Abstract
The gaps between the complex nature of cancer and therapeutics have been narrowed down due to extensive research in molecular oncology. Despite gathering massive insight into the mysteries of tumor heterogeneity and the molecular framework of tumor cells, therapy resistance and adverse side effects of current therapeutic remain the major challenge. This has shifted the attention towards therapeutics with less toxicity and high efficacy. Myricetin a natural flavonoid has been under the spotlight for its anti-cancer, anti-oxidant, and anti-inflammatory properties. The cutting-edge molecular techniques have shed light on the interplay between myricetin and dysregulated signaling cascades in cancer progression, invasion, and metastasis. However, there are limited data available regarding the nano-delivery platforms composed of myricetin in cancer. In this review, we have provided a comprehensive detail of myricetin-mediated regulation of different cellular pathways, its implications in cancer prevention, preclinical and clinical trials, and its current available nano-formulations for the treatment of various cancers.
Similar content being viewed by others
Introduction
The tumor micro-environment is diverse and involves complex molecular interactions ranging from cell-to-cell communication to metastasis and invasion [8, 35]. In cancer metastasis, cellular machinery is continuously under stress as a huge number of interfaces are taking place on a wide molecular landscape [65, 66]. A comprehensive understanding of the chain of molecular events taking place simultaneously in tumor cells helps in developing an efficacious drug and delivery system [94, 98]. The current therapeutic regimen for cancer has several drawbacks that make them aggressively toxic for both tumors as well as healthy cells [10].
Studies have found that naturally occurring phytochemicals have the therapeutic potential to treat human chronic diseases [9, 84, 100]. These phytochemicals target human long non-coding RNAs (lncRNAs) via RNAi technology. ROR, NEAT1, HI9, and PVTI are the commonest lncRNAs that are modulated by several phytochemicals including curcumin, sulforaphane, Epigallocatechin gallate (EGCG) and resveratrol. To enhance the efficacy of the treatment, conventional chemotherapeutic agents along with phytochemicals as a combination therapy can also be administered to patients [64, 69].
Myricetin, an isoflavonoid, is found in a wide variety of natural sources and is reported to contribute to alleviating numerous physiological anomalies such as cancers, cardiac diseases, inflammatory disorders, and neurological diseases. Several studies have highlighted the antioxidant and free radical scavenging properties of myricetin [6, 120]. Owing to these properties myricetin has been noted as a potential modulator of immune reactions and hypertension. Additionally, it has also been found to be an effective analgesic, anti-allergic and anti-inflammatory agent [2]. However, to perform the task, it makes molecular interaction with a wide range of proteins involved in cellular pathways related to cell survival, growth, differentiation, motility, homeostasis, and apoptosis. It is reported to alter the cell-to-cell molecular cascade in different human disorders [76, 86, 102, 116]. In healthy tissues, myricetin promotes signaling through the Akt pathway to induce cytoprotection, but in cancers, it suppresses this signaling cascade to induce apoptosis [40, 48]. In addition, it is also reported to promote TGFβ signaling in UV-exposed epidermal cells but TGFβ expression is down-regulated after myricetin treatment in the parasite-infected liver of mice [31, 71]. Thus, the current review aims to shed light on the interplay between myricetin and cellular pathways, its role in the prevention of cancer, current up-to-date knowledge regarding the nano-formulations of myricetin and selected clinical trials.
Methodology
This review examines the modules and molecular mechanisms of action of myricetin and myricetin-mediated actions in cancer. For this study, databases were analyzed such as Web of Science, TRIP Database, PubMed/MedLine, Scopus and Google Scholar using the next MeSH terms: “Antineoplastic Agents/pharmacology”, “Apoptosis”, “Cell Line”, “Tumor”, “Cell Proliferation/drug effects”, “Down-Regulation”, “Flavonoids/pharmacology”, “Free Radicals”, “Myricetin”, “Neoplasms/drug therapy”, “Neoplasms/pathology”, “Reactive Oxygen Species”, “Signal Transduction/drug effects”. The most important mechanisms are represented in figures and tables. The chemical formula has been validated according to ChemSpider.
Myricetin as anticancer agent: mediated targeting of cell signaling pathways and molecular implications
PI3K/Akt and associated mTOR pathway
PI3K/Akt pathway in the cell is responsible for controlling various cell activities that are essential for its survival, growth, proliferation, and differentiation. It is shown through immunoblotting that myricetin activates PI3K/Akt signaling by promoting phosphorylation of Akt [40]. Its cytoprotective role is reported by numerous studies but in different cancers, myricetin interacts directly with Akt and suppresses its kinase activity. On a biochemical basis, it competes with adenosine triphosphate (ATP) to bind with Akt [48]. Additionally, myricetin is also reported to target PI3K expression in both in vitro and in vivo studies [37, 75]. Further, it also down-regulates epidermal growth factor receptor (EGFR) for targeting PI3K/Akt pathway [55].
Through targeting PI3K and Akt, myricetin also abrogates the activation of downstream molecular pathways in cancer. It interacts and prevents phosphorylation of both PI3K and Akt, leading to the disrupted mTOR activation [11, 42]. Furthermore, myricetin also modulates the expression of the mTOR pathway effector, p70s6k1 [122]. Regulation of PI3K/Akt/mTOR cascade via myricetin is illustrated to induce autophagy-mediated apoptosis in human colorectal carcinoma cells [128]. Furthermore, myricetin halts signal transduction through Akt/mTOR pathway by up-regulating the protein level of SIRT3 (Akt negative regulator). SIRT3 also suppresses Akt via the LKB1/AMPK pathway [79, 112].
Raf/MEK/ERK pathway
Ras/Raf/MEK/ERK cascade is a significant signaling pathway of the biological system as it involves signals from a diverse range of molecular entities and the expression of genes associated with cell growth, progression, or death, depending on the sort of signal received [12]. Myricetin directly interacts with Raf/MEK/ERK and alters their kinase function. It binds with Raf in an ATP-noncompetitive manner and halts its activity to phosphorylate its downstream molecules [38]. Similarly, myricetin disrupts the kinase activity of MEK1 and suppresses phosphorylation of ERK, inducing an EGF stimulus [53]. It also reduces the protein levels of p38-MAPK as reported in various immunoblotting assays [127].
The myricetin-modulated Raf pathway also influences the associated signaling cascades and molecules in some diseases. For instance, in cancers, myricetin targets the expression of VEGF by directly inhibiting the activity of MEK1 and MKK4. In silico docking, the analysis revealed that myricetin binds with the ATP-binding site of MKK4 [44]. Likewise, it down-regulated MMP9 expression by inhibiting Raf [38]. FAK/ERK signaling cascade is also modulated by myricetin brings about the suppression of F-actin phosphorylation and down-regulates MMP2 and MMP9 [39]. Myricetin's potential to regulate MMPs is been exploited in cancer therapeutics, therapies related to inflammatory diseases, and anti-ageing research [13, 38, 47].
NF-κB pathway
NF-κB signaling is also directly or indirectly modulated by myricetin. This pathway, particularly, participates in inflammatory responses and mediates the cell fate towards cell growth or death [59]. Myricetin-regulated Akt or MEK/ERK pathways are essential for inhibiting the activation of associated downstream NF-κB signaling. It halts the phosphorylation of Akt or ERK1/2 and resultantly, suppresses NF-κB [51, 101]. A study further elucidated through immunoblot assay that myricetin derivate, myricetin-3-O-b-D-lactose sodium salt, significantly reduces the protein levels of phosphorylated IKKα and IκBα [126].
Through suppressing the NF-κB pathway, myricetin also controls the signal transduction from BMP signaling. COX-2 activity is mediated by NF-κB. Myricetin by downregulating NF-κB activity also suppresses COX-2 expression [14]. PGE2 is activated by COX2, which promotes BMP expression. So, myricetin suppresses the BMP pathway via the COX-2/PGE2 cascade by inhibiting NF-kB.
