood. Among the gonadotropins, low concentrations of FSH are shown to promote Amh expression in vitro mouse and human studies [56, 57]. Conversely, estrogen inhibits both Amh and Amhr2 expression in in vitro and in vivo studies [58]. In in vitro studies, addition of steroids, specifically estrogen, to FSH containing culture medium halts FSH’s positive effect on AMH expression [59, 60]. This phenomenon is supported by in vivo studies of ovarian stimulation with FSH which upregulates estrogen production which in return inhibits Amh and Amhr2 expression [61, 62]. Similar to estrogen, LH also has an inhibitory role on Amhr2 expression [58, 63]. The effect of androgens on AMH and AMHR2 expression has yet to be clearly defined.
In addition to systemic gonadotropins, an expanding list of intraovarian factors including BMPs also regulate early follicle growth as well as Amh expression. Most in vivo and vitro studies show enhancing effect of most BMPs on Amh and Amhr2 expression. Among many intraovarian factors, BMP15 is the most well studied. BMP15 alone increases both Amh and Amhr2 expressions [64, 65]. GDF9 alone hasn’t been shown to repress or stimulate AMH expression, however in the presence of BMP15, it augments BMP15’s stimulatory effect on AMH expression [64, 66,67,68]. Recombinant AMH also increases expression of GDF9 and BMP15 promoting better oocyte quality [69]. Others such as BMP2, BMP4 and BMP6 have also been shown to upregulate Amh expression in in vitro studies [65, 70, 71].
The role of environmental endocrine disruptors and metabolic factors on Amh expression and folliculogenesis remain under investigation. However, initial studies support that insulin can upregulate Amh expression in a dose dependent manner [72]. Other growth factors such as, VEGF and tumor necrosis factor alpha (TNFa) inhibit Amh mRNA levels [73]. AMHR2 levels have also been studied and demonstrate that leptin represses its expression [74], whereas IGF1 and VEGF upregulate its expression [73, 75]. Environmental factors such as abnormal vitamin D levels, oral contraceptive pills, and smoking have also been shown to alter AMH expression levels [49].
AMH is postulated to be anti-apoptotic on GCs with a protective role against follicular atresia [55] (Fig. 1). Although a casual pathway was not determined, in natural IVF cycles, AMH level was noted to be elevated while atretic antral follicle number was reduced [76] The rate of decrease in AMH over time is mirrored by an age-related rate of atresia (Fig. 2). AMH null mice exhibit increased number of atretic follicles in their ovaries [77] and Amh knockdown in macaque ovaries results in impaired secondary follicle survival [78]. Consistently, treatment with AMH reduces atretic follicle number in prepubertal [79] and adult mice [55] as well as cryopreserved mouse ovarian tissue [80] with higher primordial and primary follicular count. AMH, also a marker of ovarian reserve that was initially described by Seifer et al. in 2002, [81] is used clinically worldwide as a noninvasive, reproducible, and rapidly interpretable method associated with a high positive predictive value and low negative predictive value for number of retrieved oocytes. Due to its role in regulating the rate of follicle recruitment [82, 83] and low intracyclic variability with circulating gonadotropin levels [84], AMH is often utilized for fertility preservation counseling and individualizing ART treatment. Serum AMH levels are also used for prediction of menopause onset and for minimizing the risk of potentially life-threatening ovarian hyperstimulation syndrome in women undergoing ovulation induction [48]. In addition, in follicular fluid AMH levels can be used to determine GC apoptosis rate in ART cycles [76].
Age related changes of intraovarian small molecules and mitochondrial function within the follicle: Aging is related to decreased IGF1, decreased AMH, decreased BDNF, increased VEGF levels as well as mitochondrial dysfunction. Altered mitochondrial dynamics, disrupted mitochondrial homeostasis and increased mtDNA mutations are age related changes that occur within mitochondria and lead to impaired oxygenation-phosphorylation. Impaired oxygenation-phosphorylation leads to increased ROS accumulation which leads to follicular atresia
VEGF is crucial in gonadotropin-independent folliculogenesis through promotion of angiogenesis and is increased in aging follicles
VEGF is a potent angiogenic factor expressed in a variety of tissues, and plays a role in the complex autocrine, paracrine and endocrine regulation of the gonadotropin-independent phase of folliculogenesis [85]. VEGF, especially VEGFa, and its receptors, VEGFR1 and VEGFR2 are regulatory factors in mammalian ovarian folliculogenesis [86,87,88,89], and their expression levels increase as follicles mature [90, 91]. In the ovarian cortex, small preantral follicles do not possess their own blood supply, but rather receive their nutrition and oxygenation through passive diffusion from stromal tissue. During follicular maturation, starting with secondary follicles, outer stromal cells surrounding the oocyte differentiate into theca cells [92]. The inner theca layer, which is separated from the granulosa cell layer via a basement membrane, form a capillary network for the follicle that is essential for its growth [93, 94]. Injection of VEGF directly into the ovarian blood supply can positively affect angiogenesis, increase the number of primary and secondary follicles and reduce follicular atresia [87, 95] (Fig. 1). In cows, VEGF administration increases the number of secondary follicles [89]; and in primates, VEGF inhibitor administration decreases endothelial cells in secondary follicles and inhibits antral follicle formation [96]. VEGF has been shown to promote primordial pool activation in in vitro studies [97]. VEGF expression in the follicle progressively enhances maturational development from primary to preovulatory stages and is positively correlated to the diameter of the follicle [85]. In rodents, VegfR mRNA has been identified specifically in theca cells of secondary follicles [89]; and in humans, VEGFa and VEGFR gene expression and protein have been detected in theca cells, granulosa cells and in oocytes [98]. Thecal angiogenesis and increased vascular permeability through VEGF are thought to be crucial for follicular growth and for antrum formation by providing nutrients and oxygenation to the follicle as well as securing adequate supply of gonadotropins, steroid precursors and other folliculogenesis regulators to the developing follicle [94]. This phenomenon has been postulated to be decisive for follicular dominance with the dominant follicle containing more abundant vasculature in its theca layer compared to others in the same cohort [99].
A robust capillary network is also essential for formation of a functional CL. The mid-cycle LH surge leads to GC Vegf expression [100], proteolytic activity, and degradation of the basement membrane leading to VEGFa release into the GC layer, and new capillary formation in the developing CL [101]. Administration of a GnRH antagonist leads to decreased Vegf expression in CL of primate ovaries by inhibiting the LH surge [102].
Gonadotropins and other angiogenic factors secreted by GCs can enhance VEGF expression within the follicle [103, 104]. In addition, some intraovarian regulators such as theca derived BMP7 have been demonstrated to induce VEGFa mRNA and protein expression and increase endothelial cell sensitivity to VEGF in human GCs and enhance follicular angiogenesis [105]. Cytokines such as interleukin 6 (IL6), IGF1 and fibroblast growth factor 2 (FGF2) have been identified with their pro-angiogenic role in ovary, inducing VEGF expression [106,107,108].
In the setting of inadequate theca vasculature formation, hypoxia may lead to altered oocyte metabolism and decrease in intracellular pH in the oocyte which might both deteriorate spindle formation and organization leading to chromosomal abnormalities as well as enhanced follicular atresia [109]. For example, severe reduction in dissolved oxygen in FF among patient aged 25–37 years old, is associated with an increased number of chromosomal abnormalities on meiotic spindles in metaphase II [110]. With aging, VEGF levels in FF increase among both natural and in vitro fertilization (IVF) cycles [37, 111,112,113,114,115] (Fig. 2). This age-related increase in VEGF levels may be secondary to selective elevation of gonadotropins in older reproductive age women as well as a compensatory response to follicular hypoxia and decreased energy synthesis in the setting of disrupted mitochondrial function [10].
Mitochondrial function and dynamics are regulators for folliculogenesis and are compromised with aging
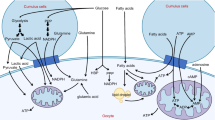
Within the follicle, glucose is the key energy source [116] and is mainly metabolized by the supporting CCs [117] which are in direct communication with the oocyte through protrusions through the zona pellucida [118]. Through this intercellular junction, oocytes receive pyruvate, an end metabolite of glycolysis, amino acids, nucleotides, glutathione and local growth factors [119] and provide CCs with oocyte derived regulators of CC gene expression [120]. Normal functioning mitochondria are essential in the cytoplasm of oocytes, not only for their role in utilization of pyruvate and adenosine triphosphate (ATP) production through oxidative phosphorylation (OXPHOS) [121], but also for apoptosis [122], amino acid metabolism, phospholipid synthesis and calcium signaling [123, 124].
Mitochondria are crucial in all stages of folliculogenesis and follicular aging. Mitochondrial number, function, and dynamics have been shown to be altered with advancing age in all compartments comprising the follicle [34, 125]. Aging has been associated with disruption in various mitochondrial functions including amount of mitochondrial DNA (mtDNA) mutations, impaired OXPHOS, accumulation of reactive oxygen species (ROS), decreased mitochondrial dynamics, as well as disruption of pathways involved in mitochondrial homeostasis [126,127,128,129,130]. Optimal mitochondrial function is important for normal chromosome distribution. Thus, mitochondrial dysfunction also contributes to chromosomal abnormalities and failure to extrude the first polar body in the oocyte through meiotic division errors, which occur at higher incidence in women with advanced age [131]. Moreover, dysfunctional mitochondria may lead to decreased ATP production and increased oxidative stress within the oocyte due to its inability to balance ROS production. Babayev et al. demonstrated that older mice have smaller mitochondria in oocytes obtained from primary follicles, increasing ROS with induced cellular stress in their mature oocytes [132]. Not only in oocytes but in CC obtained from poor ovarian response patients and women over 38 years old, follicular mitochondrial function has been shown to be severely impaired [133,134,135]. The impaired mitochondrial function in oocyte and age related ROS accumulation are directly related to decreased oocyte quality [136] with arrested maturation, halted fertilization, compromised embryo development and increased embryo aneuploidy [131] (Fig. 2).
