Abstract
Purpose
Diminished ovarian reserve (DOR) affects 10 % of women seeking fertility treatment. Although it is much more prevalent than premature ovarian failure, less is known about its etiology. The purpose of this article is to review the possible genetic causes of, and associations with, pathologic DOR.
Methods
A systematic review was conducted using PubMed from 1966 through November 2013.
Results
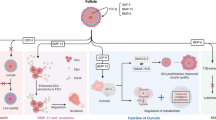
Twenty-one articles identified genes associated with DOR: one gene mutation (FMR1), three polymorphisms (GDF9, FSHR, and ESR1), and seven genes differentially expressed between women with DOR and controls (AMH, LHCGR, IGF1, IGF2, IGF1R, IGF2R and GREM1). Six candidate genes were discovered in mice, including Foxl2, Gdf9, Bmp15, Aire, Wnt4, and Gpr3. Two case reports of chromosomal translocations were also identified.
Conclusions
While the etiology of pathologic DOR is likely multifactorial, it is possible that many cases attributed to an idiopathic cause may have a genetic component. Larger studies are needed to expose the impact gene mutations, polymorphisms, and epigenetics have on pathologic DOR.
Similar content being viewed by others
References
Levi AJ, Raynault MF, Bergh PA, Drews MR, Miller BT, Scott Jr RT. Reproductive outcome in patients with diminished ovarian reserve. Fertil Steril. 2001;76(4):666–9.
Pastore LM, Young SL, Baker VL, Karns LB, Williams CD, Silverman LM. Elevated prevalence of 35–44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci. 2012;19(11):1226–31. doi:10.1177/1933719112446074.
May-Panloup P, Ferre-L’Hotellier V, Moriniere C, Marcaillou C, Lemerle S, Malinge MC, et al. Molecular characterization of corona radiata cells from patients with diminished ovarian reserve using microarray and microfluidic-based gene expression profiling. Hum Reprod. 2012;27(3):829–43. doi:10.1093/humrep/der431.
Gleicher N, Weghofer A, Oktay K, Barad DH. Is the immunological noise of abnormal autoimmunity an independent risk factor for premature ovarian aging? Menopause. 2009;16(4):760–4. doi:10.1097/gme.0b013e318193c48b.
Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod (Oxford, England). 2011;26(7):1616–24. doi:10.1093/humrep/der092.
Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol. 2011;9:23. doi:10.1186/1477-7827-9-23.
Wang TT, Wu YT, Dong MY, Sheng JZ, Leung PC, Huang HF. G546A polymorphism of growth differentiation factor-9 contributes to the poor outcome of ovarian stimulation in women with diminished ovarian reserve. Fertil Steril. 2010;94(6):2490–2. doi:10.1016/j.fertnstert.2010.03.070.
Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):30–6. doi:10.1016/j.ejogrb.2009.05.008.
Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–14. doi:10.1056/NEJMcp0808697.
Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol. 2010;45(5):257–79. doi:10.1677/jme-10-0070.
Ferrarini E, Russo L, Fruzzetti F, Agretti P, De Marco G, Dimida A, et al. Clinical characteristics and genetic analysis in women with premature ovarian insufficiency. Maturitas. 2013;74(1):61–7. doi:10.1016/j.maturitas.2012.09.017.
Lawson R, El-Toukhy T, Kassab A, Taylor A, Braude P, Parsons J, et al. Poor response to ovulation induction is a stronger predictor of early menopause than elevated basal FSH: a life table analysis. Hum Reprod. 2003;18(3):527–33.
Nikolaou D, Lavery S, Turner C, Margara R, Trew G. Is there a link between an extremely poor response to ovarian hyperstimulation and early ovarian failure? Hum Reprod. 2002;17(4):1106–11.
de Boer EJ, den Tonkelaar I, te Velde ER, Burger CW, Klip H, van Leeuwen FE, et al. A low number of retrieved oocytes at in vitro fertilization treatment is predictive of early menopause. Fertil Steril. 2002;77(5):978–85.
Shamilova NN, Marchenko LA, Dolgushina NV, Zaletaev DV, Sukhikh GT. The role of genetic and autoimmune factors in premature ovarian failure. J Assist Reprod Genet. 2013;30(5):617–22. doi:10.1007/s10815-013-9974-4.
Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis detection and clinical relevance. Hum Reprod. 2003;18(6):1137–9.
Vujovic S. Aetiology of premature ovarian failure. Menopause Int. 2009;15(2):72–5. doi:10.1258/mi.2009.009020.
Goswami D, Conway GS. Premature ovarian failure. Horm Res. 2007;68(4):196–202. doi:10.1159/000102537.
