Abstract
Purpose
Almost every female classic galactosemia patient develops primary ovarian insufficiency (POI). The unique pathophysiology of classic galactosemia, with a severely reduced follicle pool at an early age, requires a new therapeutic approach. This study evaluated the effect of dehydroepiandrosterone (DHEA) on ovarian tissue in a galactose-induced POI rat model.
Methods
Pregnant rats were fed with either a normal or a 35% galactose-containing diet from day 3 of conception continuing through weaning of the litters. Galactose-exposed female offspring were further divided into 5 groups on PND21. The first group received no application. Treatment groups were fed orally by gavage once daily with sesame oil (group 2), or DHEA at doses of 0.1 mg/kg (group 3), 1 mg/kg (group 4) or 10 mg/kg (group 5) until PND70. Fertility rates of mothers with galactosemia, body weights (BWs), and ovarian weights of the litters from PND21 to PND70 were recorded. Ovarian follicle count, immunohistochemistry for proliferation and apoptosis marker expressions and TUNEL for cell death assessment were performed in offspring ovaries.
Results
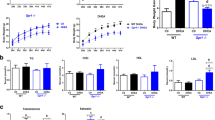
Decreased fertility, ovarian/body weights were observed under galactosemic conditions, together with decreased follicle number and increased atresia. Improved postnatal development, primordial follicle recruitment and follicular growth were observed after DHEA treatment. After DHEA treatment, the expression of Ki67 protein was found to be increased; elevated expression of cleaved-caspase-3 under galactosemia was found to be reduced.
Conclusions
Our data suggests that DHEA treatment may be a potentially useful clinical therapy to improve ovarian ageing in women with POI-induced by galactosemia.




Similar content being viewed by others
References
Shelling AN. Premature ovarian failure. Reproduction. 2010;140(5):633–41.
Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808.
Fridovich-Keil JL, Gubbels CS, Spencer JB, Sanders RD, Land JA, Rubio-Gozalbo E. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34(2):357–66.
Silva CA, Yamakami LYS, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E. Autoimmune primary ovarian insufficiency. Autoimmun Rev. 2014;13(4–5):427–30.
Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PPCA, Wodzig WKWH, Land JA. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update. 2010;16(2):177–88.
Kaufman F, et al. Ovarian failure in galactosaemia. Lancet. 1979;2(8145):737–8.
Liu G, Hale GE, Hughes CL. Galactose metabolism and ovarian toxicity. Reprod Toxicol. 2000;14(5):377–84.
Chen YT, et al. Reduction in oocyte number following prenatal exposure to a diet high in galactose. Science. 1981;214(4525):1145–7.
Swartz WJ, Mattison DR. Galactose inhibition of ovulation in mice. Fertil Steril. 1988;49(3):522–6.
Bandyopadhyay S, et al. Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed. Hum Reprod. 2003;18(10):2031–8.
Burger HG. Androgen production in women. Fertil Steril. 2002;77(Suppl 4):S3–5.
Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–10.
Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertil Steril. 2009;91(2):644–6.
Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Hum Reprod. 2006;21(11):2845–9.
Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Hum Reprod. 2000;15(10):2129–32.
Fusi FM, Ferrario M, Bosisio C, Arnoldi M, Zanga L. DHEA supplementation positively affects spontaneous pregnancies in women with diminished ovarian function. Gynecol Endocrinol. 2013;29(10):940–3.
Labrie C, et al. High bioavailability of dehydroepiandrosterone administered percutaneously in the rat. J Endocrinol. 1996;150 Suppl:S107–18.
Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta-analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–5.
Yan Z, Lee GY, Anderson E. Influence of dehydroepiandrosterone on the expression of insulin-like growth factor-1 during cystogenesis in polycystic rat ovaries and in cultured rat granulosa cells. Biol Reprod. 1997;57(6):1509–16.
van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–92.
Narkwichean A, Jayaprakasan K, Maalouf WE, Hernandez-Medrano JH, Pincott-Allen C, Campbell BK. Effects of dehydroepiandrosterone on in vivo ovine follicular development. Hum Reprod. 2014;29(1):146–54.
Sato K, Iemitsu M, Aizawa K, Mesaki N, Ajisaka R, Fujita S. DHEA administration and exercise training improves insulin resistance in obese rats. Nutr Metab (Lond). 2012;9:47.
Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306(5703):1947–50.
Liu L, Rajareddy S, Reddy P, du C, Jagarlamudi K, Shen Y, et al. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134(1):199–209.
Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, et al. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63(1):94–103.
Segal S. In utero galactose intoxication in animals. Eur J Pediatr. 1995;154(7 Suppl 2):S82–6.
Cramer DW, Harlow BL, Barbieri RL, Ng WG. Galactose-1-phosphate uridyl transferase activity associated with age at menopause and reproductive history. Fertil Steril. 1989;51(4):609–15.
Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–64.
Castrillon DH, et al. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–8.
Watkins WJ, et al. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril. 2006;86(5):1518–21.
Gallardo TD, John GB, Bradshaw K, Welt C, Reijo-Pera R, Vogt PH, et al. Sequence variation at the human FOXO3 locus: a study of premature ovarian failure and primary amenorrhea. Hum Reprod. 2008;23(1):216–21.
Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod Biol Endocrinol. 2011;9:116.
Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137(10):4392–7.
Rice S, Ojha K, Whitehead S, Mason H. Stage-specific expression of androgen receptor, follicle-stimulating hormone receptor, and anti-Mullerian hormone type II receptor in single, isolated, human preantral follicles: relevance to polycystic ovaries. J Clin Endocrinol Metab. 2007;92(3):1034–40.
Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A. 2014;111(8):3008–13.
Acknowledgements
The authors are grateful to Asli Okan (MSc) for the technical help and Andy Cox (PhD) for helpful comments and English proofreading on this article.
Funding
This study was supported by the Akdeniz University Scientific Research Fund (2013.01.0103.008)
Author information
Authors and Affiliations
Contributions
B.S., M.O., M.E., and T.G. designed the study. B.S. and M.O. created the gal-exposed animal model. B.S. performed data collection and statistical analysis. B.S. wrote the article with the help of M.E. and M.O. M.E., R.A. and N.D. provided a critical discussion of the data and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sozen, B., Ozekinci, M., Erman, M. et al. Dehydroepiandrosterone supplementation attenuates ovarian ageing in a galactose-induced primary ovarian insufficiency rat model. J Assist Reprod Genet 36, 2181–2189 (2019). https://doi.org/10.1007/s10815-019-01560-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01560-4