Mitochondria are dynamic organelles that adjust to cellular needs through fission and fusion [137]. Drp1 gene has been noted to regulate fission enabling one mitochondrion to divide into two, and it activates mitophagy (mitochondrial autophagy) mainly through the PINK1/Parkin pathway for removal of abnormal mitochondrial content. In return, fusion enables diluting damaged mitochondrial content and improves the mitochondrial pool quality. Opa1, Mfn1 and Mfn2 are important regulators of mitochondrial fusion [1]. While Opa1 controls inner mitochondrial membrane fusion, Mfn1 and 2 are responsible for outer membrane fusion [138]. Within the oocyte, optimal mitochondrial dynamics are altered with aging as evidenced by several mice studies [132, 139]. Global knockout of Opa1, Mfn1, Mfn2 or Drp1 in mice have been shown to be lethal in embryogenesis [140,141,142]. Oocyte specific knock out of Drp1 in mice demonstrated that fission of mitochondria is important for follicular maturation and ovulation [143]. Zhang et al. demonstrated that lack of MFN1 specifically at mice oocytes leads to infertility through accelerated follicular pool depletion secondary to defective follicular maturation with arrest at secondary stage as well as impaired oocyte maturation and increased apoptosis [144]. Lack of another fusion protein, MFN2 in oocytes also lead to abnormal follicle development and impaired oocyte maturation through shortened telomere length leading to subfertility and DOR in female mice, mimicking aging related changes [145].
Mitochondria are known to carry their own double stranded mtDNA; its content increases during oogenesis though is constant during oocyte maturation, fertilization, and blastocyst formation [146,147,148]. With increasing age, the body’s antioxidant capacity decreases and dormant follicles are exposed to accumulated ROS for prolonged periods of time leading to potential mutations in mtDNA and deficits in adequate functioning [149]. Given the lack of histones and an effective DNA repair system and its proximity to ROS produced within mitochondria, mtDNA is postulated to be more susceptible to oxidative damage than nuclear DNA [150]. It has been shown that mtDNA deletions, which cause deficits in proper OXPHOS may be responsible for increased GC apoptosis within aged follicles [151, 152]. Specifically, mt4944, the most commonly studied mtDNA mutation, is noted to accumulate in aging ovarian tissue [153] and within both granulosa cells [135] and the oocyte [154]. However, it is interesting that the studies assessing abnormal mtDNA number, whether high or low, and its impact on oocyte quality, fertilization, and implantation potential have been conflicting [155,156,157,158,159,160,161]. In one study, Fragouli et al. demonstrated that increased mtDNA is detected in embryos obtained from advanced maternal age patients, euploid embryos that failed to implant, and aneuploidy embryos regardless of maternal age [162]. Whereas, Diez-Juan et al. did not observe any difference in mtDNA content within embryos obtained from old and young females [163]. In two other studies conducted by Victor et al. and Klimczak et al., mtDNA content was not significantly altered with age, chromosomal aneuploidy, or implantation potential [158, 161].
In addition, mitochondria have an unfolded protein response (mtUPR) system, which enables a response to cellular stress and enhances mitochondrial hemostasis with maintaining the balance of a healthy mitochondrial pool. It identifies and eliminates misfolded proteins and promotes appropriate protein folding within the mitochondria [164]. mtUPR also promotes coenzymeQ10 (CoQ10) synthesis, glycolysis, and mitochondrial dynamics [1]. In an aging mouse model, expression of Hspd1, a mtUPR gene was downregulated in oocytes obtained from old mice with prior proven fertility [132]. Disruption of this mtUPR system leads to mitochondrial malfunction. Global deletion of Clpp, a mitochondrial protease involved in mtUPR system, leads to infertility in female mice with accelerated ovarian reserve depletion consistent with the DOR phenotype (Fig. 2). These mice demonstrate a decreased number of follicular responses to gonadotropin stimulation and fail to make blastocysts. This is believed to be secondary to impaired mitochondrial functioning, disrupted OXPHOS, and ROS accumulation within the oocyte [165]. mtDNA content on oocytes obtained from CLPP deficient mice also noted to be higher signaling mitochondrial distress [165]. They also noted a higher mtDNA content in oocytes. In another study with targeted Clpp deletion solely in cumulus cells, expression of mitochondrial dynamics and cellular metabolism genes were altered and apoptosis in cumulus cells was increased with a decreased number of cumulus cells within the follicle [166].
IGF1 is an important paracrine modulator of gonadotropin-dependent folliculogenesis and its levels in FF decrease with age as well as DOR
The IGF system consists of IGF1, IGF2, their specific receptors (IGF1-R and IGF2-R), and IGF binding proteins (IGFBPs), which are inhibitors of IGF expression. IGF1 and IGF2 are growth factors that are mainly secreted by hepatic cells through growth hormone (GH) influence [167]. They are potent mitogens, promoting DNA synthesis, involved in cell proliferation and differentiation [168, 169] and are known to suppress apoptosis [170]. All members of IGF system have also been identified in the mammalian ovary [171,172,173]. They are primarily synthesized in GCs of preovulatory follicles [174, 175] and are important intraovarian modulators of folliculogenesis [176]. In an autocrine manner, IGF1 and IGF2 enhance GC proliferation and differentiation [177, 178], promote steroidogenesis by acting synergistically with gonadotropins [179,180,181], and prevent follicular atresia, enhancing survival of small growing follicles [172, 182]. In in vitro grown rat GCs, supplementation of IGF1 increases aromatase activity, amplifies FSH induced estrogen production [174, 183] and promotes progesterone synthesis [184]. In mouse models, IGF1 knock out leads to dwarfism and infertility with complete arrest in growing follicle pool and this phenomenon could not be rescued with gonadotropin stimulation [185]. Additionally, IGF1 has been identified in FF. In vitro cultured mature oocytes that successfully fertilized had more FF IGF1 expression compared to those that did not fertilize [186]. In human studies, FF IGF1 levels noted to correlate with follicle development, embryo quality and clinical pregnancy rates [187]. In the same study, estrogen levels noted to be higher in group with higher FF IGF1, supporting earlier finding of IGF1’s role in promoting aromatase activity [187].
Ovarian IGF production is affected by multiple factors. Similar to liver, GH promotes IGF1 production in GCs [188] and enhances FSH action with increasing FSH receptor expression [189]. Sequential treatment with GH and IGF1, promotes in vitro follicular growth with normal parameters of viability and ultrastructure [190]. DHEA supplementation in women undergoing IVF increases IGF1 expression especially in poor responders [191, 192].
Furthermore, IGF1 induces follicular angiogenesis by stimulating VEGF production within the follicle and promoting proliferation and differentiation of endothelial cells [107, 193].
After puberty, serum IGF1 expression was noted to decrease progressively [194, 195] (Fig. 2). To further explore IGF’s role in ovarian aging, Greenseid et al. examined IGF1 and IGF2 expression in GCs obtained from DOR patient and noted a significant decline in their expression compared to GCs obtained from control groups [181]. This was corroborated by Pashaiasl et al. who assessed key regulating genes in ovarian aging and noted downregulated expression of IGF2 in DOR patients [196]. Moreover, it has been observed that young women with DOR or older women compared to younger control, had significantly lower values of FF IGF1 [37, 197]. Interestingly, FF IGF1 levels were also used to assess clinical outcomes. For example, in young patients (24–35 years old) who obtained clinical pregnancy after single attempt IVF treatment compared to those who did not, FF IGF1 levels were shown to be higher [198]. Given IGF1’s role in aromatase activity and estrogen production, significantly downregulated IGF1 and IGF2 expression can explain suboptimal estrogen levels in the DOR population with gonadotropin stimulation during IVF treatment.
Neurotropin family is a regulator of gonadotropin-independent and dependent stages of folliculogenesis and BDNF levels decrease within the aging follicles
Neurotropins are a family of growth factors, first described for their role in brain neurogenesis [199] and are identified as regulators of ovarian function in mammals, including humans [200,201,202,203,204]. The family is comprised of brain derived neurotropic factor (BDNF), nerve growth factor (NGF), neurotropin 3 (NT3), and neurotropin 4/5 (NT 4/5). They are known to have paracrine roles and support early germ cell survival, follicular development, steroidogenesis, oocyte maturation through polar body extrusion, and ovulation [204,205,206,207,208,209,210,211]. Among neurotropins, BDNF is the only one at present that has been demonstrated to be affected by ovarian aging [212].
BDNF has been identified in fetal and neonatal mammalian ovaries and plays role in various stages of folliculogenesis in adult ovaries [201, 203, 204, 207, 213,214,215,216,217]. Chow et al. demonstrated increasing BDNF levels with oogenesis in fetal growth [218]. BDNF expression also changes during the menstrual cycle and decreases with age and menopause [219]. It is important for oocyte maturation [214] and follicular maturation starting from the primordial stage. BDNF levels continuously increase while positively affecting Gdf9 expression and GC proliferation rates [218]. In secondary follicles, BDNF upregulates FSH-R expression [218]. In human antral follicles, CC are responsible for BDNF secretion as evidenced by immunohistochemistry and in vitro studies [201, 203]. BDNF secreted by CC exerts its effect by binding to TrkB receptors located in the oocyte [201]. Its expression levels are upregulated following the LH surge [214]. In human CC treated with cAMP (whose expression also increases with exogenous gonadotropins), higher BDNF concentration was identified, though this wasn’t able to be replicated in GC or oocytes [201, 217]. In addition, BDNF was found in FF of antral follicles obtained from normally cycling women as well as in women after ovulation induction prior to IVF [201, 216] and the expression level was reflected by oocyte maturation rate [220]. Seifer et al. observed a higher level of BDNF expression in FF after ovulation induction compared to FF in natural cycles [216]. BDNF treatment of in vitro cultured mice oocytes had improved rate of maturation and first polar body extrusion [201, 214]. In mice, chronic stress was exhibited to reduce retrieved oocyte number, lower blastocyst formation rate and decrease BDNF expression in antral follicles; and treatment with BDNF was able to negate these effects [221]. In women with DOR and in endometriosis patients, FF BDNF levels obtained from antral follicles were observed to be lower [212]. With aging, especially after menopause, BDNF expression in follicles also noted to be downregulated significantly (Fig. 2). In a more recently published study, injection of agonistic TrkB inhibitor, Ab5B19, was able to attenuate the age related reduction in antral follicular count in vitro, indicating a possible role of BDNF in the follicular aging process [222].