Fusco F, Paciolla M, Chen E, Li X, Genesio R, Conti A, et al. Genetic and molecular analysis of a new unbalanced X;18 rearrangement: localization of the diminished ovarian reserve disease locus in the distal Xq POF1 region. Hum Reprod. 2011;26(11):3186–96. doi:10.1093/humrep/der266.
Sun W, Stegmann BJ, Henne M, Catherino WH, Segars JH. A new approach to ovarian reserve testing. Fertil Steril. 2008;90(6):2196–202. doi:10.1016/j.fertnstert.2007.10.080.
Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: is there a link? Eur J Obstet Gynecol Reprod Biol. 1997;74(1):63–6.
Vegetti W, Grazia Tibiletti M, Testa G, de Lauretis Y, Alagna F, Castoldi E, et al. Inheritance in idiopathic premature ovarian failure: analysis of 71 cases. Hum Reprod. 1998;13(7):1796–800.
Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86(4):110. doi:10.1095/biolreprod.111.097501.
Tran S, Zhou X, Lafleur C, Calderon MJ, Ellsworth BS, Kimmins S, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407–21. doi:10.1210/me.2012-1286.
Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–5. doi:10.1038/383531a0.
McMahon HE, Hashimoto O, Mellon PL, Shimasaki S. Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology. 2008;149(6):2807–15. doi:10.1210/en.2007-1550.
Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–66.
Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89(9):4569–74. doi:10.1210/jc.2004-0347.
Barasoain M, Barrenetxea G, Huerta I, Telez M, Carrillo A, Perez C, et al. Study of FMR1 gene association with ovarian dysfunction in a sample from the Basque country. Gene. 2013;521(1):145–9. doi:10.1016/j.gene.2013.03.032.
Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117(4):376–82. doi:10.1007/s00439-005-1326-8.
Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod. 2010;25(5):1335–8. doi:10.1093/humrep/deq058.
Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-mullerian hormone. Fertil Steril. 2009;91(5):1700–6.
Hoogendoorn B, Coleman SL, Guy CA, Smith K, Bowen T, Buckland PR, et al. Functional analysis of human promoter polymorphisms. Hum Mol Genet. 2003;12(18):2249–54. doi:10.1093/hmg/ddg246.
Wang TT, Ke ZH, Song Y, Chen LT, Chen XJ, Feng C, et al. Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013;28(9):2473–81. doi:10.1093/humrep/det291.
Sheikhha MH, Eftekhar M, Kalantar SM. Investigating the association between polymorphism of follicle-stimulating hormone receptor gene and ovarian response in controlled ovarian hyperstimulation. J Hum Reprod Sci. 2011;4(2):86–90. doi:10.4103/0974-1208.86089.
Livshyts G, Podlesnaja S, Kravchenko S, Sudoma I, Livshits L. A distribution of two SNPs in exon 10 of the FSHR gene among the women with a diminished ovarian reserve in Ukraine. J Assist Reprod Genet. 2009;26(1):29–34. doi:10.1007/s10815-008-9279-1.
Altmae S, Haller K, Peters M, Hovatta O, Stavreus-Evers A, Karro H, et al. Allelic estrogen receptor 1 (ESR1) gene variants predict the outcome of ovarian stimulation in in vitro fertilization. Mol Hum Reprod. 2007;13(8):521–6. doi:10.1093/molehr/gam035.
M’Rabet N, Moffat R, Helbling S, Kaech A, Zhang H, de Geyter C. The CC-allele of the PvuII polymorphic variant in intron 1 of the alpha-estrogen receptor gene is significantly more prevalent among infertile women at risk of premature ovarian aging. Fertil Steril. 2012;98(4):965–72.e1-5. doi:10.1016/j.fertnstert.2012.05.048.
Livshyts G, Podlesnaja S, Kravchenko S, Livshits L. Association of PvuII polymorphism in ESR1 gene with impaired ovarian reserve in patients from Ukraine. Reprod Biol. 2013;13(1):96–9. doi:10.1016/j.repbio.2013.01.178.
Yoon SH, Choi YM, Hong MA, Lee GH, Kim JJ, Im HJ, et al. Estrogen receptor {alpha} gene polymorphisms in patients with idiopathic premature ovarian failure. Hum Reprod. 2010;25(1):283–7. doi:10.1093/humrep/dep375.
Chin KV, Seifer DB, Feng B, Lin Y, Shih WC. DNA microarray analysis of the expression profiles of luteinized granulosa cells as a function of ovarian reserve. Fertil Steril. 2002;77(6):1214–8.