JAK/STAT pathway
JAK/STAT pathway has a central role in mediating cell inflammation and immune responses. It also employs NF-κB signaling to perform its designated function [90]. It is unveiled through pull-down assay that myricetin interacts with and inhibits JAK1, thus, consequently attenuating the phosphorylation-activation of STAT3. Myricetin treatment in JB6 cells inhibited cell transformation by down-regulating the JAK1-STAT3 pathway [49]. Suppression of STAT3 through myricetin action is also documented in cholangiocarcinoma cells [109]. STAT1 is specifically targeted by myricetin in ischemia-induced cardiac injury [88]. Another study revealed reduced phosphorylated-STAT3concentration, along with decreased HAMP mRNA levels, after myricetin treatment has alleviating role in iron-related toxicity in cells [15].
Its presence in a wide amount (8.80 mg/g) is determined in phenolic extracts of red vines through the Folin-Ciocalteu assay. In vitro analysis revealed that red vine extracts containing myricetin have an anti-inflammatory influence on colorectal HT-29 cells. These extracts mold phosphorylated-JAK1 protein levels and attenuate the nuclear localization of STAT3 [70].
TNFα/TNFR signaling
TNFα signaling in a cell is primarily related to inflammation-induced cytotoxicity. Myricetin targets TNFα and disrupts the initiation of the TNFα/TNFR signaling cascade, resulting in down-regulation of downstream Akt, mTOR, and NF-κB pathways [126]. ERK also gets activated upon TNFα stimulus that contributes to cytotoxicity by activating JNK and NF-κB signaling [40, 101]. Hence, by targeting TNFα, myricetin abrogates signal transduction through all these pathways and contributes to cytoprotection. TNFα adaptor protein, TRAF6, is also modulated by myricetin. It induces ubiquitination of TRAF6 and inhibits downstream phosphorylation cascade TAK1/P38/JNK1/2 [58].
Nrf2 pathway
Contrary to JAK-STAT, TNFα, and NF-κB signaling, the Nrf2 pathway acts to curb inflammation and cytotoxicity (induced by oxidative stress) [3]. The activity of Nrf2 is promoted while the NF-κB function is reciprocally suppressed by myricetin [58]. Myricetin modulates the Nrf2 pathway by preventing its ubiquitination-mediated destruction, leading to Nrf2 accumulation in the cytoplasm [78]. Accumulated Nrf2 translocation in a nucleus is also mediated by myricetin leads to activation of ARE which up-regulates ARE-associated genes’ expression [70, 78]. Derivative of myricetin also enhances the expression of Nrf2 associated gene, HO-1 which in turn, inhibits pro-inflammatory cytokines and induces expression of the anti-inflammatory cytokine, IL-10 [14].
TGFβ/Smad pathway
TGFβ pathway has a major role in cell homeostasis and inflammation reduction. Signaling through TGFβ/Smad cascade is induced by myricetin [71]. Myricetin targets TFGβ1 expression to ameliorate noise-induced hearing loss [6]. Similarly, during pulmonary fibrosis, myricetin abrogates interaction between the cytoplasmic tail of the TGFβ receptor and HSP90β, leading to the inhibition of the Smad2/Smad5 complex [56]. A derivate of myricetin named as Myricetin-3-O-b-D-Lactose Sodium Salt also up-regulates the expression of TGFβ [126].
BMP/SMAD and BMP-downstream pathways
BMPs belong to the superfamily of TGFβ protein. It is involved in the differentiation of mesenchymal stem cells into bone. Upon stimulus BMP receptors assemble a heterotetrameric form of SMADs and phosphorylate them, leading to the complex formation and their nuclear translocation. This signaling is also referred to as the canonical BMP pathway [114]. Myricetin is known to enhance protein levels of BMP1 that bolster signaling through both canonical (BMP-SMAD1/5/8) and non-canonical pathways (BMP/P38-MAPK) during osteoblast differentiation and maturation [28]. Further, myricetin also activates ERK, JNK, and P38-MAPK signaling cascades by enhancing the expression of BMP2 [43].
BMP pathway also plays a role in iron metabolism, by inducing hepcidin expression. Hepcidin acts as a modulator of iron metabolism and causes iron accumulation in cells, by down-regulating an iron transporter named ferroportin. Myricetin alleviates iron-associated cytotoxicity by suppressing gene expression of both ferroportin and hepcidin. Mechanistically, myricetin targets SMAD1 that in turn halts transcription of the HAMP gene [15].
Wnt/β-catenin pathways
The Wnt pathway is involved in the regulation of cell fate determination, embryonic organogenesis, cell polarity, and cell migration [104]. Several studies indicate the targeting of the canonical wnt pathway by myricetin. It modulates the phosphorylation status of β-catenin and GSK-3β [43]. In breast carcinoma MCF-7 cells, myricetin promotes dephosphorylation of GSK-3β through inhibiting PAK1 protein expression. PAK1 attenuation abrogates the MEK/ERK signaling cascade, elevating the cytoplasmic concentration of unphosphorylated GSK-3β that captures β-catenin in the cytoplasm and subjects it to proteasomal degradation [36].
Intrinsic apoptotic pathway
Pieces of evidence indicate that myricetin promotes apoptosis through different molecular pathways in different diseases. In resistant cancer cell lines, it enhances the activity of p53 and brings about cell death through both intrinsic and extrinsic pathways [30]. Furthermore, it also has the potential to trigger a caspase-independent cell death pathway. Kim et al. reported that myricetin promotes cell death by inducing Bax and AIF expression. AIF is further associated with the caspase-independent cell death pathway [7, 45].
Myricetin particularly promotes intrinsic apoptosis by directly promoting the expression and mitochondrial translocation of Bax and it is also reported to stimulate Bax-caspase3 signaling [36]. A study in hepatocellular carcinoma cell line HepG2 further explained the myricetin’s molecular mechanism in inducing intrinsic apoptosis. According to a study, myricetin promotes Bcl-2 down-regulation, it also induces Bax translocation to mitochondria and brings about cytochrome c release from the mitochondrial membrane. Myricetin triggers this process by attenuating Akt/ p70s6k1 signaling [122].
The apoptotic potential of myricetin is specific for affected cells. In healthy cells, it activates PI3K/Akt signaling and inhibits ERK/JNK pathway to induce cytoprotective influence. Mechanistically, it causes phosphorylation of Akt and prevents p38-MAPK phosphorylation. Through modulating these pathways, myricetin enhances Bcl-2 levels and prevents cytochrome c release from mitochondria, leading to the cell’s increased capability to tolerate and curb oxidative stress [40].
Other pathways
Few studies have been conducted to delineate myricetin modulatory influence on the Hippo pathway. Its stimulus facilitates the Hippo pathway in its activation. It enhances the kinase function of LAT1/2 that phosphorylates YAP and subjects it to degradation [54]. Likewise, its role in the cell cycle is also reported. For instance, in cancer cell lines, it down-regulates cyclinD1 expression [36]. Similarly, attenuation of cyclinE1 is reported in endometriosis upon myricetin exposure [73]. However, these studies are scarce to understand myricetin's contribution to cell cycle regulation. This opens room for more investigation in determining myricetin's role in cell cycle regulation which could be extremely helpful in exploring its application in cancer treatment studies.
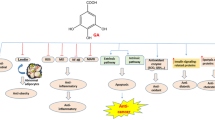
Figure 1 shows the myricetin regulatory influence on several cellular pathways.
Myricetin regulatory influence on several cellular pathways. Myricetin modulates essential cellular pathways that support cell survival, growth, proliferation, cell cycle division, and apoptosis. PI3K/Akt and RAF/MEK/ERK signaling cascades are mainly influenced by myricetin action. Further, it acts as a negative modulator of the NFkB pathway, either by directly acting on it or by promoting signaling through the Nrf2 pathway. It also blocks JAK/STAT pathway. Myricetin also interacts with cell surface receptors of the RTKs family. It directly interacts with the TGF receptor and hinders TGF signaling. It also has a role in preventing beta-catenin accumulation in the cytoplasm by activating GSK 3b. Furthermore, it also turns on the hippo pathway by promoting LATS activity directly or through JNK. Myricetin's pro-apoptotic function is also known. It promotes Bax mitochondrial translocation through p53 activation or via Akt pathway inhibition
Myricetin as chemopreventive agent
It has been reported that myricetin is involved in the inhibition of cancer growth via regulating the expression of key enzymes involved in cancer progression and proliferation [60] (Table 1). A study conducted by Kim et al. reported that myricetin induces apoptosis in the HCT-15 cell lines of human colon cancer, in a dose-dependent manner. Myricetin promoted cytotoxicity in HCT-15 cells via activating the apoptotic genes such as the Bcl-2 associated X Protein. Dose-dependent (5-100 µM) presence of myricetin increased the Bax/Bcl-2 ratio ultimately leading to the recruitment of apoptotic machinery and consequently leading to cell death in HCT-15 cells [45]. Myricetin holds tremendous potential to be used as a potential therapeutic solution for colon cancer. Within molecular docking, cancer cell-based assays, and inhibitory mechanism studies it was found that myricetin prevents activation of human flap endonuclease 1 (hFEN1). The hFEN-1 is an endonuclease responsible for DNA replication and repair. The study showed that myricetin at a specific concentration (IC 50, 690 mM) prevents the attachment of the hFEN1 to Arg100 and Lys93 and consequently inhibits the proliferation of colon cancer [61].