Potential modifiers of age-dependent changes in folliculogenesis
CoQ10 levels decrease with age and CoQ10 supplementation may mitigate rate of decline of ovarian reserve with aging
ROS at physiological levels play an essential role in the female reproductive system [223]. When overabundant or imbalanced, ROS may lead to female infertility [224]. CoQ10 is a lipid soluble, natural antioxidant ubiquitously expressed in multiple organ systems including the cumulus oophorus complex and the FF [225]. It is an essential component of oxidative phosphorylation and ATP production in mitochondria and serves as a free radical scavenger with preventing lipid peroxidation and DNA oxidation functioning as an antioxidant [226]. In females, CoQ10 levels decrease with age [227] and levels in FF correlate with the quality of the oocyte contained within the follicle [225, 228]. In the aged oocyte, OXPHOS and ATP synthesis are noted to be reduced in human and mouse samples [229]. CoQ10 supplementation has been demonstrated to protect ovarian reserve from aging in rats and alleviate effects of aging in fertility outcomes [230]. In the aged mice model, CoQ10 treatment delayed ovarian reserve depletion and improved oocyte mitochondrial gene expression while improving mitochondrial activity [229]. This was correlated with an increase in ovulation and improvement in pregnancy rates [229]. One of the mechanisms by which CoQ10 ameliorates the effects of aging is by inhibiting DNA oxidation in mitochondria, thus inhibiting oocyte apoptosis [231]. As previously stated, post ovulatory oocytes were noted to have increased apoptosis and abnormal meiotic assembly [232]. Disrupted mitochondrial function and the increased DNA fragmentation rate in aged oocytes were alleviated with CoQ10 supplementation (Table 1). Negative impacts of mitochondrial dysfunction on meiotic spindle assembly and chromosomal misalignment were also improved with CoQ10 [229].
Furthermore, as reviewed earlier in this article, aging leads to a reduced number of cumulus cells surrounding the oocyte with increased apoptosis. This is accompanied by decreased expression of enzymes involved in CoQ10 synthesis in mice and human CCs [34]. Moreover, with aging, a notable reduction in the mitochondrial pool, glucose uptake [34], mitochondrial respiratory complex activity [250], and decreased expression of several OXPHOS genes are observed. Ben-Meir et al. was able to demonstrate reduced CC apoptosis and increased oocyte quality and quantity after oral CoQ10 supplementation in mice models [34, 229]. Administration of CoQ10 to the aged mice was able to improve mitochondrial metabolism, decrease apoptosis, restore CC number, stimulate glucose uptake, and increase progesterone synthesis [34]. Ben-Meir et al. was also able demonstrate improved mitochondrial OXPHOS gene expression after in vitro CoQ10 supplementation in aged human GCs [250]. In addition, in a systemic review and meta-analysis of women, five randomized control trials involving oral CoQ10 supplementation, it was concluded that oral supplementation of CoQ10 (in varying doses and durations: 600 mg daily for 8 weeks, 600 mg twice daily for 12 weeks or 200 mg three times daily for 8 weeks) increases clinical pregnancy rate with significant results in DOR patients (27.3% vs.17.5%; OR 1.83, 95% CI 1.04–3.24, p = 0.04; I2 0%) [251] (Table 1).
Dehydroepiandrosterone supplementation may improve fertility in aged ovaries
DHEA is an essential prohormone, produced during synthesis of testosterone and estradiol from cholesterol by the zona reticularis layer of adrenal cortex and theca cells of ovary [252]. Its levels are observed to be high especially in early reproductive years and decline with age [253, 254]. Androgen receptors (AR) have been identified at several stages of folliculogenesis [255]. Androgens were shown to increase primary follicle number by activating primordial follicles in primate [256, 257] and mouse models [258]. They also have a role in follicle maturation and preovulatory follicle formation and by increasing FSH-R mRNA synthesis in mice and primate models [259, 260] and by enhancing GC proliferation demonstrated in in vitro [261] and GC specific AR knockout mouse models [262]. Global AR knockout mouse was shown to exhibit subfertility and defective folliculogenesis [263].
Similar to androgens, DHEA was demonstrated to be important for folliculogenesis, and administration of DHEA may improve IVF outcomes especially in populations with DOR or poor ovarian response. Casson et al. was among the first to describe beneficial effects of DHEA with DOR. They noted an increase in ovarian response to gonadotropin stimulation as well as increase in peak estradiol levels [192, 236]. Following Casson’s initial study, multiple mice and human studies with poor responders were conducted using various doses (10 mg – 80 mg per day) of DHEA administration for different durations of time (preIVF treatment or concurrently with ovarian stimulation) and noted improved ovarian function with increasing ovarian response while decreasing number of atretic follicles [192, 237, 238]. Li et al. exhibited increased oocyte quality and improved energy production in CCs of women older than 38 years who were pretreated with DHEA for at least 8 weeks prior to their IVF treatment [233]. In another study conducted by Sozen et al., a galactose induced premature ovarian insufficiency rat model with subfertility, decreased follicular number and increased atresia was used. DHEA was able to promote primordial follicular recruitment and stimulate follicular growth [240] (Table 1).
One of the mechanism that enables positive effects of DHEA was noted to be secondary to its role in increasing IGF1 which in turn may enhance response to gonadotropins and may have positive effects on oocyte quality, especially in poor responders [192, 241,242,243,244]. Furthermore, Zhang et al. corroborated improved BMP15 levels with 2-month of DHEA supplementation in poor ovarian response cases [264]. In addition, DHEA is noted to regulate AR expression and increase follicular recruitment and growth [262]. Another mechanism is attributed to DHEA’s effect in improving mitochondrial hemostasis and transport of oxidative phosphorylation and increasing cumulus cells mitochondrial oxygen consumption while shifting the energy production to aerobic metabolism from anaerobic metabolism which is commonly used in aging follicles [233, 234]. Its alleviating effect on preventing mitochondrial dysfunction with alternating expression of mitochondrial dynamics genes was also described by Wu [235] and Lin [133] (Table 1). PINK1 and PRKN, essential proteins for mitophagy, were noted to be downregulated and MFN1, a mitochondrial fusion gene expression was noted to be upregulated with DHEA treatment in human CCs [234]. DHEA also can slow down apoptosis of CCs of aging follicles [239]. The enhanced ovarian microenvironment with DHEA is hypothesized to decrease age related embryonic aneuploidy [265].
Testosterone is another androgen that can be used as a potential modifier of folliculogenesis. Within the ovary, it is produced theca layer of growing follicles [256]. It has been demonstrated promote FSH-R activity on GCs, leading to increase antral follicle response to gonadotropin stimulation [266]. In rhesus monkeys, testosterone administration was shown to promote primordial pool recruitment and increase primary follicular mass by inducing IGF1 production [257]. Same group also demonstrated increased preantral and small antral follicle numbers with testosterone supplementation in primates [256]. In human studies, baseline testosterone levels have been positively correlated with number of oocytes obtained after ovarian stimulation, although no direct correlation was noted with pregnancy outcome rates [267, 268]. Transdermal testosterone pretreatment on known poor responders was able to increase number of retrieved and fertilized oocytes, good quality embryos, and clinical pregnancy rates [269,270,271]. Reproductive aging has been associated with decreased serum testosterone levels [267, 272]. However, there is currently no available data assessing the impact of testosterone supplementation in ovarian aging mammalian models or among aged women undergoing IVF. Letrozole is an aromatase inhibitor which blocks conversion of testosterone to estrogen, thus leading to increase in testosterone levels. In poor responders, it has been demonstrated to increase number of retrieved oocytes and implantation rate [273] and decrease IVF costs through lowering gonadotropin dosage [274, 275]. However, there is insufficient data on its effect on clinical pregnancy or live birth rates [274, 276].
Vitamin D level is positively correlated with reproductive potential and outcomes
Vitamin D (VD) is a fat-soluble secosteroid that is synthesized predominantly in the skin upon sunlight exposure but may also be obtained from dietary sources. It is metabolized in the liver to 25-hydroxyvitamin D, which is then metabolized in the kidneys by the enzyme 25-hydroxyvitamin D-1α-hydroxylase to its active form, 1,25-dihydroxyvitamin D [277]. This process is tightly regulated by plasma parathyroid hormone and serum calcium and phosphorus levels [277]. In target cells, VD binds to specific VD receptors (VDR) to regulate transcription of genes involved in diverse cellular processes, including pro-differentiation, anti-proliferation, pro-apoptosis, immunosuppression, and anti-inflammation roles [277]. Along with its essential role in bone physiology and health, there is an increasing recognition that VD is critical for normal folliculogenesis and optimizing reproductive potential in women [277, 278].
VD biosynthesis and signaling systems are expressed in ovarian follicles and detected in FF [245, 279,280,281]. In vitro studies on the effects of VD exposure on secondary preantral follicles isolated from rhesus monkeys revealed that VD supplementation improved follicle survival, growth, and function as well as oocyte maturation, and AMH production [245, 246]. They also confirmed that VD biosynthesis and signaling regulate follicular development in a stage-dependent manner, suggesting a potential endocrine and paracrine/autocrine actions of VD in the ovary and a stage-specific trophic effect on follicles [245, 246]. In goat and hen models, levels of VDR mRNA and protein expression correlate positively with antral follicle size [281, 282], suggesting that VD is involved in follicular maturation. VDR null mutant mice exhibit decreased aromatase activity in the ovary, resulting in impaired folliculogenesis [283]. Moreover, results of a study among Cyp27b1 (the rate-limiting enzyme in the synthesis of VD) null mice and wild-type mice randomized to VD-replete or -deficient diets supplemented with calcium, revealed that mice on VD-deficient diets had arrested follicular development and prolonged estrous cycles, with less oocytes retrieved from oviducts following gonadotropin stimulation [284].
VD levels have also been shown to be associated with ovarian reserve, as reflected in serum AMH levels. In premenopausal women with regular menstrual cycles, there was a correlation between circulating VD and AMH in women aged ≥40 years, suggesting that VD deficiency may be associated with lower ovarian reserve in late-reproductive-aged women [248]. In addition, VD acts as a regulator of Amh gene expression in vitro [249]. AMH gene was up-regulated by VD via functional VD response elements that bind the VDR in human prostate cancer cells [249]. Additionally, co-expression of steroidogenic factor 1, a key regulator of AMH, increased basal AMH promoter activity that was also found to be further stimulated by VD [249]. An inverse relationship between serum VD levels and ovarian reserve was also identified in patients with uterine fibroids [285]. Moreover, a VDR polymorphism among patients undergoing ovarian stimulation was correlated with decreased antral follicle counts [286] (Table 1).
