Abstract
Fisetin, a natural flavonoid, possesses numerous biological activities that have been extensively studied in various diseases. When it comes to cancer, fisetin exhibits a range of biological effects, such as suppressing cell growth, triggering programmed cell death, reducing the formation of new blood vessels, protecting against oxidative stress, and inhibiting cell migration. Moreover, fisetin has the ability to enhance the effectiveness of chemotherapy. The anticancer properties of fisetin can be attributed to a diverse array of molecules and signaling pathways, including vascular endothelial growth factor (VEGF), mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), PI3K/Akt/mTOR, and Nrf2/HO-1. Consequently, fisetin holds promise as a therapeutic agent for anticancer treatment. In this review, we place emphasis on the biological functions and various molecular targets of fisetin in anticancer therapy.
Similar content being viewed by others
Introduction
Cancer poses a significant global health challenge due to its high mortality rates [1, 2]. The development of cancer involves complex processes, including excessive cell proliferation, evasion of apoptosis, sustained angiogenesis, increased migration, and invasion [3, 4]. Unfortunately, current therapies are often expensive, harmful to normal cells, and only provide limited improvements in survival and symptom reduction. Despite advancements in medical technology and surgical techniques, the overall prognosis for many cancer patients remains poor. As a result, there is a pressing need to target anticancer therapy as a crucial area of medical research. Over the past few decades, an increasing number of researchers have focused on unraveling the underlying mechanisms of cancer and exploring new and effective treatment approaches.
Many centuries ago, Hippocrates, often referred to as the father of medicine, famously stated, “Let food be thy medicine and medicine be thy food.” The use of plants for medicinal purposes has been practiced for thousands of years [5]. Flavonoids, which are naturally occurring compounds found in vegetables and fruits, have been extensively studied and shown to have beneficial effects in various diseases, including cancer [6, 7]. Due to their effectiveness, affordability, safety, and the convenience of oral administration, flavonoids have attracted significant research attention to explore their biological activities and underlying mechanisms [8]. Among these flavonoids, fisetin has emerged as a promising candidate. Fisetin, also known as 3,30,40,7-tetrahydroxyflavone, is a natural flavonoid with a well-defined chemical structure. It is widely present in a variety of vegetables and fruits, ranging from 2 to 160 μg/g, including, cucumber, persimmon, strawberry and apple [9]. Fisetin possess diverse biological activities, including inhibiting oxidative stress, anti-inflammation, neuroprotection and anticancer properties [10]. The effects of fisetin have been demonstrated in various types of cancers (Table 1).
Fisetin exhibits several beneficial effects in cancer cells, including the suppressing proliferation, inducing apoptosis, reducing of angiogenesis, preventing oxidative stress, inhibiting migration, and enhancing chemotherapeutic effects. Moreover, the anticancer properties of fisetin are attributed to the involvement of numerous molecules and signaling pathways, including vascular endothelial growth factor (vegf), mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), PI3K/Akt/mTOR and Nrf2/HO-1. This review summarizes the functions and mechanisms of fisetin as a therapeutic agent in anticancer therapy.
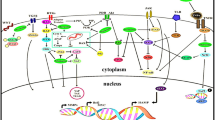
Biological function of fisetin (Fig. 1)
Suppress proliferation
Proliferation plays a central role in the progression of cancer and contributes to cancer invasion, metastasis, and relapse [11]. Inhibiting proliferation is a promising strategy for anticancer therapy. The inhibition of proliferation involves various mechanisms, including preventing cells from undergoing mitosis, arresting the cell cycle, and inducing the Warburg effect. Additionally, the regulation of growth factors and activation of cell adhesion molecules are crucial for controlling proliferation. Moreover, multiple signaling pathways and molecules are involved in the process of proliferation in cancer, including MAPK, ERK, VEGF, PI3K/AKT/mTOR and MMPs [12].