Myricetin has also been reported to suppress angiogenesis in ovarian cancer cell lines. A study using in vitro and in vivo approaches showed that myricetin inhibited angiogenesis in a dose-dependent manner in OVCAR-3 cells [29]. The underlying mechanism involved in the suppression of angiogenesis is still unknown. Future investigations unveiling VEGF and other angiogenesis factors modulation through myricetin will enhance understanding of myricetin therapeutic potential.
In another ovarian cancer cell line SKOV-3, myricetin directly influenced cell viability in a dose-dependent manner. At a dose of 40 µg/ml, myricetin triggered apoptosis in SKOV-3 cells via activation of endoplasmic reticulum stress and DNA double-strand breaks [110]. Similar results were found in the study conducted by Zheng et al. in A2780 and OVCAR-3 cell lines of ovarian cancer [125]. From these findings, it can be justified that flavonoid myricetin has significant potential to be used as a potent inhibitor of ovarian cancer.
A recent study reported that myricetin can inhibit the proliferation and invasion of malignant cells in skin cancer. As potential anticancer mechanism, myricetin inhibited the activation of mitogen-activated protein kinase-1 (MEK1), which in turn prevented the activation of the downstream signal of the ERK/RSK AP-1 axis, thereby exerting inhibition of neoplastic transformation of skin cells. [41, 53]. Moreover, it has come to light less lately that myricetin can bind to central kinases such as the PI3K/Akt/JAK1, RAF1, MEK1, MKK4, and FYN. These kinases are involved in the regulation of a plethora of cellular processes and myricetin can directly inhibit such processes. It has also been investigated that myricetin inhibited the expression of tPA and EGF and explicitly inhibited growth in skin cancer cells in a dose-dependent manner [106]. Myricetin inhibits the growth of human lung adenocarcinoma cell line A549 cells in vitro. Myricetin directly targeted ERK signaling pathway and prevented the invasion and migration of A459 cells in a time-dependent manner [101]. It has also been demonstrated that a combination of myricetin and radiotherapy enhanced the sensitivity of tumor cells (A549 and H1299) toward radiation [121]. Another study has shown that a combination of myricetin and chemotherapy (5-fluorouracil) is efficient to prevent growth, invasion, and metastasis in the esophageal cancer cell EC9706 [113]. In addition to this, findings have also demonstrated the role of myricetin in the modulation and inhibition of bladder cancer. In human T24 bladder cancer cells, myricetin inhibited tumor growth and viability in a dose-dependent and time-dependent manner [105].
Bioavailability
For centuries, plant extracts are used to treat almost all kinds of incurred illnesses, however, anciently, the source plants were solely chosen from past experiences or on a trial basis [89, 96, 99]. The plant extracts contained flavonoids and had been proved useful in treating various anomalies including cancers [72, 87]. In more recent times, the plant extracts are refined and detailed studies are carried out to further unearth the efficacy of these flavonoids and their specified use in treatment strategies[83, 85]. One such flavonoid, myricetin (3,5,7,3’,4’,5’-hexahydroxyflavone) has been found effective against several diseases including cancers. It is readily found in numerous edible parts of plants, including herbs and teas as well as fruits such as oranges, grapes, and berries. Myricetin is a lipophilic and weak acidic compound that works best at pH 2.0 and with low aqueous solubility (16.60 g/mL), making it insoluble in the gastrointestinal tract and thus limiting its efficacy via oral absorption [102, 117].
Despite clinical advancements in human health and cancer treatment, optimal gut absorption and solubility are the two stumbling blocks that have significantly hampered the drug efficacy in treating cancer. The drug concentration and mode of delivery greatly affect its efficacy, thus various strategies have been proposed [81, 95].
Recent developments have attempted to enhance myricetin bioavailability by designing a drug delivery system based on nanotechnology [115]. Such efforts have been made previously on several natural compounds whose therapeutic efficacy was hampered by poor aqueous solubility [33, 34].
Ready-to-go strategies to increase bioavailability and anticancer efficacy: nanoformulations of myricetin
To overcome the low bioavailability and increase oral delivery of myricetin, alternative approaches such as nanoformulation of myricetin are done to improve solubility, drug delivery, and efficacy. In this section, we will discuss different strategies for nanoformulation of myricetin. Many studies have achieved significant improvements to curb various cancers by using myricetin nanoformulations.
Nanoformulation is carried out in numerous ways owing to a wide range of shapes and forms of nanoscale particles [19, 63]. Nanoformulations including nanocrystals, nanoemulsions, polymeric nanoparticles, dendrimers, carbon nanotubes, polymeric micelles, and lipid nanocarriers, are exploited for their ability to circumvent the poor oral bioavailability of insoluble drugs [74]. So various studies have exploited certain advantages of each type and have made a wide range of myricetin-nanoformulations available for use in cancer and other treatments (Fig. 2). The nanoformulation could also provide an added advantage of targeted delivery of drugs. It is achieved by furnishing formulations to target a specific molecule, thus reducing the risk of wastage of dose at one side and protecting other sites from the toxicity and side effects of the drug. Moreover, high efficacy is achieved with low dosage [82]. The synthesis of metal nanoparticles encapsulating drugs including flavonoids is well established and tested practice.
In a recent study, the development of nano-emulsifying drug delivery systems (SNEDDS) of myricetin was achieved, which was formulated in various phases of excipients, constructing pseudo-ternary phase diagrams, and optimizing based on droplet size and emulsification efficacy after drug loading [77].
Another strategy is the microemulsion formulation to improve the issues of drugs. A microemulsion formulation was successfully developed by Guo et al. resulted in efficiently delivering and increased antiproliferative activity by myricetin in human cancer HepG2 cells without damaging normal cells of surroundings. This formulation consisted of Cremophor RH40 (12%), Tween 80 (6%), Transcutol HP (9%), WL 1349 (18%) and distilled water (55%). This microemulsion significantly increased the solubility of myricetin 1225 times more than myricetin alone [24].
Myricetin micelles (Myr-MCs) have also been formulated using a range of micelles compounds for their utilization in the treatment of various anomalies. One such nanocarrier was HS15-Myr micelle based on polyoxyl 15 hydroxystearate micelles encapsulating myricetin. The resulting ultra-small-sized micelles (12.17 ± 0.73 nm) demonstrated higher aqueous stability, storage temperature range, and membrane permeation than the free myricetin solution among the accepted pH ranges for eyedrops. These were also seen to permeate into the cornea of a mouse without any damage and showed anti-inflammatory properties [27].
A recent study has synthesized myricetin-mediated silver nanoparticles (Myr-AgNPs) using a green approach to aim for improved therapeutic efficacy of myricetin. They found that the resulted NPs were spherical and showed promising antibacterial effects [57]. In another example, spherical myricetin-gold nanoparticles (Myr-AuNPs) with sizes less than 50 nm were synthesized by adopting a simple and stable ultrasound-assisted method. Interestingly, the graph-theoretical network analysis revealed mTOR as an effective target for Myr-AuNPs in breast cancer cells which was further confirmed by in silico molecular docking. Further studies to assess the anti-cancerous effect of Myr-AuNPs revealed that it could decrease cell viability by acting as a pro-apoptotic agent and could also depolarize mitochondrial membrane potential and elevate reactive oxygen species [67].