VD levels also appear to affect reproductive potential and outcomes. In a study assessing infertile women undergoing IVF, higher FF levels of 25-hydroxyvitamin D were noted to be associated with significantly increased clinical pregnancy and implantation rates [287]. In several prospective studies, serum VD levels correlated positively with the number of mature oocytes retrieved and oocyte fertilization rates in patients undergoing the IVF [288,289,290]. In the same study, multivariable logistic regression analysis adjusting for age, BMI, ethnicity, and number of embryos transferred, identified FF levels of VD as an independent predictor of success of an IVF cycle [287]. In another study assessing a cohort of women undergoing IVF, the adjusted odds ratio for clinical pregnancy in women with vitamin D levels ≥20 ng/mL was significantly increased when compared to women with serum levels < 20 ng/mL [247]. In a subgroup analysis, it was concluded that women with the highest serum levels (> 30 ng/mL) had the highest chances of pregnancy [247]. However, studies assessing the prognostic value of FF VD on IVF outcomes have been inconsistent. Even though women with higher serum and FF vitamin D levels were found to be significantly more likely to achieve clinical pregnancy following IVF-embryo transfer in a prospective cohort study [283], another study noted lower quality embryos and significantly lower clinical pregnancy rates with higher levels of follicular VD levels [284].
Summary
Forming an antral follicle that contains a healthy mature oocyte that is ready to be fertilized from a dormant primordial follicle is a well-orchestrated, complex process requiring exquisite synchronized timing of small intraovarian molecules and mitochondria. In this review, we summarized specific molecules and mechanisms that are crucial to the process of folliculogenesis and described how such molecules may be significantly impacted with aging. We then discuss well-studied modifiers, namely CoQ10, DHEA, and VD, that may be used to potentially improve fertility outcomes from aged follicles.
Availability of data and materials
Not applicable.
Abbreviations
- AFC:
-
Antral follicle count
- AMH:
-
Antimullerian Hormone
- AR:
-
Androgen receptors
- ART:
-
Assisted reproductive technology
- ATP:
-
Adenosine triphosphate
- BMP:
-
Bone morphogenetic protein
- BDNF:
-
Brain derived neurotropic factor
- CL:
-
Corpus luteum
- CC:
-
Cumulus cells
- CoQ10:
-
Coenzyme Q10
- Cyp19a1:
-
Cytochrome P450 family 19 subfamily A member 1 (aromatase gene)
- DHEA:
-
Dehydroepiandrosterone
- DOR:
-
Diminished ovarian reserve
- FF:
-
Follicular fluid
- FGF2:
-
Fibroblast growth factor 2
- FSH:
-
Follicle-stimulating hormone
- GC:
-
Granulosa cells
- GDF:
-
Growth differentiating factor
- GH:
-
Growth hormone
- IGF1:
-
Insulin-like growth factor 1
- IGFBP:
-
Insulin-like growth factor binding protein
- IL6:
-
Interleukin 6
- IUI:
-
Intrauterine insemination
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- Lhcgr:
-
Luteinizing hormone/choriogonadotropin receptor
- mtDNA:
-
Mitochondrial DNA
- mtUPR:
-
Mitochondrial unfolded protein response system
- OXPHOS:
-
Oxidative phosphorylation
- NT:
-
Neurotropin
- ROS:
-
Reactive oxygen species
- Star:
-
Steroidogenic acute regulatory protein
- TNFa:
-
Tumor necrosis factor alpha
- VD:
-
Vitamin D
- VDR:
-
Vitamin D receptor
- VEGF:
-
Vascular endothelial growth factor
References
Kasapoglu I, Seli E. Mitochondrial dysfunction and ovarian aging. Endocrinol. 2020;161:2.
Esencan E, Simsek B, Seli E. Analysis of female demographics in the United States: life expectancy, education, employment, family building decisions, and fertility service utilization. Curr Opin Obstet Gynecol. 2021;33(3):170–7.
Qiao J, Wang ZB, Feng HL, Miao YL, Wang Q, Yu Y, et al. The root of reduced fertility in aged women and possible therapentic options: current status and future perspects. Mol Asp Med. 2014;38:54–85.
Jacobs SL, Metzger DA, Dodson WC, Haney AF. Effect of age on response to human menopausal gonadotropin stimulation. J Clin Endocrinol Metab. 1990;71(6):1525–30.
Tal R, Seifer DB. Ovarian reserve testing: a user's guide. Am J Obstet Gynecol. 2017;217(2):129–40.
Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev. 1996;17(2):121–55.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Sci. 2002;296(5576):2178–80.
Anderson E, Wilkinson RF, Lee G, Meller S. A correlative microscopical analysis of differentiating ovarian follicles of mammals. J Morphol. 1978;156(3):339–66.
Liu C, Peng J, Matzuk MM, Yao HH. Lineage specification of ovarian theca cells requires multicellular interactions via oocyte and granulosa cells. Nat Commun. 2015;6:6934.
Babayev E, Duncan FE. Age-associated changes in cumulus cells and follicular fluid: the local oocyte microenvironment as a determinant of gamete quality. Biol Reprod. 2022;106(2):351–65.
Zeleznik AJ. Follicle selection in primates: "many are called but few are chosen". Biol Reprod. 2001;65(3):655–9.
Canipari R, Palombi F, Riminucci M, Mangia F. Early programming of maturation competence in mouse oogenesis. Dev Biol. 1984;102(2):519–24.
Chesnel F, Eppig JJ. Induction of precocious germinal vesicle breakdown (GVB) by GVB-incompetent mouse oocytes: possible role of mitogen-activated protein kinases rather than p34cdc2 kinase. Biol Reprod. 1995;52(4):895–902.
Eppig JJ, Chesnel F, Hirao Y, O'Brien MJ, Pendola FL, Watanabe S, et al. Oocyte control of granulosa cell development: how and why. Hum Reprod. 1997;12(11):127–32.
Sorensen RA, Wassarman PM. Relationship between growth and meiotic maturation of the mouse oocyte. Dev Biol. 1976;50(2):531–6.
Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712.
Richards JS, Pangas SA. New insights into ovarian function. Handb Exp Pharmacol. 2010;198:3–27.
Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986;114(2):453–62.
Erickson GF, Shimasaki S. The role of the oocyte in folliculogenesis. Trends Endocrinol Metab. 2000;11(5):193–8.
Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nat. 1997;385(6616):525–9.
Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reprod. 2001;122(6):829–38.
Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13(11):569–74.
Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci U S A. 2002;99(5):2890–4.
Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103(2):303–16.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nat. 1996;383(6600):531–5.
McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444–9.
McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9(1):131–6.
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–66.
Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, et al. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet. 2000;25(3):279–83.
Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13(6):1018–34.
Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15(6):707–24.
Eichenlaub-Ritter U. Oocyte ageing and its cellular basis. Int J Dev Biol. 2012;56(10–12):841–52.
Kordowitzki P. Oxidative Stress Induces Telomere Dysfunction and Shortening in Human Oocytes of Advanced Age Donors. Cells. 2021;10(8):1866. https://doi.org/10.3390/cells10081866.
Ben-Meir A, Kim K, McQuaid R, Esfandiari N, Bentov Y, Casper RF, Jurisicova A. Co-Enzyme Q10 Supplementation Rescues Cumulus Cells Dysfunction in a Maternal Aging Model. Antioxidants (Basel). 2019;8(3):58. https://doi.org/10.3390/antiox8030058.
Raman RS, Chan PJ, Corselli JU, Patton WC, Jacobson JD, Chan SR, et al. Comet assay of cumulus cell DNA status and the relationship to oocyte fertilization via intracytoplasmic sperm injection. Hum Reprod. 2001;16(5):831–5.
Seifer DB, Gardiner AC, Ferreira KA, Peluso JJ. Apoptosis as a function of ovarian reserve in women undergoing in vitro fertilization. Fertil Steril. 1996;66(4):593–8.
Klein NA, Battaglia DE, Woodruff TK, Padmanabhan V, Giudice LC, Bremner WJ, et al. Ovarian follicular concentrations of activin, follistatin, inhibin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-2 (IGFBP-2), IGFBP-3, and vascular endothelial growth factor in spontaneous menstrual cycles of normal women of advanced reproductive age. J Clin Endocrinol Metab. 2000;85(12):4520–5.
Seifer DB, Charland C, Berlinsky D, Penzias AS, Haning RV Jr, Naftolin F, et al. Proliferative index of human luteinized granulosa cells varies as a function of ovarian reserve. Am J Obstet Gynecol. 1993;169(6):1531–5.
Pellicer A, Mari M, de los Santos MJ, Simon C, Remohi J, Tarin JJ. Effects of aging on the human ovary: the secretion of immunoreactive alpha-inhibin and progesterone. Fertil Steril. 1994;61(4):663–8.
Jost A. The age factor in the castration of male rabbit fetuses. Proc Soc Exp Biol Med. 1947;66(2):302.
Josso N, Picard JY, Rey R, di Clemente N. Testicular anti-Mullerian hormone: history, genetics, regulation and clinical applications. Pediatr Endocrinol Rev. 2006;3(4):347–58.
Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79(3):415–25.
Josso N, Belville C, di Clemente N, Picard JY. AMH and AMH receptor defects in persistent Mullerian duct syndrome. Hum Reprod Update. 2005;11(4):351–6.
Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinol. 2002;143(3):1076–84.
Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84(10):3836–44.
di Clemente N, Racine C, Pierre A, Taieb J. Anti-Mullerian hormone in female reproduction. Endocr Rev. 2021;42(6):753–82.
La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16(2):113–30.
Seifer DB, Tal R. Anti-Müllerian Hormone: Biology, Role in Ovarian Function and Clinical Significance; 2016.
Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of anti-Mullerian hormone (AMH) on ovarian primordial follicle assembly. Plos one. 2011;6(5):e20087.
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinol. 1999;140(12):5789–96.
Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89(5):1364–70.
Dumont A, Robin G, Catteau-Jonard S, Dewailly D. Role of anti-Mullerian hormone in pathophysiology, diagnosis and treatment of polycystic ovary syndrome: a review. Reprod Biol Endocrinol. 2015;13:137.
Durlinger AL, Gruijters MJ, Kramer P, Karels B, Kumar TR, Matzuk MM, et al. Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinol. 2001;142(11):4891–9.
Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, et al. Intra-cellular mechanism of anti-Mullerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016;433:56–65.