The anticancer effects of fisetin on proliferation have been reported in various types of cancers, including astrocytes, oral cancer, gastric cancer, renal carcinoma, and others [13]. Fisetin’s antiproliferative activity is attributed to its ability to suppress DNA synthesis and induce G0/G1 cell cycle arrest in cancer cells [14]. Specifically, fisetin has been shown to inhibit the CDK6/cyclin D complex, which plays a critical role in the G1 phase of the cell cycle. Furthermore, fisetin can induce premature initiation of chromosome segregation and exit from mitosis without normal cytokinesis in cancer cells. Additionally, fisetin interferes with the ligand binding domain of androgen receptor (AR), resulting in a reduction in cancer cell growth. Various signaling pathways are involved in fisetin’s suppression of proliferation in cancer cells. For example, fisetin inhibits the phosphorylation of AKT and MAPK in colon cancer, which are both implicated in cancer cell proliferation.
Reduce angiogenesis
Angiogenesis is a crucial process involving the formation of new blood vessels from pre-existing vessels within cancerous growth. It represents a significant step in cancer development. Targeting angiogenesis and reducing the formation of new blood vessels is an effective strategy that has shown success in numerous types of cancer cells [15]. In many cancers, the activation of proteases and signaling pathways by specific mediators plays a vital role in driving angiogenesis. These mediators include VEGF, EGF, PDGF, TGF-β, wingless, Wnt signaling, and others [16]. Among these molecules, VEGF is considered the most important factor for angiogenesis in cancer cells [17].
Fisetin is found to have powerful antiangiogenic potential by blocking neovascularization and disrupting the utility of existing blood vessels [18]. Fisetin exhibits its antiangiogenic role by regulating a number of important angiogenesis-related factors in cancer cells, such as VEGF, MMP2/9, eNOS, wingless and Wnt-signaling. VEGF-caused angiogenic response in cancer cells can be strongly inhibited by fisetin [10]. In prostate cancer, fisetin inhibits angiogenesis by reducing NF-κB activity and VEGF expression. Fisetin also inhibits hyaluronan synthesis to upregulate the expression of high-molecular-mass-HA, an antiangiogenic protein [13]. In addition, fisetin can inhibit endothelial cells proliferation and invasion, which are significant in angiogenesis.
Induce apoptosis
Cell apoptosis, a programmed cell death process, plays a critical role in tumor formation and can be initiated through either the extrinsic pathway or the intrinsic pathway [19]. The extrinsic pathway is triggered by the interaction between death receptors and specific ligands, while the intrinsic pathway involves stimuli that act directly on mitochondria [20]. In both pathways, caspases, which are protease enzymes, play crucial roles and ultimately lead to the cleavage of substrates and fragmentation of DNA. Cancer cells often evade apoptosis by upregulating anti-apoptotic proteins and downregulating pro-apoptotic proteins. Therefore, a therapeutic agent that can sensitize cancer cells to undergo apoptosis represents a promising approach for anticancer therapy [21].
Multiple studies have demonstrated that fisetin has the ability to induce apoptosis in cancer cells, and various mechanisms are involved, including the activation of MAPK, NF-κB, p53, and the generation of reactive oxygen species (ROS) [22]. Fisetin treatment leads to increased expression of pro-apoptotic proteins (Bak, Bax, and Bad), decreased expression of anti-apoptotic proteins (Bcl-xl, Bcl-2, and Mcl-1), and the release of cytochrome c [23, 24]. Fisetin also activates caspase 3/8 and calpain, leading to DNA fragmentation and apoptosis in cancer cells [25]. In bladder cancer, fisetin disrupts mitochondrial integrity and initiates the intrinsic pathway to induce apoptosis. Similarly, fisetin induces mitochondrial-mediated apoptosis in lung cancer by generating ROS, which triggers mitochondrial membrane depolarization, cell apoptosis and DNA fragmentation. The ERK1/2 and NF-κB pathways play pivotal roles in the anti-apoptotic effects of fisetin. In cervical cancer, fisetin could induce apoptosis through the phosphorylation of ERK1/2. In cancer cells, fisetin interferes with NF-κB signaling, resulting in the reduction of survivin, TRAF1, Bcl-xl, Bcl-2, and IAP1/2 levels, ultimately inhibiting apoptosis [8]. In colon cancer, fisetin inhibits COX2 expression, leading to the down-regulation of PGE2 secretion and inactivation of β-catenin, thereby inducing apoptosis. Moreover, fisetin markedly induces apoptosis in renal carcinoma through increased expression of DR5, which is regulated by p53.