Gaber et al. encapsulated myricetin into Gelucire-based solid lipid nanoparticles (SLNs). They proposed that fat-soluble antioxidant in SLN helps the drug survive at a higher temperature. Moreover, cell media is usually not suitable for the survival of the flavonoid, so physiological buffers, as well as simulated fluids, should be supplemented with stabilizers additives. With all their recommendations, they found 300-fold lesser drug degradation rates and 4500-fold increased half-life of myricetin [21], thus clearly indicating the importance of nanoformulation of myricetin and other flavonoids.
The development of inhalable myricetin solid lipid nanoparticles (SLNs) for lung cancer therapy has produced encouraging results. The formulation could be done in two steps, producing complexation of myricetin with phospholipid Lipoid-S100 and nanoencapsulation in Gelucire-based, surfactant-free SLNs, which could result in 75.98 nm diameter nanoparticles with a zeta-potential of -22.5 mV, and encapsulation efficiency of 84.5%. Subsequent experiments with these inhalable myricetin solid lipid nanoparticles showed enhanced cellular uptake and increased efficacy of myricetin with a threefold reduction in IC50 values [68].
Myricetin-loaded NLCs (nanostructured lipid carriers) have also been utilized in combination with another drug. For example, myricetin-loaded NLCs in combination with docetaxel (DXT) in MDA-MBA231 breast cancer cells increased the percentage of apoptosis. The expression of anti-apoptotic genes, Cyclin B1 and Mcl1 was reduced, while the expression of pro-apoptotic factors such as Bax and Bid was significantly increased. [62].
To increase the low solubility of myricetin, Hong et al. synthesized nanosuspensions with myricetin, the particle size ranging from 300 to 500 nm. The results of the study showed that myricetin nanosuspensions had a higher solubility in vitro and efficacy compared to simple myricetin powder [25].
Folic acid (FA)-conjugated bovine serum albumin (BSA) nanoparticles (NPs) encapsulating myricetin could be targeted to bind to folate receptor (FR) positive breast cancer cells. The Myr-loaded BSA NPs were further assembled by a modified desolvation cross-linking technique. An FA-conjugated carrier, N-hydroxysuccinimide (NHS)-FA ester, was successfully synthesized by Kunjiappan et al. This resulted in NPS non-covalently bound to folate receptors and demonstrated fast release of myricetin in targeted breast cancer cells where they appeared to cause a significant decrease in cell viability of targeted cells [50]. In another study, myricetin-loaded mesoporous silica nanoparticles (MSN) combined with multidrug resistance protein (MRP-1) siRNA showed excellent target accuracy in non-small cell lung cancer (NSCLC) cells. Again, FA-conjugation directed the nanoformulations to target lung cancer cells exclusively and the release of the drug into the cells resulted in a decrease in cell viability in myr-nanoformulations treatment combined with MRP 1 than myricetin alone in A549 and NCI-H1299 cell lines [103].
The bioavailability of myricetin can also be increased by the formulation of nanophytosomes. A group of researchers developed nanophytosomes with myricetin using phosphatidylcholine. Myricetin nanophytosomes were formulated using a thin-layer hydration-sonication method with a varied ratio of myricetin, phosphatidylcholine, and cholesterol, respectively. The results of the study showed that the formulation myricetin: phosphatidylcholine: cholesterol in the ratio 1: 1: 0.4 has the highest bioavailability. [17].
Formulation of polymeric carrier based on chitosan-functionalized Pluronic P123/F68 micelles encapsulating myricetin has also shown promising results against glioblastoma cancer. These myricetin micelles (Myr-MCs) exhibited improved cellular uptake and antitumor activity compared to free myricetin in vitro and in vivo. These micelles could also affect apoptotic proteins such as Bcl-2, Bad and Bax in mice [111].
Clinical perspectives on anticancer potential of myricetin
Recent clinical studies
Myricetin in combination with different phytochemicals has been clinically tested for the treatment of diabetes. In a clinical trial spanning over 4 weeks, Blueberin and myricetin were administrated in a quantity of 250 mg blueberry leaves and 50 mg myricetin (300 mg together) on daily basis. This placebo-controlled trial revealed that blueberin in combination with myricetin successfully reduced plasma sugar levels in type-2 diabetic patients. The use of blueberin reduced plasma sugar levels from 143 ± 5.2 mg/L to 104 ± 5.7 mg/L. Blueberin was also found to reduce the serum levels of alanine aminotransferase (ALT), AST, and glutamyltransferase (GGT) and also minimized the levels of C-reactive protein (CRP) which could ultimately prevent inflammation [1]. A blend of chlorogenic acid, myricetin, and quercetin is also known as Emulin has been reported to decrease blood glucose levels in type-2 diabetes patients. The clinical trial of Emulin was conducted on 40 subjects having a range of BSF (blood sugar fasting) between 126 and 249 mg/mL [4]. In addition to its role in diabetes, myricetin has been reported to be tested for its chemopreventive abilities and as an inhibitor of cell proliferation and signaling [16]. In one of the surveys, it was found that the use of myricetin as a dietary supplement prevented the symptoms of prostate cancer and reduced risk [23]. In another clinical study, intake of myricetin in combination with other flavonoids such as apigenin, kaempherol, quercetin, and luteolin prevented the incidence of ovarian cancer [22] however, no exact cause of disease incidence was correlated with any of the above flavonoids [16, 22]. Myricetin as a dietary supplement has also been reported to decrease the risk of the development of lung cancer. In a meta-analysis, it was demonstrated that myricetin and other flavonoids could prevent the risk of lung cancer by 30–40% in 5073 lung cancer patients that were examined [108].
Therapeutic limitations and clinical gaps of myricetin as anticancer agent
Traditional medicinal herbs have majorly contributed to the pharmaceutical industry. But these products possess challenges for drug discovery and drug optimization. In this current era, these challenges are addressed by scientific and technological developments including advancements in microbial culturing, genome mining, and enhanced efficacy of analytical tools. Due to this, the emerging chemotherapeutic resistance challenge can be addressed [5]. According to the studies, reactive oxygen species (ROS) majorly contribute to the initiation, proliferation, metastasis, and anti-apoptotic behavior of cancer cells. Several cells signaling pathways are responsible for the oncogenic behavior of ROS [92, 97]. Radiotherapy, chemotherapy, and surgery are the only effective techniques to combat advanced stages of cancer approximately till the age of 50 years [18] Traditional herbs and their structural analogues are the alternatives and biocompatible approaches to combat cancer cell proliferation and metastasis, thus, directly affecting the prognosis and overall survival rate in cancer patients [26, 32]. There has been a recent surge in the field of phytochemistry that promoted the exploration of a broad range of phytochemicals with the potential to reduce the toxicity caused by the current drugs used for the treatment of cancer [80, 91, 93, 107].
Myricetin has plenty of health benefits and despite its evident role in the treatment of cancer, very limited data is available that justifies its thorough clinical implications. However, emerging preclinical evidence has begun to shed light on the therapeutic efficacy of myricetin. Although emerging preclinical evidence has begun to shed light on its potential anticancer efficacy, there are numerous limitations which need further studies in the future.
An important therapeutic limitation is the lack of translational studies to establish the routes of administration, the exact effective doses for each type of cancer, or combinations with other cytostatic drugs. Another clinical limitation is due to the poor pharmacokinetic characteristics and low potency, as a result, nanoformulations with myricetin to be transported directly to targeted cancer cells were the most promising strategy for increasing its bioavailability. Also, stronger analogues of myricetin may be developed to increase its anticancer potency.
More research is needed to understand in-depth the potential anticancer mechanisms of myricetin, and it is unclear whether these effects are the result of a direct effect on malignant cells or as a result of binding to extracellular targets or effects on angiogenesis.