Taieb J, Grynberg M, Pierre A, Arouche N, Massart P, Belville C, et al. FSH and its second messenger cAMP stimulate the transcription of human anti-Mullerian hormone in cultured granulosa cells. Mol Endocrinol. 2011;25(4):645–55.
Scheetz D, Folger JK, Smith GW, Ireland JJ. Granulosa cells are refractory to FSH action in individuals with a low antral follicle count. Reprod Fertil Dev. 2012;24(2):327–36.
Pierre A, Taieb J, Giton F, Grynberg M, Touleimat S, El Hachem H, et al. Dysregulation of the anti-Mullerian hormone system by steroids in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(11):3970–8.
Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, et al. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240–5.
Voutilainen R, Miller WL. Human mullerian inhibitory factor messenger ribonucleic acid is hormonally regulated in the fetal testis and in adult granulosa cells. Mol Endocrinol. 1987;1(9):604–8.
Dumesic DA, Lesnick TG, Stassart JP, Ball GD, Wong A, Abbott DH. Intrafollicular antimullerian hormone levels predict follicle responsiveness to follicle-stimulating hormone (FSH) in normoandrogenic ovulatory women undergoing gonadotropin releasing-hormone analog/recombinant human FSH therapy for in vitro fertilization and embryo transfer. Fertil Steril. 2009;92(1):217–21.
Fanchin R, Schonauer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Mullerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18(2):328–32.
Pierre A, Peigne M, Grynberg M, Arouche N, Taieb J, Hesters L, et al. Loss of LH-induced down-regulation of anti-Mullerian hormone receptor expression may contribute to anovulation in women with polycystic ovary syndrome. Hum Reprod. 2013;28(3):762–9.
Pierre A, Estienne A, Racine C, Picard JY, Fanchin R, Lahoz B, et al. The bone morphogenetic protein 15 up-regulates the anti-Mullerian hormone receptor expression in granulosa cells. J Clin Endocrinol Metab. 2016;101(6):2602–11.
Poole DH, Ocon-Grove OM, Johnson AL. Anti-Mullerian hormone (AMH) receptor type II expression and AMH activity in bovine granulosa cells. Theriogenol. 2016;86(5):1353–60.
Zhao Z, Guo F, Sun X, He Q, Dai Z, Chen X, et al. BMP15 regulates AMH expression via the p38 MAPK pathway in granulosa cells from goat. Theriogenol. 2018;118:72–9.
Convissar S, Armouti M, Fierro MA, Winston NJ, Scoccia H, Zamah AM, et al. Regulation of AMH by oocyte-specific growth factors in human primary cumulus cells. Reprod. 2017;154(6):745–53.
Roy S, Gandra D, Seger C, Biswas A, Kushnir VA, Gleicher N, et al. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinol. 2018;159(9):3433–45.
Zhang Y, Shao L, Xu Y, Cui Y, Liu J, Chian RC. Effect of anti-Mullerian hormone in culture medium on quality of mouse oocytes matured in vitro. Plos one. 2014;9(6):e99393.
Estienne A, Pierre A, di Clemente N, Picard JY, Jarrier P, Mansanet C, et al. Anti-Mullerian hormone regulation by the bone morphogenetic proteins in the sheep ovary: deciphering a direct regulatory pathway. Endocrinol. 2015;156(1):301–13.
Shi J, Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, et al. Bone morphogenetic protein-6 stimulates gene expression of follicle-stimulating hormone receptor, inhibin/activin beta subunits, and anti-Mullerian hormone in human granulosa cells. Fertil Steril. 2009;92(5):1794–8.
Liu XY, Yang YJ, Tang CL, Wang K, Chen JJ, Teng XM, et al. Elevation of antimullerian hormone in women with polycystic ovary syndrome undergoing assisted reproduction: effect of insulin. Fertil Steril. 2019;111(1):157–67.
Fang Y, Lu X, Liu L, Lin X, Sun M, Fu J, et al. Vascular endothelial growth factor induces antiMullerian hormone receptor 2 overexpression in ovarian granulosa cells of in vitro fertilization/intracytoplasmic sperm injection patients. Mol Med Rep. 2016;13(6):5157–62.
Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S Jr, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–9.
Wang T, Liu Y, Lv M, Xing Q, Zhang Z, He X, et al. miR-323-3p regulates the steroidogenesis and cell apoptosis in polycystic ovary syndrome (PCOS) by targeting IGF-1. Gene. 2019;683:87–100.
Jancar N, Virant-Klun I, Osredkar J, Vrtacnik BE. Apoptosis, reactive oxygen species and follicular anti-Mullerian hormone in natural versus stimulated cycles. Reprod BioMed Online. 2008;16(5):640–8.
Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinol. 2007;148(5):2301–8.
Xu J, Xu F, Lawson MS, Tkachenko OY, Ting AY, Kahl CA, et al. Anti-Mullerian hormone is a survival factor and promotes the growth of rhesus macaque preantral follicles during matrix-free culture. Biol Reprod. 2018;98(2):197–207.
Racine C, Genet C, Bourgneuf C, Dupont C, Plisson-Petit F, Sarry J, et al. New anti-Mullerian hormone target genes involved in granulosa cell survival in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2021;106(3):e1271–e89.
Kong HS, Kim SK, Lee J, Youm HW, Lee JR, Suh CS, et al. Effect of exogenous anti-Mullerian hormone treatment on cryopreserved and transplanted mouse ovaries. Reprod Sci. 2016;23(1):51–60.
Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum mullerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–71.
van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–71.
de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–62.
Nelson SM, Larsson P, Mannaerts B, Nyboe Andersen A, Fauser B. Anti-Mullerian hormone variability and its implications for the number of oocytes retrieved following individualized dosing with follitropin delta. Clin Endocrinol. 2019;90(5):719–26.
Araujo VR, Duarte AB, Bruno JB, Pinho Lopes CA, de Figueiredo JR. Importance of vascular endothelial growth factor (VEGF) in ovarian physiology of mammals. Zygote. 2013;21(3):295–304.
Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB. Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab. 1996;81(1):353–9.
Quintana R, Kopcow L, Sueldo C, Marconi G, Rueda NG, Baranao RI. Direct injection of vascular endothelial growth factor into the ovary of mice promotes follicular development. Fertil Steril. 2004;82(3):1101–5.
Roberts AE, Arbogast LK, Friedman CI, Cohn DE, Kaumaya PT, Danforth DR. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol Reprod. 2007;76(2):218–23.
Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Mol Reprod Dev. 2007;74(9):1095–104.
Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, et al. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1999;84(10):3845–51.
Harata T, Ando H, Iwase A, Nagasaka T, Mizutani S, Kikkawa F. Localization of angiotensin II, the AT1 receptor, angiotensin-converting enzyme, aminopeptidase a, adipocyte-derived leucine aminopeptidase, and vascular endothelial growth factor in the human ovary throughout the menstrual cycle. Fertil Steril. 2006;86(2):433–9.
Bruno JB, Celestino JJ, Lima-Verde IB, Lima LF, Matos MH, Araujo VR, et al. Expression of vascular endothelial growth factor (VEGF) receptor in goat ovaries and improvement of in vitro caprine preantral follicle survival and growth with VEGF. Reprod Fertil Dev. 2009;21(5):679–87.
Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Nagura H. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum Reprod. 1998;13(4):953–9.
Kaczmarek MM, Schams D, Ziecik AJ. Role of vascular endothelial growth factor in ovarian physiology - an overview. Reprod Biol. 2005;5(2):111–36.
Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary. Biol Reprod. 2003;68(5):1736–41.
Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor trap R1R2. Endocrinol. 2002;143(7):2797–807.
Mattioli M, Barboni B, Turriani M, Galeati G, Zannoni A, Castellani G, et al. Follicle activation involves vascular endothelial growth factor production and increased blood vessel extension. Biol Reprod. 2001;65(4):1014–9.
Abir R, Ao A, Zhang XY, Garor R, Nitke S, Fisch B. Vascular endothelial growth factor a and its two receptors in human preantral follicles from fetuses, girls, and women. Fertil Steril. 2010;93(7):2337–47.
Aerts JM, Bols PE. Ovarian follicular dynamics: a review with emphasis on the bovine species. Part I: Folliculogenesis and pre-antral follicle development. Reprod Domest Anim. 2010;45(1):171–9.
Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5(12):1115–21.
Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reprod. 2009;138(6):869–81.
Ravindranath N, Little-Ihrig L, Phillips HS, Ferrara N, Zeleznik AJ. Vascular endothelial growth factor messenger ribonucleic acid expression in the primate ovary. Endocrinol. 1992;131(1):254–60.
Koos RD. Increased expression of vascular endothelial growth/permeability factor in the rat ovary following an ovulatory gonadotropin stimulus: potential roles in follicle rupture. Biol Reprod. 1995;52(6):1426–35.
Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90(4):1550–8.
Akiyama I, Yoshino O, Osuga Y, Shi J, Harada M, Koga K, et al. Bone morphogenetic protein 7 increased vascular endothelial growth factor (VEGF)-a expression in human granulosa cells and VEGF receptor expression in endothelial cells. Reprod Sci. 2014;21(4):477–82.
Zhang H, Yang Y, Ma W, Wu H, Zheng X, Hei C, et al. The revascularization and follicular survival of mouse ovarian grafts treated with FSH during cryopreservation by vitrification. Cryo Letters. 2016;37(2):88–102.
Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol. 2004;24(3):435–44.
Gospodarowicz D, Cheng J, Lui GM, Baird A, Esch F, Bohlen P. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinol. 1985;117(6):2383–91.
Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-fc (vascular endothelial growth factor trap(A40)). Endocrinol. 2001;142(7):3244–54.
Van Blerkom J, Antczak M, Schrader R. The developmental potential of the human oocyte is related to the dissolved oxygen content of follicular fluid: association with vascular endothelial growth factor levels and perifollicular blood flow characteristics. Hum Reprod. 1997;12(5):1047–55.
Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G, et al. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science. 1996;273(5279):1219–21.
Friedman CI, Danforth DR, Herbosa-Encarnacion C, Arbogast L, Alak BM, Seifer DB. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril. 1997;68(4):607–12.
Manau D, Balasch J, Jimenez W, Fabregues F, Civico S, Casamitjana R, et al. Follicular fluid concentrations of adrenomedullin, vascular endothelial growth factor and nitric oxide in IVF cycles: relationship to ovarian response. Hum Reprod. 2000;15(6):1295–9.