Skiadas CC, Duan S, Correll M, Rubio R, Karaca N, Ginsburg ES, et al. Ovarian reserve status in young women is associated with altered gene expression in membrana granulosa cells. Mol Hum Reprod. 2012;18(7):362–71. doi:10.1093/molehr/gas008.
Greenseid K, Jindal S, Hurwitz J, Santoro N, Pal L. Differential granulosa cell gene expression in young women with diminished ovarian reserve. Reprod Sci. 2011;18(9):892–9. doi:10.1177/1933719111398502.
McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19(12):2869–74. doi:10.1093/humrep/deh535.
Jindal S, Greenseid K, Berger D, Santoro N, Pal L. Impaired gremlin 1 (GREM1) expression in cumulus cells in young women with diminished ovarian reserve (DOR). J Assist Reprod Genet. 2012;29(2):159–62. doi:10.1007/s10815-011-9684-8.
Jiang H, Huang X, Su Z, Rao L, Wu S, Zhang T, et al. Genetic analysis of the forkhead transcriptional factor 2 gene in three Chinese families with blepharophimosis syndrome. Mol Vis. 2013;19:418–23.
Park M, Shin E, Won M, Kim JH, Go H, Kim HL, et al. FOXL2 interacts with steroidogenic factor-1 (SF-1) and represses SF-1-induced CYP17 transcription in granulosa cells. Mol Endocrinol. 2010;24(5):1024–36. doi:10.1210/me.2009-0375.
Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: II Different genotype and phenotype for genetic and autoimmune etiologies. Fertil Steril. 2009;91(5):1707–11. doi:10.1016/j.fertnstert.2008.01.099.
Gleicher N, Weghofer A, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. Is androgen production in association with immune system activation potential evidence for existence of a functional adrenal/ovarian autoimmune system in women? Reprod Biol Endocrinol. 2013;11:58. doi:10.1186/1477-7827-11-58.
Lawrenz B, Henes J, Henes M, Neunhoeffer E, Schmalzing M, Fehm T, et al. Impact of systemic lupus erythematosus on ovarian reserve in premenopausal women: evaluation by using anti-Muellerian hormone. Lupus. 2011;20(11):1193–7. doi:10.1177/0961203311409272.
Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. Faseb j. 2010;24(8):3010–25. doi:10.1096/fj.09-145789.
Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356(1–2):65–73. doi:10.1016/j.mce.2011.11.002.
Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, et al. Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci U S A. 2005;102(25):8922–6. doi:10.1073/pnas.0503840102.
Kummer N, Martin JR, Pal L. Diminished ovarian reserve in a woman with a balanced 13;21 translocation. Fertil Steril. 2009;91(3):931 e3-5. doi:10.1016/j.fertnstert.2008.07.1726.
Cosgrave MP, Tyrrell J, McCarron M, Gill M, Lawlor BA. Age at onset of dementia and age of menopause in women with Down’s syndrome. J Intellect Disabil Res. 1999;43(Pt 6):461–5.
Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009;27(5):351–7. doi:10.1055/s-0029-1237423.
Skinner MK, Anway MD. Seminiferous cord formation and germ-cell programming: epigenetic transgenerational actions of endocrine disruptors. Ann N Y Acad Sci. 2005;1061:18–32. doi:10.1196/annals.1336.004.
Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. doi:10.1371/journal.pone.0036129.
Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: an ovarian perspective. Front Neuroendocrinol. 2010;31(4):420–39. doi:10.1016/j.yfrne.2010.06.003.
Layman LC. The genetic basis of female reproductive disorders: etiology and clinical testing. Mol Cell Endocrinol. 2013;370(1–2):138–48. doi:10.1016/j.mce.2013.02.016.
Acknowledgments
We would like to thank Dr. Alan DeCherney and Dr. Peter McGovern for their support.
Funding
This research was supported, in part, by the Program in Reproductive and Adult Endocrinology, NICHD, NIH and ZIA HD-008737- to JHS.
Conflicts of interest
The authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Diminished ovarian reserve (DOR) can be a normal process of aging. In other cases DOR occurs at earlier ages, pathologic DOR, a multifactorial condition which may be caused by genetic or epigenetic anomalies. We review what is currently known about the contribution of genetic causes to the clinical condition of DOR.
Rights and permissions
About this article
Cite this article
Greene, A.D., Patounakis, G. & Segars, J.H. Genetic associations with diminished ovarian reserve: a systematic review of the literature. J Assist Reprod Genet 31, 935–946 (2014). https://doi.org/10.1007/s10815-014-0257-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0257-5