Overall conclusion
Cancer is a multifactorial disease and several cellular pathways responsible for cell growth, survival and proliferation are modulated simultaneously in disease development and progression. Effective drugs for cancer must have the potential to target different molecular players belonging to different cell signaling cascades. Myricetin is an isoflavonoid that is usually present as glycoside in different fruits, vegetables, nuts, berries and herbs. It has also been found as an important constituent of wine, tea, and some medicinal plants. Several cellular pathways modulated by myricetin in humans are discussed in detail in this paper. Myricetin has been demonstrated to modulate cell pathways essential for supporting tumor cell survival such as PI3K/Akt pathway, nrf signaling, canonical and non-canonical wnt pathway, mTOR pathway, Ras/Raf pathway and JAK/STAT pathway. The interplay between myricetin and signaling pathways sheds light on its importance as a therapeutic solution for cancer. However, further exploration is still required to sketch out the most probable targeting of the cellular network. The major stumbling block to using myricetin at the clinical level is its poor water solubility, pH and low bioavailability. Plenty of research is in the phase of pre-clinical trials to establish nano-formulations of myricetin that can enhance its bioavailability and absorption but still requires herculean efforts to bring it to clinical trials and human use. Considering the importance of myricetin as an important modulator in-depth analysis of its interaction with non-coding RNAs will bring up a new avenue in the smart targeting of cancer cells. Considering such benefits putting myricetin to use as a therapeutic agent has been challenging because of its limited bioavailability and solubility. Nano formulation at in vitro and in vivo levels has proven its significance in enhancing the bioavailability and treatment efficacy of myricetin. The incorporation of myricetin into nanoformulations can be a promising therapeutic option. Further research delineating the dosage safety of myricetin nanoformulation will be a step forward in its clinical application.
Availability of data and materials
Not Applicable.
Abbreviations
- lncRNAs:
-
Human long non-coding RNAs
- RNAi:
-
RNAi
- NEAT1:
-
Nuclear paraspeckle assembly transcript 1
- EGCG:
-
Epigallocatechin gallate
- PI3K:
-
Phosphatidylinositol-3-kinase
- mTOR:
-
Mammalian target of rapamycin
- ATP:
-
Adenosine triphosphate
- EGFR:
-
Epidermal growth factor receptor
- MEK:
-
Mitogen-activated protein kinase kinase
- ERK:
-
Extracellular signal-regulated kinase
- MKK4:
-
Mitogen-activated Protein Kinase Kinase 4
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- IKKα:
-
Inhibitory Kappa B Kinase α
- IκBα:
-
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- BMP:
-
Bone morphogenetic protein
- COX-2:
-
Cyclooxygenase-2
- PGE2:
-
Prostaglandin E2
- JAK/STAT:
-
Janus kinase-signal transducer and activator of transcription
- STAT:
-
Signal transducer and activator of transcription
- HAMP:
-
Human hepcidin gene
- TNF:
-
Tumor necrosis factor
- TRAF6:
-
Tumor necrosis factor receptor (TNFR)-associated factor 6
- TAK1:
-
Transforming growth factor-β-activated kinase 1
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- TGF-β:
-
Transforming growth factor-β
- SMAD:
-
Suppressor of Mothers against Decapentaplegic
- GSK3β:
-
Glycogen synthase kinase
- Bax:
-
B-cell Leukaemia/Lymphoma 2-associated X Protein
- AIF:
-
Apoptosis-inducing factor
- Bcl-2:
-
B-cell lymphoma 2
- LAT1:
-
L-Type Amino Acid Transporter
- TGF:
-
Transforming Growth Factor
- JNK:
-
Jun N-terminal Kinase.
- hFEN1:
-
Human flap endonuclease 1
- VEGF:
-
Vascular endothelial growth factor
- DNA:
-
Deoxyribonucleic Acid
- RSK:
-
Ribosomal protein S6 kinase
- tPA:
-
Tissue plasminogen activator
- EGF:
-
Epidermal growth factor
- ERK:
-
Extracellular signal-regulated kinase
- Myr-AuNPs:
-
Spherical myricetin-gold nanoparticles
- SLNs:
-
Solid lipid nanoparticles
- NLCs:
-
Nanostructured lipid carriers
- MCL1:
-
Myeloid Cell Leukemia 1
References
Abidov M, Ramazanov A, Jimenez Del Rio M, Chkhikvishvili I. Effect of Blueberin on fasting glucose, C-reactive protein and plasma aminotransferases, in female volunteers with diabetes type 2: double-blind, placebo controlled clinical study. Georgian Med News. 2006;141:66–72.
Afroze N, Pramodh S, Hussain A, Waleed M, Vakharia K. A review on myricetin as a potential therapeutic candidate for cancer prevention. 3 Biotech. 2020;10:1–12.
Ahmed SMU, Luo L, Namani A, Wang XJ, Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochimica et Biophysica Acta (BBA) Mol Basis Dis. 2017;1863:585–97.
Ahrens MJ, Thompson DL. Effect of emulin on blood glucose in type 2 diabetics. J Med Food. 2013;16:211–5.
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discovery. 2021;20:200–16.
Bahaloo M, Rezvani ME, Yazd EF, Mehrjerdi FZ, Davari MH, Roohbakhsh A, Mollasadeghi A, Nikkhah H, Vafaei M, Mehrparvar AH. Effect of myricetin on the gene expressions of NOX3, TGF-β1, prestin, and HSP-70 and anti-oxidant activity in the cochlea of noise-exposed rats. Iran J Basic Med Sci. 2020;23:594.
Bröker LE, Kruyt FA, Giaccone G. Cell death independent of caspases: a review. Clin Cancer Res. 2005;11:3155–62.
Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol Lett. 2019;17:4170–5.
Calina D, Buga AM, Mitroi M, Buha A, Caruntu C, Scheau C, Bouyahya A, El Omari N, El Menyiy N, Docea AO. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. J Clin Med. 2020;9:28.
Cano A, Espina M, García ML. Recent advances on antitumor agents-loaded polymeric and lipid-based nanocarriers for the treatment of brain cancer. Curr Pharm Des. 2020;26:1316–30.
Cao J, Chen H, Lu W, Wu Y, Wu X, Xia D, Zhu J. Myricetin induces protective autophagy by inhibiting the phosphorylation of mTOR in HepG2 cells. Anat Rec. 2018;301:786–95.
Chang F, Steelman L, Lee J, Shelton J, Navolanic P, Blalock WL, Franklin R, Mccubrey J. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;08876924:17.
Ci Y, Zhang Y, Liu Y, Lu S, Cao J, Li H, Zhang J, Huang Z, Zhu X, Gao J. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phytother Res. 2018;32:1373–81.
D’Ambrosio M, Bigagli E, Cinci L, Gori A, Brunetti C, Ferrini F, Luceri C. Ethyl acetate extract from Cistus x incanus L. leaves enriched in myricetin and quercetin derivatives, inhibits inflammatory mediators and activates Nrf2/HO-1 pathway in LPS-stimulated RAW 264.7 macrophages. Zeitschrift für Naturforschung C. 2021;76:79–86.
Deng H, Liu S, Pan D, Jia Y, Ma Z-G. Myricetin reduces cytotoxicity by suppressing hepcidin expression in MES23.5 cells. Neural Regen Res. 2021;16:1105.
Devi KP, Rajavel T, Habtemariam S, Nabavi SF, Nabavi SM. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015;142:19–25.
Dewi NA, Eko G, Dzakwan M. Development of the myricetin nano-phytosome formula with phosphatidylcholine variations. Aqua. 2020;1050:5.
Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, Szopa A, Sharifi-Rad J, Docea AO, Mardare I, Calina D, Cho WC. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22:206.
Docea AO, Calina D, Buga AM, Zlatian O, Paoliello MMB, Mogosanu GD, Streba CT, Popescu EL, Stoica AE, Birca AC, Vasile BS, Grumezescu AM, Mogoanta L. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. Int J Mol Sci. 2020;21:17.
Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang W, Zhao G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem. 2015;408:163–70.
Gaber DM, Nafee N, Abdallah OY. Myricetin solid lipid nanoparticles: stability assurance from system preparation to site of action. Eur J Pharm Sci. 2017;109:569–80.
Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–32.
Geybels MS, Verhage BA, Arts IC, Van Schooten FJ, Goldbohm RA, Van Den Brandt PA. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol. 2013;177:1388–98.
Guo RX, Fu X, Chen J, Zhou L, Chen G. Preparation and characterization of microemulsions of myricetin for improving its antiproliferative and antioxidative activities and oral bioavailability. J Agric Food Chem. 2016;64:6286–94.
Hong C, Dang Y, Lin G, Yao Y, Li G, Ji G, Shen H, Xie Y. Effects of stabilizing agents on the development of myricetin nanosuspension and its characterization: an in vitro and in vivo evaluation. Int J Pharm. 2014;477:251–60.