Prevent oxidative stress
Oxidative stress primarily occurs due to the high expression of reactive oxygen species (ROS), including H2O2, hydroxyl radicals, and superoxide anions. ROS are byproducts of cellular metabolism and are normally present at low levels in physiological conditions [26]. However, when ROS levels become elevated due to pathological factors, it triggers oxidative stress, which can damage cellular structures and contribute to the development of cancer and other diseases [27]. The ideal approach for antioxidants is to minimize ROS expression in cancer cells while preserving the physiological effects of ROS [28].
Fisetin demonstrates promising antioxidant properties in cancer [29]. It has the ability to reverse the decreased expression of endogenous antioxidants, thereby maintaining cellular redox homeostasis and neutralizing ROS from various sources [30]. Fisetin reduces the levels of hydroxyl radicals and superoxide anions, with mitochondrial origin being a common source of their generation [31]. Additionally, fisetin treatment can rescue the decrease in cell viability and the increase in ROS production caused by hydrogen peroxide. Furthermore, fisetin possesses antioxidant properties that enable it to counteract the adverse effects of chemotherapy. A previous study indicated that fisetin mitigated cisplatin-induced nephrotoxicity by inhibiting oxidative stress and scavenging free radicals [32].
Inhibit invasion and migration
Oncogenesis is characterized by the aggressive invasive and migratory abilities of cancer cells [33]. The high invasiveness of cancer cells contributes to the process of cancer metastasis and malignant transformation [34]. Once cancer cells metastasize to other organs in the body, treatment becomes challenging, and it becomes a leading cause of high mortality [35]. Matrix metalloproteinases (MMPs), which are responsible for the degradation of the extracellular matrix, play a critical role in regulating the metastasis of cancer cells. Furthermore, several signaling pathways, including MAPK, NF-κB, PI3K/AKT/mTOR, and EGFR, are known to be involved in the invasion and migration of cancer cells [36, 37].
The inhibitory effects of fisetin on invasion and migration have been demonstrated in various types of cancer cells [38]. For instance, fisetin has been shown to inhibit the metastasis of PC3 prostate cancer cells by reducing the activity of the PI3K/AKT and JNK pathways, resulting in the suppression of MMP-2 and MMP-9 expression [39]. In astrocytoma, fisetin can inhibit cell migration and reduce focal adhesion kinase (FAK) phosphorylation levels, which play a significant role in cell spreading and migration processes. Furthermore, fisetin significantly suppresses the invasion of U-2 cells by decreasing the expression of NF-κB, urokinase-type plasminogen activator (uPA), FAK, and MMP-2/9 [40].
Furthermore, reactivated epithelial–mesenchymal transition (EMT) often facilitates the metastatic spread of cancer cells, which is a process where fully differentiated epithelial cells undergo a transition to poorly differentiated and migratory mesenchymal cells [41, 42]. Fisetin has been shown to have the ability to reverse EMT, thereby inhibiting the invasion and migration of cancer cells [43].
Enhance chemotherapeutic effects
Chemotherapy is a cornerstone of anticancer treatment [44]. However, it is often associated with unwanted toxic effects and the development of chemoresistance, leading to a poor prognosis [45]. To address these challenges, researchers have investigated the combination of phytochemicals with chemotherapeutic agents, which has shown promising results in enhancing the efficacy of anticancer therapy. By combining phytochemicals with conventional chemotherapy, it is possible to achieve higher treatment efficiency and reduce the required drug doses, thereby minimizing toxic effects. This approach has the potential to prolong survival and improve the quality of life for cancer patients [46, 47].