Kawano Y, Zeineh Hasan K, Fukuda J, Mine S, Miyakawa I. Production of vascular endothelial growth factor and angiogenic factor in human follicular fluid. Mol Cell Endocrinol. 2003;202(1–2):19–23.
Fujii EY, Nakayama M. The measurements of RAGE, VEGF, and AGEs in the plasma and follicular fluid of reproductive women: the influence of aging. Fertil Steril. 2010;94(2):694–700.
Herrick JR, Lane M, Gardner DK, Behboodi E, Memili E, Blash S, et al. Metabolism, protein content, and in vitro embryonic development of goat cumulus-oocyte complexes matured with physiological concentrations of glucose and L-lactate. Mol Reprod Dev. 2006;73(2):256–66.
Harris SE, Leese HJ, Gosden RG, Picton HM. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev. 2009;76(3):231–8.
Wen J, Wang GL, Yuan HJ, Zhang J, Xie HL, Gong S, et al. Effects of glucose metabolism pathways on nuclear and cytoplasmic maturation of pig oocytes. Sci Rep. 2020;10(1):2782.
Lubusky M, Prochazka M, Dhaifalah I, Horak D, Geierova M, Santavy J. Fetal enterolithiasis: prenatal sonographic and MRI diagnosis in two cases of urorectal septum malformation (URSM) sequence. Prenat Diagn. 2006;26(4):345–9.
Downs SM. A gap-junction-mediated signal, rather than an external paracrine factor, predominates during meiotic induction in isolated mouse oocytes. Zygote. 2001;9(1):71–82.
Perkins GA, Frey TG. Recent structural insight into mitochondria gained by microscopy. Micron. 2000;31(1):97–111.
McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16(14):R551–60.
Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015;13:89.
Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13(9):566–78.
Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J Assist Reprod Genet. 2011;28(9):773–83.
Passos JF, Saretzki G, Ahmed S, Nelson G, Richter T, Peters H, et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5(5):e110.
Moiseeva O, Bourdeau V, Roux A, Deschenes-Simard X, Ferbeyre G. Mitochondrial dysfunction contributes to oncogene-induced senescence. Mol Cell Biol. 2009;29(16):4495–507.
Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY). 2012;4(1):3–12.
Sun N, Youle RJ, Finkel T. The mitochondrial basis of aging. Mol Cell. 2016;61(5):654–66.
Kauppila TES, Kauppila JHK, Larsson NG. Mammalian mitochondria and aging: an update. Cell Metab. 2017;25(1):57–71.
van der Reest J, Nardini Cecchino G, Haigis MC, Kordowitzki P. Mitochondria: their relevance during oocyte ageing. Ageing Res Rev. 2021;70:101378.
Babayev E, Wang T, Szigeti-Buck K, Lowther K, Taylor HS, Horvath T, et al. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas. 2016;93:121–30.
Lin LT, Wang PH, Wen ZH, Li CJ, Chen SN, Tsai EM, et al. The application of Dehydroepiandrosterone on improving mitochondrial function and reducing apoptosis of cumulus cells in poor ovarian responders. Int J Med Sci. 2017;14(6):585–94.
Tsui KH, Wang PH, Lin LT, Li CJ. DHEA protects mitochondria against dual modes of apoptosis and necroptosis in human granulosa HO23 cells. Reprod. 2017;154(2):101–10.
Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78(5):1046–8.
Ottolenghi C, Uda M, Hamatani T, Crisponi L, Garcia JE, Ko M, et al. Aging of oocyte, ovary, and human reproduction. Ann N Y Acad Sci. 2004;1034:117–31.
Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99.
Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–87.
Sebastian D, Palacin M, Zorzano A. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol Med. 2017;23(3):201–15.
Davies VJ, Hollins AJ, Piechota MJ, Yip W, Davies JR, White KE, et al. Opa1 deficiency in a mouse model of autosomal dominant optic atrophy impairs mitochondrial morphology, optic nerve structure and visual function. Hum Mol Genet. 2007;16(11):1307–18.
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160(2):189–200.
Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11(8):958–66.
Udagawa O, Ishihara T, Maeda M, Matsunaga Y, Tsukamoto S, Kawano N, et al. Mitochondrial fission factor Drp1 maintains oocyte quality via dynamic rearrangement of multiple organelles. Curr Biol. 2014;24(20):2451–8.
Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott Iii R, et al. Mitofusin 1 is required for female fertility and to maintain ovarian follicular reserve. Cell Death Dis. 2019;10(8):560.
Zhang M, Bener MB, Jiang Z, Wang T, Esencan E, Scott R, et al. Mitofusin 2 plays a role in oocyte and follicle development, and is required to maintain ovarian follicular reserve during reproductive aging. Aging (Albany NY). 2019;11(12):3919–38.
Michaels GS, Hauswirth WW, Laipis PJ. Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev Biol. 1982;94(1):246–51.
Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83(1):52–62.
St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update. 2010;16(5):488–509.
Lin PH, Lin LT, Li CJ, Kao PG, Tsai HW, Chen SN, Wen ZH, Wang PH, Tsui KH. Combining Bioinformatics and Experiments to Identify CREB1 as a Key Regulator in Senescent Granulosa Cells. Diagnostics (Basel). 2020;10(5):295. https://doi.org/10.3390/diagnostics10050295.
Druzhyna NM, Wilson GL, LeDoux SP. Mitochondrial DNA repair in aging and disease. Mech Ageing Dev. 2008;129(7–8):383–90.
Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1(8639):637–9.
Cortopassi GA, Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990;18(23):6927–33.
Suganuma N, Kitagawa T, Nawa A, Tomoda Y. Human ovarian aging and mitochondrial DNA deletion. Horm Res. 1993;39(Suppl 1):16–21.
Keefe DL, Niven-Fairchild T, Powell S, Buradagunta S. Mitochondrial deoxyribonucleic acid deletions in oocytes and reproductive aging in women. Fertil Steril. 1995;64(3):577–83.
Ravichandran K, McCaffrey C, Grifo J, Morales A, Perloe M, Munne S, et al. Mitochondrial DNA quantification as a tool for embryo viability assessment: retrospective analysis of data from single euploid blastocyst transfers. Hum Reprod. 2017;32(6):1282–92.
Fragouli E, McCaffrey C, Ravichandran K, Spath K, Grifo JA, Munne S, et al. Clinical implications of mitochondrial DNA quantification on pregnancy outcomes: a blinded prospective non-selection study. Hum Reprod. 2017;32(11):2340–7.
Treff NR, Zhan Y, Tao X, Olcha M, Han M, Rajchel J, et al. Levels of trophectoderm mitochondrial DNA do not predict the reproductive potential of sibling embryos. Hum Reprod. 2017;32(4):954–62.
Victor AR, Brake AJ, Tyndall JC, Griffin DK, Zouves CG, Barnes FL, et al. Accurate quantitation of mitochondrial DNA reveals uniform levels in human blastocysts irrespective of ploidy, age, or implantation potential. Fertil Steril. 2017;107(1):34–42 e3.
Shang W, Zhang Y, Shu M, Wang W, Ren L, Chen F, et al. Comprehensive chromosomal and mitochondrial copy number profiling in human IVF embryos. Reprod BioMed Online. 2018;36(1):67–74.
Lledo B, Ortiz JA, Morales R, Garcia-Hernandez E, Ten J, Bernabeu A, et al. Comprehensive mitochondrial DNA analysis and IVF outcome. Hum Reprod Open. 2018;2018(4):hoy023.
Klimczak AM, Pacheco LE, Lewis KE, Massahi N, Richards JP, Kearns WG, et al. Embryonal mitochondrial DNA: relationship to embryo quality and transfer outcomes. J Assist Reprod Genet. 2018;35(5):871–7.
Fragouli E, Spath K, Alfarawati S, Kaper F, Craig A, Michel CE, et al. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015;11(6):e1005241.
Diez-Juan A, Rubio C, Marin C, Martinez S, Al-Asmar N, Riboldi M, et al. Mitochondrial DNA content as a viability score in human euploid embryos: less is better. Fertil Steril. 2015;104(3):534–41 e1.
Wang T, Zhang M, Jiang Z, Seli E. Mitochondrial dysfunction and ovarian aging. Am J Reprod Immunol. 2017;77(5). https://doi.org/10.1111/aji.12651.
Wang T, Babayev E, Jiang Z, Li G, Zhang M, Esencan E, et al. Mitochondrial unfolded protein response gene Clpp is required to maintain ovarian follicular reserve during aging, for oocyte competence, and development of pre-implantation embryos. Aging Cell. 2018;17(4):e12784.
Esencan E, Cozzolino M, Imamoglu G, Seli E. Mitochondrial Stress Response Gene Clpp Is Not Required for Granulosa Cell Function. Antioxidants (Basel). 2020;10(1):1. https://doi.org/10.3390/antiox10010001.
Hart RJ. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Front Endocrinol (Lausanne). 2019;10:500.
Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34.
Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76(4):1005–26.
Makarevich AV, Markkula M. Apoptosis and cell proliferation potential of bovine embryos stimulated with insulin-like growth factor I during in vitro maturation and culture. Biol Reprod. 2002;66(2):386–92.
Hernandez ER, Roberts CT Jr, LeRoith D, Adashi EY. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinol. 1989;125(1):572–4.
Oliver JE, Aitman TJ, Powell JF, Wilson CA, Clayton RN. Insulin-like growth factor I gene expression in the rat ovary is confined to the granulosa cells of developing follicles. Endocrinol. 1989;124(6):2671–9.
Zhou J, Chin E, Bondy C. Cellular pattern of insulin-like growth factor-I (IGF-I) and IGF-I receptor gene expression in the developing and mature ovarian follicle. Endocrinol. 1991;129(6):3281–8.
Adashi EY, Resnick CE, D'Ercole AJ, Svoboda ME, Van Wyk JJ. Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev. 1985;6(3):400–20.
Gates GS, Bayer S, Seibel M, Poretsky L, Flier JS, Moses AC. Characterization of insulin-like growth factor binding to human granulosa cells obtained during in vitro fertilization. J Recept Res. 1987;7(6):885–902.
Giudice LC. Insulin-like growth factors and ovarian follicular development. Endocr Rev. 1992;13(4):641–69.