Hossain R, Ray P, Sarkar C, Islam MS, Khan RA, Khalipha ABR, Islam MT, Cho WC, Martorell M, Sharifi-Rad J, Butnariu M, Umbetova A, Calina D. Natural compounds or their derivatives against breast cancer: a computational study. Biomed Res Int. 2022;2022:5886269.
Hou Y, Zhang F, Lan J, Sun F, Li J, Li M, Song K, Wu X. Ultra-small micelles based on polyoxyl 15 hydroxystearate for ocular delivery of myricetin: optimization, in vitro, and in vivo evaluation. Drug Deliv. 2019;26:158–67.
Hsu Y-L, Chang J-K, Tsai C-H, Chien T-TC, Kuo P-L. Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem Pharmacol. 2007;73:504–14.
Huang H, Chen AY, Rojanasakul Y, Ye X, Rankin GO, Chen YC. Dietary compounds galangin and myricetin suppress ovarian cancer cell angiogenesis. J Funct Foods. 2015;15:464–75.
Huang H, Chen AY, Ye X, Li B, Rojanasakul Y, Rankin GO, Chen YC. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int J Oncol. 2015;47:1494–502.
Huang P, Zhou M, Cheng S, Hu Y, Gao M, Ma Y, Limpanont Y, Zhou H, Dekumyoy P, Cheng Y. Myricetin possesses anthelmintic activity and attenuates hepatic fibrosis via modulating TGFβ1 and Akt signaling and shifting Th1/Th2 balance in Schistosoma japonicum-infected mice. Front Immunol. 2020;11:593.
Islam MT, Quispe C, El-Kersh DM, Shill MC, Bhardwaj K, Bhardwaj P, Sharifi-Rad J, Martorell M, Hossain R, Al-Harrasi A, Al-Rawahi A, Butnariu M, Rotariu LS, Suleria HAR, Taheri Y, Docea AO, Calina D, Cho WC. A literature-based update on Benincasa hispida (Thunb.) Cogn.: traditional uses, nutraceutical, and phytopharmacological profiles. Oxid Med Cell Longev. 2021;2021:6349041.
Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Sadia H, Qadri QR, Raza S, Irshad A, Akbar A. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021;21:1–12.
Javed Z, Khan K, Rasheed A, Sadia H, Shahwani MN, Irshad A, Raza S, Salehi B, Sharifi-Rad J, Suleria HA. Targeting androgen receptor signaling with MicroRNAs and curcumin: a promising therapeutic approach for Prostate Cancer Prevention and intervention. Cancer Cell Int. 2021;21:1–13.
Javed Z, Khan K, Sadia H, Raza S, Salehi B, Sharifi-Rad J, Cho WC. LncRNA & Wnt signaling in colorectal cancer. Cancer Cell Int. 2020;20:1–10.
Jiao D, Zhang XD. Myricetin suppresses p21-activated kinase 1 in human breast cancer MCF-7 cells through downstream signaling of the β-catenin pathway. Oncol Rep. 2016;36:342–8.
Jung SK, Lee KW, Byun S, Lee EJ, Kim J-E, Bode AM, Dong Z, Lee HJ. Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis. 2010;31:911–7.
Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, Heo Y-S, Kang NJ, Bode AM, Dong Z. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010;79:1455–61.
Kang HR, Moon JY, Ediriweera MK, Song YW, Cho M, Kasiviswanathan D, Cho SK. Dietary flavonoid myricetin inhibits invasion and migration of radioresistant lung cancer cells (A549-IR) by suppressing MMP-2 and MMP-9 expressions through inhibition of the FAK-ERK signaling pathway. Food Sci Nutr. 2020;8:2059–67.
Kang KA, Wang ZH, Zhang R, Piao MJ, Kim KC, Kang SS, Kim YW, Lee J, Park D, Hyun JW. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 2015;16(1):1482–3.
Kang NJ, Jung SK, Lee KW, Lee HJ. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann N Y Acad Sci. 2011;1229:124–32.
Kim GD. Myricetin inhibits angiogenesis by inducing apoptosis and suppressing PI3K/Akt/mTOR signaling in endothelial cells. J Cancer Prev. 2017;22:219.
Kim H-Y, Park S-Y, Choung S-Y. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP-2/Smad and ERK/JNK/p38 mitogen-activated protein kinase signaling pathway. Eur J Pharmacol. 2018;834:84–91.
Kim J-E, Kwon JY, Lee DE, Kang NJ, Heo Y-S, Lee KW, Lee HJ. MKK4 is a novel target for the inhibition of tumor necrosis factor-α-induced vascular endothelial growth factor expression by myricetin. Biochem Pharmacol. 2009;77:412–21.
Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Res. 2014;34:701–6.
Knickle A, Fernando W, Greenshields AL, Rupasinghe HV, Hoskin DW. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol. 2018;118:154–67.
Ko S-Y. Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells. Arch Oral Biol. 2012;57:1623–32.
Kumamoto T, Fujii M, Hou D-X. Akt is a direct target for myricetin to inhibit cell transformation. Mol Cell Biochem. 2009;332:33–41.
Kumamoto T, Fujii M, Hou D-X. Myricetin directly targets JAK1 to inhibit cell transformation. Cancer Lett. 2009;275:17–26.
Kunjiappan S, Govindaraj S, Parasuraman P, Sankaranarayanan M, Arunachalam S, Palanisamy P, Mohan UP, Babkiewicz E, Maszczyk P, Vellaisamy S. Design, in silico modelling and functionality theory of folate-receptor-targeted myricetin-loaded bovine serum albumin nanoparticle formulation for cancer treatment. Nanotechnology. 2020;31: 155102.
Lee CS. Flavonoid myricetin inhibits TNF-α-stimulated production of inflammatory mediators by suppressing the Akt, mTOR and NF-κB pathways in human keratinocytes. Eur J Pharmacol. 2016;784:164–72.
Lee JH, Choi YJ, Park S-H, Nam MJ. Potential role of nucleoside diphosphate kinase in myricetin-induced selective apoptosis in colon cancer HCT-15 cells. Food Chem Toxicol. 2018;116:315–22.
Lee KW, Kang NJ, Rogozin EA, Kim H-G, Cho YY, Bode AM, Lee HJ, Surh Y-J, Bowden GT, Dong Z. Myricetin is a novel natural inhibitor of neoplastic cell transformation and MEK1. Carcinogenesis. 2007;28:1918–27.
Li M, Chen J, Yu X, Xu S, Li D, Zheng Q, Yin Y. Myricetin suppresses the propagation of hepatocellular carcinoma via down-regulating expression of YAP. Cells. 2019;8:358.
Li W, Xu C, Hao C, Zhang Y, Wang Z, Wang S, Wang W. Inhibition of herpes simplex virus by myricetin through targeting viral gD protein and cellular EGFR/PI3K/Akt pathway. Antiviral Res. 2020;177: 104714.
Li X, Yu H, Liang L, Bi Z, Wang Y, Gao S, Wang M, Li H, Miao Y, Deng R. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem Pharmacol. 2020;178: 114097.
Li Z, Ma W, Ali I, Zhao H, Wang D, Qiu J. Green and facile synthesis and antioxidant and antibacterial evaluation of dietary myricetin-mediated silver nanoparticles. ACS Omega. 2020;5:32632–40.
Liao HH, Zhang N, Meng YY, Feng H, Yang JJ, Li WJ, Chen S, Wu HM, Deng W, Tang QZ. Myricetin alleviates pathological cardiac hypertrophy via TRAF6/TAK1/MAPK and Nrf2 signaling pathway. Oxid Med Cell Longev. 2019;2019:6304058. https://doi.org/10.1155/2019/6304058.
Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:1–9.
Lu J, Papp LV, Fang J, Rodriguez-Nieto S, Zhivotovsky B, Holmgren A. Inhibition of mammalian thioredoxin reductase by some flavonoids: implications for myricetin and quercetin anticancer activity. Can Res. 2006;66:4410–8.
Ma L, Cao X, Wang H, Lu K, Wang Y, Tu C, Dai Y, Meng Y, Li Y, Yu P. Discovery of Myricetin as a potent inhibitor of human flap endonuclease 1, which potentially can be used as sensitizing agent against HT-29 human Colon Cancer cells. J Agric Food Chem. 2019;67:1656–65.
Maroufi NF, Vahedian V, Mazrakhondi SAM, Kooti W, Khiavy HA, Bazzaz R, Ramezani F, Pirouzpanah SM, Ghorbani M, Akbarzadeh M. Sensitization of MDA-MBA231 breast cancer cell to docetaxel by myricetin loaded into biocompatible lipid nanoparticles via sub-G1 cell cycle arrest mechanism. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1–11.
Matei A-M, Caruntu C, Tampa M, Georgescu SR, Matei C, Constantin MM, Constantin TV, Calina D, Ciubotaru DA, Badarau IA, Scheau C, Caruntu A. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl Sci. 2021;11:4915.
Mishra S, Verma SS, Rai V, Awasthee N, Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G, Gupta SC. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol Life Sci. 2019;76:1947–66.
Mitrur et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022.
Mitrut P, Docea AO, Kamal AM, Mitrut R, Calina D, Gofita E, Padureanu V, Gruia C, Streba L. Colorectal cancer and inflammatory bowel disease. 2016
Mohan UP, Sriram B, Panneerselvam T, Devaraj S, Mubarakali D, Parasuraman P, Palanisamy P, Premanand A, Arunachalam S, Kunjiappan S. Utilization of plant-derived Myricetin molecule coupled with ultrasound for the synthesis of gold nanoparticles against breast cancer. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1963–76.
Nafee N, Gaber DM, Elzoghby AO, Helmy MW, Abdallah OY. Promoted antitumor activity of myricetin against lung carcinoma via nanoencapsulated phospholipid complex in respirable microparticles. Pharm Res. 2020;37:1–24.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803.
Nunes C, Teixeira N, Serra D, Freitas V, Almeida L, Laranjinha J. Red wine polyphenol extract efficiently protects intestinal epithelial cells from inflammation via opposite modulation of JAK/STAT and Nrf2 pathways. Toxicol Res. 2016;5:53–65.
Oh JH, Karadeniz F, Lee JI, Park SY, Seo Y, Kong C-S. Anticatabolic and anti-inflammatory effects of myricetin 3-O-β-d-galactopyranoside in UVA-irradiated dermal cells via repression of MAPK/AP-1 and activation of TGFβ/Smad. Molecules. 2020;25:1331.
Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, Daştan SD, Taheri Y, Calina D, Cho WC. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med Cell Longev. 2022;2022:2910411.
Park S, Song G, Lim W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J Nutr Biochem. 2020;78: 108328.
Pathak K, Raghuvanshi S. Oral bioavailability: issues and solutions via nanoformulations. Clin Pharmacokinet. 2015;54:325–57.
Phillips P, Sangwan V, Borja-Cacho D, Dudeja V, Vickers S, Saluja A. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Lett. 2011;308:181–8.
Pluta R, Januszewski S, Czuczwar SJ. Myricetin as a promising molecule for the treatment of post-ischemic brain neurodegeneration. Nutrients. 2021;13:342.
Qian J, Meng H, Xin L, Xia M, Shen H, Li G, Xie Y. Self-nanoemulsifying drug delivery systems of myricetin: formulation development, characterization, and in vitro and in vivo evaluation. Colloids Surf B. 2017;160:101–9.
Qin S, Chen J, Tanigawa S, Hou DX. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol Nutr Food Res. 2013;57:435–46.
Quan Y, Wang N, Chen Q, Xu J, Cheng W, Di M, Xia W, Gao W-Q. SIRT3 inhibits prostate cancer by destabilizing oncoprotein c-MYC through regulation of the PI3K/Akt pathway. Oncotarget. 2015;6:26494.
Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, Dua K, Chellappan DK, Pabreja K, Satija S, Mehta M, Sureda A, Martorell M, Satmbekova D, Yeskaliyeva B, Sharifi-Rad J, Rasool N, Butnariu M, Bagiu IC, Bagiu RV, Calina D, Cho WC. Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev. 2022;2022:8615242.
Quispe C, Herrera-Bravo J, Javed Z, Khan K, Raza S, Gulsunoglu-Konuskan Z, Daştan SD, Sytar O, Martorell M, Sharifi-Rad J, Calina D. Therapeutic applications of curcumin in diabetes: a review and perspective. Biomed Res Int. 2022;2022:1375892.
Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Castillo CMS, Martorell M, Martins N, Iriti M, Suleria HAR, Sharifi-Rad J. Curcumin’s nanomedicine formulations for therapeutic application in neurological diseases. J Clin Med. 2020;9:35.
Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, Sawicka B, Głowacka A, Abu-Darwish MS, Hussein Tarawneh A, Gadetskaya AV, Cabral C, Salgueiro L, Victoriano M, Martorell M, Docea AO, Abdolshahi A, Calina D, Sharifi-Rad J. Ficus plants: State of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35:1187–217.
Salehi B, Quispe C, Chamkhi I, El Omari N, Balahbib A, Sharifi-Rad J, Bouyahya A, Akram M, Iqbal M, Docea AO, Caruntu C, Leyva-Gómez G, Dey A, Martorell M, Calina D, López V, Les F. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Front Pharmacol. 2021;11:592654–592654.
Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, Calina D, Docea AO, Kamiloglu S, Kregiel D, Antolak H, Pawlikowska E, Sen S, Acharya K, Bashiry M, Selamoglu Z, Martorell M, Sharopov F, Martins N, Namiesnik J, Cho WC. Cucurbita plants: from farm to industry. Appl Sci Basel. 2019;9:21.
Sang H, Huang Y, Tian Y, Liu M, Chen L, Li L, Liu S, Yang J. Multiple modes of action of myricetin in influenza A virus infection. Phytother Res. 2021;35:2797–806.
Sani TA, Mohammadpour E, Mohammadi A, Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M, Etemad L, Shahsavand S. Cytotoxic and apoptogenic properties of Dracocephalum kotschyi aerial part different fractions on calu-6 and mehr-80 lung cancer cell lines. Farmacia. 2017;65:189–99.
Scarabelli TM, Mariotto S, Abdel-Azeim S, Shoji K, Darra E, Stephanou A, Chen-Scarabelli C, Marechal JD, Knight R, Ciampa A. Targeting STAT1 by myricetin and delphinidin provides efficient protection of the heart from ischemia/reperfusion-induced injury. FEBS Lett. 2009;583:531–41.
Scheau C, Caruntu C, Badarau IA, Scheau AE, Docea AO, Calina D, Caruntu A. Cannabinoids and inflammations of the gut-lung-skin barrier. J Pers Med. 2021;11:494.
Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:1–13.
Semwal P, Painuli S, Abu-Izneid T, Rauf A, Sharma A, Daştan SD, Kumar M, Alshehri MM, Taheri Y, Das R, Mitra S, Emran TB, Sharifi-Rad J, Calina D, Cho WC. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid Med Cell Longev. 2022;2022:1035441.
Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Goloshvili T, Cirone Silva NC, Bouyahya A, Vitalini S, Varoni EM, Martorell M, Abdolshahi A, Docea AO, Iriti M, Calina D, Les F, López V, Caruntu C. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol. 2021;12:600139–600139.
Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, Nicola S, Lapava N, Herrera-Bravo J, Salazar LA, Changan S, Kumar M, Calina D. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus bulbophyllum. Evid Based Complement Alternat Med. 2022;2022:6727609.
Sharifi-Rad J, Quispe C, Butnariu M, Rotariu LS, Sytar O, Sestito S, Rapposelli S, Akram M, Iqbal M, Krishna A, Kumar NVA, Braga SS, Cardoso SM, Jafernik K, Ekiert H, Cruz-Martins N, Szopa A, Villagran M, Mardones L, Martorell M, Docea AO, Calina D. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021;21:318–318.
Sharifi-Rad J, Quispe C, Durazzo A, Lucarini M, Souto EB, Santini A, Imran M, Moussa AY, Mostafa NM, El-Shazly M, Sener B, Schoebitz M, Martorell M, Dey A, Calina D, Cruz-Martins N. Resveratrol’ biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herb Med. 2022;32: 100550.