Zhou J, Refuerzo J, Bondy C. Granulosa cell DNA synthesis is strictly correlated with the presence of insulin-like growth factor I and absence of c-fos/c-Jun expression. Mol Endocrinol. 1995;9(7):924–31.
Di Blasio AM, Vigano P, Ferrari A. Insulin-like growth factor-II stimulates human granulosa-luteal cell proliferation in vitro. Fertil Steril. 1994;61(3):483–7.
Christman GM, Randolph JF Jr, Peegel H, Menon KM. Differential responsiveness of luteinized human granulosa cells to gonadotropins and insulin-like growth factor I for induction of aromatase activity. Fertil Steril. 1991;55(6):1099–105.
Greene AD, Patounakis G, Segars JH. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet. 2014;31(8):935–46.
Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18(9):892–9.
Chun SY, Eisenhauer KM, Minami S, Billig H, Perlas E, Hsueh AJ. Hormonal regulation of apoptosis in early antral follicles: follicle-stimulating hormone as a major survival factor. Endocrinol. 1996;137(4):1447–56.
Adashi EY, Resnick CE, Svoboda ME, Van Wyk JJ. A novel role for somatomedin-C in the cytodifferentiation of the ovarian granulosa cell. Endocrinol. 1984;115(3):1227–9.
Adashi EY, Resnick CE, Svoboda ME, Van Wyk JJ. Somatomedin-C synergizes with follicle-stimulating hormone in the acquisition of progestin biosynthetic capacity by cultured rat granulosa cells. Endocrinol. 1985;116(6):2135–42.
Dosouto C, Calaf J, Polo A, Haahr T, Humaidan P. Growth hormone and reproduction: lessons learned from animal models and clinical trials. Front Endocrinol (Lausanne). 2019;10:404.
Jimena P, Castilla JA, Peran F, Molina R, Ramirez JP, Acebal M, et al. Insulin and insulin-like growth factor I in follicular fluid after induction of ovulation in women undergoing in vitro fertilization. J Reprod Fertil. 1992;96(2):641–7.
Mehta BN, Chimote NM, Chimote MN, Chimote NN, Nath NM. Follicular fluid insulin like growth factor-1 (FF IGF-1) is a biochemical marker of embryo quality and implantation rates in in vitro fertilization cycles. J Hum Reprod Sci. 2013;6(2):140–6.
Hsu CJ, Hammond JM. Concomitant effects of growth hormone on secretion of insulin-like growth factor I and progesterone by cultured porcine granulosa cells. Endocrinol. 1987;121(4):1343–8.
Nakamura E, Otsuka F, Inagaki K, Miyoshi T, Matsumoto Y, Ogura K, et al. Mutual regulation of growth hormone and bone morphogenetic protein system in steroidogenesis by rat granulosa cells. Endocrinol. 2012;153(1):469–80.
Jimenez CR, de Azevedo JL, Ciro Alexandre Alves T, Penitente-Filho JM, Goncalves WG. Sequential medium with GH and IGF-1 improved in vitro development of bovine preantral follicles enclosed in ovarian tissue. Reprod Domest Anim. 2018;53(5):1103–13.
Fouany MR, Sharara FI. Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet. 2013;30(9):1239–44.
Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–32.
Schams D, Kosmann M, Berisha B, Amselgruber WM, Miyamoto A. Stimulatory and synergistic effects of luteinising hormone and insulin like growth factor 1 on the secretion of vascular endothelial growth factor and progesterone of cultured bovine granulosa cells. Exp Clin Endocrinol Diabetes. 2001;109(3):155–62.
Gleicher N, Darmon SK, Molinari E, Patrizio P, Barad DH. Importance of IGF-I levels in IVF: potential relevance for growth hormone (GH) supplementation. J Assist Reprod Genet. 2022;39(2):409–16. https://doi.org/10.1007/s10815-021-02379-8.
Gomez JM, Mourot B, Fostier A, Le Gac F. Growth hormone receptors in ovary and liver during gametogenesis in female rainbow trout (Oncorhynchus mykiss). J Reprod Fertil. 1999;115(2):275–85.
Pashaiasl M, Ebrahimi M, Ebrahimie E. Identification of the key regulating genes of diminished ovarian reserve (DOR) by network and gene ontology analysis. Mol Biol Rep. 2016;43(9):923–37.
Stadtmauer L, Vidali A, Lindheim SR, Sauer MV. Follicular fluid insulin-like growth factor-I and insulin-like growth factor-binding protein-1 and -3 vary as a function of ovarian reserve and ovarian stimulation. J Assist Reprod Genet. 1998;15(10):587–93.
Mendoza C, Ruiz-Requena E, Ortega E, Cremades N, Martinez F, Bernabeu R, et al. Follicular fluid markers of oocyte developmental potential. Hum Reprod. 2002;17(4):1017–22.
Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77(5):627–38.
Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem Res. 1996;21(8):929–38.
Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87(2):655–9.
Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Neurotrophin-4/5 and neurotrophin-3 are present within the human ovarian follicle but appear to have different paracrine/autocrine functions. J Clin Endocrinol Metab. 2002;87(10):4569–71.
Seifer DB, Feng B, Shelden RM. Immunocytochemical evidence for the presence and location of the neurotrophin-Trk receptor family in adult human preovulatory ovarian follicles. Am J Obstet Gynecol. 2006;194(4):1129–34.
Spears N, Molinek MD, Robinson LL, Fulton N, Cameron H, Shimoda K, et al. The role of neurotrophin receptors in female germ-cell survival in mouse and human. Development. 2003;130(22):5481–91.
Jensen T, Johnson AL. Expression and function of brain-derived neurotrophin factor and its receptor, TrkB, in ovarian follicles from the domestic hen (Gallus gallus domesticus). J Exp Biol. 2001;204(Pt 12):2087–95.
Dissen GA, Hill DF, Costa ME, Les Dees CW, Lara HE, Ojeda SR. A role for trkA nerve growth factor receptors in mammalian ovulation. Endocrinol. 1996;137(1):198–209.
Dissen GA, Hirshfield AN, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinol. 1995;136(10):4681–92.
Waraksa JA, Lindsay RM, Ip NY, Hutz RJ. Neurotrophin-3 augments steroid secretion by hamster ovarian follicles in vitro. Zool Sci. 1995;12(4):499–502.
Mayerhofer A, Dissen GA, Parrott JA, Hill DF, Mayerhofer D, Garfield RE, et al. Involvement of nerve growth factor in the ovulatory cascade: trkA receptor activation inhibits gap junctional communication between thecal cells. Endocrinol. 1996;137(12):5662–70.
Ojeda SR, Romero C, Tapia V, Dissen GA. Neurotrophic and cell-cell dependent control of early follicular development. Mol Cell Endocrinol. 2000;163(1–2):67–71.
Paredes A, Romero C, Dissen GA, DeChiara TM, Reichardt L, Cornea A, et al. TrkB receptors are required for follicular growth and oocyte survival in the mammalian ovary. Dev Biol. 2004;267(2):430–49.
Buyuk E, Seifer DB. Follicular-fluid neurotrophin levels in women undergoing assisted reproductive technology for different etiologies of infertility. Fertil Steril. 2008;90(5):1611–5.
Childs AJ, Bayne RA, Murray AA, Martins Da Silva SJ, Collins CS, Spears N, et al. Differential expression and regulation by activin of the neurotrophins BDNF and NT4 during human and mouse ovarian development. Dev Dyn. 2010;239(4):1211–9.
Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci U S A. 2005;102(26):9206–11.
Feng B, Chen S, Shelden RM, Seifer DB. Effect of gonadotropins on brain-derived neurotrophic factor secretion by human follicular cumulus cells. Fertil Steril. 2003;80(3):658–9.
Seifer DB, Lambert-Messerlian G, Schneyer AL. Ovarian brain-derived neurotrophic factor is present in follicular fluid from normally cycling women. Fertil Steril. 2003;79(2):451–2.
Zhao P, Qiao J, Huang S, Zhang Y, Liu S, Yan LY, et al. Gonadotrophin-induced paracrine regulation of human oocyte maturation by BDNF and GDNF secreted by granulosa cells. Hum Reprod. 2011;26(3):695–702.
Chow R, Wessels JM, Foster WG. Brain-derived neurotrophic factor (BDNF) expression and function in the mammalian reproductive tract. Hum Reprod Update. 2020;26(4):545–64.
Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, et al. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod. 2007;22(4):995–1002.
Wang X, Sun Z, Zhen J, Yu Q. Brain-derived neurotrophic factor from follicular fluid is positively associated with rate of mature ooocytes collected and cleavage rate in intracytoplasmic sperm injection patients. J Assist Reprod Genet. 2011;28(11):1053–8.
Wu LM, Hu MH, Tong XH, Han H, Shen N, Jin RT, et al. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. Plos one. 2012;7(12):e52331.
Qin X, Zhao Y, Zhang T, Yin C, Qiao J, Guo W, et al. TrkB agonist antibody ameliorates fertility deficits in aged and cyclophosphamide-induced premature ovarian failure model mice. Nat Commun. 2022;13(1):914.
Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43.
Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16(1):80.
Akarsu S, Gode F, Isik AZ, Dikmen ZG, Tekindal MA. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J Assist Reprod Genet. 2017;34(5):599–605.
Hernandez-Camacho JD, Bernier M, Lopez-Lluch G, Navas P. Coenzyme Q10 supplementation in aging and disease. Front Physiol. 2018;9:44.
Morre DM, Guo F, Morre DJ. An aging-related cell surface NADH oxidase (arNOX) generates superoxide and is inhibited by coenzyme Q. Mol Cell Biochem. 2003;254(1–2):101–9.
Turi A, Giannubilo SR, Bruge F, Principi F, Battistoni S, Santoni F, et al. Coenzyme Q10 content in follicular fluid and its relationship with oocyte fertilization and embryo grading. Arch Gynecol Obstet. 2012;285(4):1173–6.
Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–95.
Ozcan P, Ficicioglu C, Kizilkale O, Yesiladali M, Tok OE, Ozkan F, et al. Can coenzyme Q10 supplementation protect the ovarian reserve against oxidative damage? J Assist Reprod Genet. 2016;33(9):1223–30.
Zhang M, ShiYang X, Zhang Y, Miao Y, Chen Y, Cui Z, et al. Coenzyme Q10 ameliorates the quality of postovulatory aged oocytes by suppressing DNA damage and apoptosis. Free Radic Biol Med. 2019;143:84–94.