Sharifi-Rad J, Quispe C, Herrera-Bravo J, Martorell M, Sharopov F, Tumer TB, Kurt B, Lankatillake C, Docea AO, Moreira AC, Dias DA, Mahomoodally MF, Lobine D, Cruz-Martins N, Kumar M, Calina D. A pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem Res. 2021;46(9):2205–25.
Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, Zhilina OM, Garsiya ER, Smeriglio A, Trombetta D, Pons DG, Martorell M, Cardoso SM, Razis AFA, Sunusi U, Kamal RM, Rotariu LS, Butnariu M, Docea AO, Calina D. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136.
Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, Živković J, Cruz-Martins N, Klimek-Szczykutowicz M, Ekiert H, Choudhary MI, Ayatollahi SA, Tynybekov B, Kobarfard F, Muntean AC, Grozea I, Daştan SD, Butnariu M, Szopa A, Calina D. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021:3687700.
Sharifi-Rad J, Quispe C, Rahavian A, Pereira Carneiro JN, Rocha JE, Alves Borges Leal AL, Bezerra Morais Braga MF, Melo Coutinho HD, Ansari Djafari A, Alarcón-Zapata P, Martorell M, Antika G, Tumer TB, Cruz-Martins N, Helon P, Paprocka P, Koch W, Docea AO, Calina D. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Front Pharmacol. 2021;12:1886.
Sharifi-Rad J, Quispe C, Shaheen S, El Haouari M, Azzini E, Butnariu M, Sarac I, Pentea M, Ramírez-Alarcón K, Martorell M, Kumar M, Docea AO, Cruz-Martins N, Calina D. Flavonoids as potential anti-platelet aggregation agents: from biochemistry to health promoting abilities. Crit Rev Food Sci Nutr. 2021;13:1–14. https://doi.org/10.1080/10408398.2021.1924612
Shih Y-W, Wu P-F, Lee Y-C, Shi M-D, Chiang T-A. Myricetin suppresses invasion and migration of human lung adenocarcinoma A549 cells: possible mediation by blocking the ERK signaling pathway. J Agric Food Chem. 2009;57:3490–9.
Song X, Tan L, Wang M, Ren C, Guo C, Yang B, Ren Y, Cao Z, Li Y, Pei J. Myricetin: a review of the most recent research. Biomed Pharmacother. 2021;134: 111017.
Song Y, Zhou B, Du X, Wang Y, Zhang J, Ai Y, Xia Z, Zhao G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed Pharmacother. 2020;125: 109561.
Steinhart Z, Angers S. Wnt signaling in development and tissue homeostasis. Development. 2018;145:dev146589.
Sun F, Zheng XY, Ye J, Wu TT, Wang JL, Chen W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr Cancer. 2012;64:599–606.
Sun W, Tao Y, Yu D, Zhao T, Wu L, Yu W, Han W. Myricetin exerts potent anticancer effects on human skin tumor cells. Trop J Pharm Res. 2018;17:1067–72.
Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, Kiliç M, Sen S, Acharya K, Nasiri A, Cruz-Martins N, Tsouh Fokou PV, Ydyrys A, Bassygarayev Z, Daştan SD, Alshehri MM, Calina D, Cho WC. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2022;2022:4024331.
Tang N-P, Zhou B, Wang B, Yu R-B, Ma J. Flavonoids intake and risk of lung cancer: a meta-analysis. Jpn J Clin Oncol. 2009;39:352–9.
Tuponchai P, Kukongviriyapan V, Prawan A, Kongpetch S, Senggunprai L. Myricetin ameliorates cytokine-induced migration and invasion of cholangiocarcinoma cells via suppression of STAT3 pathway. J Cancer Res Ther. 2019;15:157.
Varela-Rodríguez L, Sánchez-Ramírez B, Hernández-Ramírez VI, Varela-Rodríguez H, Castellanos-Mijangos RD, González-Horta C, Chávez-Munguía B, Talamás-Rohana P. Effect of Gallic acid and Myricetin on ovarian cancer models: a possible alternative antitumoral treatment. BMC Complement Med Ther. 2020;20:1–16.
Wang G, Wang J-J, Tang X-J, Du L, Li F. In vitro and in vivo evaluation of functionalized chitosan–pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomed Nanotechnol Biol Med. 2016;12:1263–78.
Wang G, Wang J-J, Wang Y-Z, Feng S, Jing G, Fu X-L. Myricetin nanoliposomes induced SIRT3-mediated glycolytic metabolism leading to glioblastoma cell death. Artif Cells Nanomed Biotechnol. 2018;46:S180–91.
Wang L, Feng J, Chen X, Guo W, Du Y, Wang Y, Zang W, Zhang S, Zhao G. Myricetin enhance chemosensitivity of 5-fluorouracil on esophageal carcinoma in vitro and in vivo. Cancer Cell Int. 2014;14:1–8.
Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105.
Xia W, Zheng B, Li T, Lian F, Lin Y, Liu R. Fabrication, characterization and evaluation of myricetin adsorption onto starch nanoparticles. Carbohyd Polym. 2020;250: 116848.
Xie Y, Wang Y, Xiang W, Wang Q, Cao Y. Molecular mechanisms of the action of myricetin in cancer. Mini Rev Med Chem. 2020;20:123–33.
Yao Y, Lin G, Xie Y, Ma P, Li G, Meng Q, Wu T. Preformulation studies of myricetin: a natural antioxidant flavonoid. Die Pharmazie Int J Pharm Sci. 2014;69:19–26.
Ye C, Zhang C, Huang H, Yang B, Xiao G, Kong D, Tian Q, Song Q, Song Y, Tan H. The natural compound myricetin effectively represses the malignant progression of prostate cancer by inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction. Cell Physiol Biochem. 2018;48:1230–44.
Zang W, Wang T, Wang Y, Li M, Xuan X, Ma Y, Du Y, Liu K, Dong Z, Zhao G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumor Biol. 2014;35:12583–92.
Zhang Q, Zhao Y, Zhang M, Zhang Y, Ji H, Shen L. Recent advances of vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J Pharm Anal. 2020;11(5):555–63.
Zhang S, Wang L, Liu H, Zhao G, Ming L. Enhancement of recombinant myricetin on the radiosensitivity of lung cancer A549 and H1299 cells. Diagn Pathol. 2014;9:1–7.
Zhang X-H, Chen S-Y, Tang L, Shen Y-Z, Luo L, Xu C-W, Liu Q, Li D. Myricetin induces apoptosis in HepG2 cells through Akt/p70S6K/bad signaling and mitochondrial apoptotic pathway. Anti-Cancer Agents Med Chem Former Curr Med Chem Anti Cancer Agents. 2013;13:1575–81.
Zhang X-H, Zou Z-Q, Xu C-W, Shen Y-Z, Li D. Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting the activity of the cyclin B/Cdc2 complex. Mol Med Rep. 2011;4:273–7.
Zhang X, Ling Y, Yu H, Ji Y. Studies on mechanism of myricetin-induced apoptosis in human hepatocellular carcinoma HepG-2 cells. Zhongguo Zhong yao za zhi Zhongguo zhongyao zazhi China J Chin Mater Med. 2010;35:1046–50.
Zheng AW, Chen YQ, Zhao LQ, Feng JG. Myricetin induces apoptosis and enhances chemosensitivity in ovarian cancer cells. Oncol Lett. 2017;13:4974–8.
Zhou X-L, Yang J, Qu X-J, Meng J, Miao R-R, Cui S-X. M10, a myricetin-3-ObD-Lactose sodium salt, prevents ulcerative colitis through inhibiting necroptosis in mice. Front Pharmacol. 2020;11:1458.
Zhou Z, Mao W, Li Y, Qi C, He Y. Myricetin inhibits breast tumor growth and angiogenesis by regulating VEGF/VEGFR2 and p38MAPK signaling pathways. Anat Rec. 2019;302:2186–92.
Zhu M-L, Zhang P-M, Jiang M, Yu S-W, Wang L. Myricetin induces apoptosis and autophagy by inhibiting PI3K/Akt/mTOR signalling in human colon cancer cells. BMC Complement Med Ther. 2020;20:1–9.
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation or all these areas; took part in drafting, revising, or critically reviewing the article; gave the final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Javed, Z., Khan, K., Herrera-Bravo, J. et al. Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int 22, 239 (2022). https://doi.org/10.1186/s12935-022-02663-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02663-2