Qiu D, Hou X, Han L, Li X, Ge J, Wang Q. Sirt2-BubR1 acetylation pathway mediates the effects of advanced maternal age on oocyte quality. Aging Cell. 2018;17(1):e12698. https://doi.org/10.1111/acel.12698.
Li CJ, Lin LT, Tsui KH. Dehydroepiandrosterone Shifts Energy Metabolism to Increase Mitochondrial Biogenesis in Female Fertility with Advancing Age. Nutrients. 2021;13(7):2449. https://doi.org/10.3390/nu13072449.
Li CJ, Chen SN, Lin LT, Chern CU, Wang PH, Wen ZH, Tsui KH. Dehydroepiandrosterone Ameliorates Abnormal Mitochondrial Dynamics and Mitophagy of Cumulus Cells in Poor Ovarian Responders. J Clin Med. 2018;7(10):293. https://doi.org/10.3390/jcm7100293.
Wu X, Li F, Wang X, Li C, Meng Q, Wang C, et al. Antibiotic bedaquiline effectively targets growth, survival and tumor angiogenesis of lung cancer through suppressing energy metabolism. Biochem Biophys Res Commun. 2018;495(1):267–72.
Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–5.
Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9.
Neves AR, Montoya-Botero P, Polyzos NP. The role of androgen supplementation in women with diminished ovarian reserve: time to randomize, not Meta-analyze. Front Endocrinol (Lausanne). 2021;12:653857.
Lin LT, Cheng JT, Wang PH, Li CJ, Tsui KH. Dehydroepiandrosterone as a potential agent to slow down ovarian aging. J Obstet Gynaecol Res. 2017;43(12):1855–62.
Sozen B, Ozekinci M, Erman M, Gunduz T, Demir N, Akouri R. Dehydroepiandrosterone supplementation attenuates ovarian ageing in a galactose-induced primary ovarian insufficiency rat model. J Assist Reprod Genet. 2019;36(10):2181–9.
Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–10.
Yan Z, Lee GY, Anderson E. Influence of dehydroepiandrosterone on the expression of insulin-like growth factor-1 during cystogenesis in polycystic rat ovaries and in cultured rat granulosa cells. Biol Reprod. 1997;57(6):1509–16.
Xie M, Zhong Y, Xue Q, Wu M, Deng X, Santos HO, et al. Impact of dehydroepianrosterone (DHEA) supplementation on serum levels of insulin-like growth factor 1 (IGF-1): a dose-response meta-analysis of randomized controlled trials. Exp Gerontol. 2020;136:110949.
Sonmezer M, Ozmen B, Cil AP, Ozkavukcu S, Tasci T, Olmus H, et al. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reprod BioMed Online. 2009;19(4):508–13.
Xu J, Lawson MS, Xu F, Du Y, Tkachenko OY, Bishop CV, et al. Vitamin D3 regulates follicular development and Intrafollicular vitamin D biosynthesis and signaling in the primate ovary. Front Physiol. 2018;9:1600.
Xu J, Hennebold JD, Seifer DB. Direct vitamin D3 actions on rhesus macaque follicles in three-dimensional culture: assessment of follicle survival, growth, steroid, and antimullerian hormone production. Fertil Steril. 2016;106(7):1815–20 e1.
Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol. 2010;8:91.
Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: Women's interagency HIV study. Fertil Steril. 2012;98(1):228–34.
Malloy PJ, Peng L, Wang J, Feldman D. Interaction of the vitamin D receptor with a vitamin D response element in the Mullerian-inhibiting substance (MIS) promoter: regulation of MIS expression by calcitriol in prostate cancer cells. Endocrinol. 2009;150(4):1580–7.
Ben-Meir A, Yahalomi S, Moshe B, Shufaro Y, Reubinoff B, Saada A. Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging. Fertil Steril. 2015;104(3):724–7.
Florou P, Anagnostis P, Theocharis P, Chourdakis M, Goulis DG. Does coenzyme Q10 supplementation improve fertility outcomes in women undergoing assisted reproductive technology procedures? A systematic review and meta-analysis of randomized-controlled trials. J Assist Reprod Genet. 2020;37(10):2377–87.
Burger HG. Androgen production in women. Fertil Steril. 2002;77(4):S3–5.
Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91(2):644–6.
Gleicher N, Barad DH. Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR). Reprod Biol Endocrinol. 2011;9:67.
Walters KA. Role of androgens in normal and pathological ovarian function. Reprod. 2015;149(4):R193–218.
Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622–9.
Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod. 1999;61(2):353–7.
Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, et al. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinol. 2010;151(2):774–82.
Fujibe Y, Baba T, Nagao S, Adachi S, Ikeda K, Morishita M, et al. Androgen potentiates the expression of FSH receptor and supports preantral follicle development in mice. J Ovarian Res. 2019;12(1):31.
Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84(8):2951–6.
Laird M, Thomson K, Fenwick M, Mora J, Franks S, Hardy K. Androgen stimulates growth of mouse Preantral follicles in vitro: interaction with follicle-stimulating hormone and with growth factors of the TGFbeta superfamily. Endocrinol. 2017;158(4):920–35.
Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–403.
Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, et al. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101(31):11209–14.
Zhang HH, Xu PY, Wu J, Zou WW, Xu XM, Cao XY, et al. Dehydroepiandrosterone improves follicular fluid bone morphogenetic protein-15 and accumulated embryo score of infertility patients with diminished ovarian reserve undergoing in vitro fertilization: a randomized controlled trial. J Ovarian Res. 2014;7:93.
Gleicher N, Weghofer A, Barad DH. Dehydroepiandrosterone (DHEA) reduces embryo aneuploidy: direct evidence from preimplantation genetic screening (PGS). Reprod Biol Endocrinol. 2010;8:140.
Meldrum DR, Chang RJ, Giudice LC, Balasch J, Barbieri RL. Role of decreased androgens in the ovarian response to stimulation in older women. Fertil Steril. 2013;99(1):5–11.
Barbieri RL, Sluss PM, Powers RD, McShane PM, Vitonis A, Ginsburg E, et al. Association of body mass index, age, and cigarette smoking with serum testosterone levels in cycling women undergoing in vitro fertilization. Fertil Steril. 2005;83(2):302–8.
Qin Y, Zhao Z, Sun M, Geng L, Che L, Chen ZJ. Association of basal serum testosterone levels with ovarian response and in vitro fertilization outcome. Reprod Biol Endocrinol. 2011;9:9.
Kim CH, Howles CM, Lee HA. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertil Steril. 2011;95(2):679–83.
Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis L, et al. The use of androgens or androgen-modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(2):127–45.
Nagels HE, Rishworth JR, Siristatidis CS, Kroon B. Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction. Cochrane Database Syst Rev. 2015;11:CD009749.
Frattarelli JL, Gerber MD. Basal and cycle androgen levels correlate with in vitro fertilization stimulation parameters but do not predict pregnancy outcome. Fertil Steril. 2006;86(1):51–7.
Garcia-Velasco JA, Moreno L, Pacheco A, Guillen A, Duque L, Requena A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84(1):82–7.
Ozmen B, Sonmezer M, Atabekoglu CS, Olmus H. Use of aromatase inhibitors in poor-responder patients receiving GnRH antagonist protocols. Reprod BioMed Online. 2009;19(4):478–85.
Goswami SK, Das T, Chattopadhyay R, Sawhney V, Kumar J, Chaudhury K, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19(9):2031–5.
Sunkara SK, Pundir J, Khalaf Y. Effect of androgen supplementation or modulation on ovarian stimulation outcome in poor responders: a meta-analysis. Reprod BioMed Online. 2011;22(6):545–55.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
Xu F, Wolf S, Green O, Xu J. Vitamin D in follicular development and oocyte maturation. Reprod. 2021;161(6):R129–R37.
Potashnik G, Lunenfeld E, Levitas E, Itskovitz J, Albutiano S, Yankowitz N, et al. The relationship between endogenous oestradiol and vitamin D3 metabolites in serum and follicular fluid during ovarian stimulation for in-vitro fertilization and embryo transfer. Hum Reprod. 1992;7(10):1357–60.
Thill M, Becker S, Fischer D, Cordes T, Hornemann A, Diedrich K, et al. Expression of prostaglandin metabolising enzymes COX-2 and 15-PGDH and VDR in human granulosa cells. Anticancer Res. 2009;29(9):3611–8.
Yao X, Zhang G, Guo Y, Ei-Samahy M, Wang S, Wan Y, et al. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenol. 2017;102:162–73.
Wojtusik J, Johnson PA. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol Reprod. 2012;86(3):91.
Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinol. 2000;141(4):1317–24.
Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, et al. Peripubertal vitamin D (3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod. 2012;87(2):51.
Jukic AM, Steiner AZ, Baird DD. Association between serum 25-hydroxyvitamin D and ovarian reserve in premenopausal women. Menopause. 2015;22(3):312–6.
Reginatto MW, Pizarro BM, Antunes RA, Mancebo ACA, Hoffmann L, Fernandes P, et al. Vitamin D receptor TaqI polymorphism is associated with reduced follicle number in women utilizing assisted reproductive technologies. Front Endocrinol (Lausanne). 2018;9:252.
Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–9.
Abadia L, Gaskins AJ, Chiu YH, Williams PL, Keller M, Wright DL, et al. Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am J Clin Nutr. 2016;104(3):729–35.
Liu X, Zhang W, Xu Y, Chu Y, Wang X, Li Q, et al. Effect of vitamin D status on normal fertilization rate following in vitro fertilization. Reprod Biol Endocrinol. 2019;17(1):59.
Wu L, Kwak-Kim J, Zhang R, Li Q, Lu FT, Zhang Y, et al. Vitamin D level affects IVF outcome partially mediated via Th/Tc cell ratio. Am J Reprod Immunol. 2018;80(6):e13050.
Acknowledgements
Not applicable.
Funding
This research received no departmental or external funding.
Author information
Authors and Affiliations
Contributions
D.B.S. conceived the idea for the manuscript. E.E. and G.B. wrote the main manuscript text. E.E. prepared the figures and the table. D.B.S revised the manuscript critically for important intellectual content. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Esencan, E., Beroukhim, G. & Seifer, D.B. Age-related changes in Folliculogenesis and potential modifiers to improve fertility outcomes - A narrative review. Reprod Biol Endocrinol 20, 156 (2022). https://doi.org/10.1186/s12958-022-01033-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-022-01033-x