It has been demonstrated that fisetin has the ability to enhance the effects of chemotherapy [48]. When combined with chemotherapeutic agents, fisetin improves the anticancer effects compared to using either agent alone [49]. For example, Eiman et al. showed combining fisetin with cabazitaxel synergistically inhibited proliferation and metastasis, and promoted apoptosis, thereby enhancing the overall anticancer activities. In lung cancer, the combination of fisetin with carnosic acid demonstrated a stronger effect in regulating apoptosis-related proteins, increasing caspase-3/8/9, Bax, DR, and p53, while decreasing Bcl-2 and Bcl-xl [50]. Fisetin has also been found to enhance the pro-apoptotic effects of cisplatin in teratocarcinoma, as well as sensitize resistant lung cancer and breast cancer cells to cisplatin [49, 51]. Tripathi et al. reported that the combination of fisetin with cisplatin resulted in a fourfold increase in anticancer potential compared to individual treatments [52]. Furthermore, fisetin could arrest cell cycle by increasing cell proportion in G2/S-phase and reducing cell proportion in G2-phase when combined with etoposide [53]. Importantly, numerous studies have shown that fisetin can exert its biological activities without inducing any significant toxic effects [54, 55].
Signaling pathways of fisetin (Fig. 2)
VEGF
Endothelial cells play a crucial role in the process of angiogenesis [56]. Vascular endothelial growth factor (VEGF) is a key proangiogenic factor that activates endothelial cells and promotes the formation of new blood vessels in cancer cells [57]. In addition to its role in stimulating angiogenesis, VEGF also regulates cancer cell growth, making it an attractive target for anticancer therapy [58]. Moreover, higher levels of VEGF expression are associated with more advanced stages of cancer and poorer prognosis in cancer patients [59, 60]. Therefore, the development of a non-toxic agent that inhibits VEGF is of great importance in anticancer therapy, as it can help control cancer cell growth and migration.
Fisetin has been shown to effectively inhibit VEGF and reduce its expression, thereby inhibiting angiogenesis and proliferation [61]. In breast cancer and colorectal cancer, fisetin suppresses angiogenesis by decreasing both the expression and release of VEGF from cancer cells [10]. The antiproliferative effect of fisetin, which involves arresting cell cycle in G1 phase and inducing a mild G2/M arrest, is attributed to its inhibitory effect on VEGF expression.
MAPK
MAPK is a signaling pathway that includes ERK1/2, p38, and JNK1/2, which can be activated through specific phosphorylation cascades [62, 63]. The activation of MAPK is crucial for mediating cancer cell proliferation, apoptosis, and invasion [64]. Phosphorylation of ERK1/2 can result in cell cycle arrest and induction of apoptosis [64, 65]. There is a delicate balance between ERK1/2 and JNK/p38, which plays a critical role in determining whether cancer cells undergo apoptosis or survival [66].
It has been demonstrated that fisetin has the ability to suppress ERK1/2 activation and activate JNK/p38 pathways [67, 68]. The MAPK pathway plays a significant role in the apoptosis process and endoplasmic reticulum (ER) stress induced by fisetin in cancer cells [29]. Fisetin inhibits cell proliferation by targeting the ERK-dependent signaling pathway in astrocytes and gastric cancer [14, 69]. In breast cancer cells, fisetin reduces the expression of MMP-9 by inhibiting PKCα/ROS/ERK1/2 and p38 MAPK activation, thereby leading to a decrease in cell migration [70]. Furthermore, fisetin has been found to regulate MAPK signaling to inhibit cell proliferation and induce apoptosis in acute promyelocytic leukemia cells [71].
NF-κB
NF-κB is a heterodimeric transcription factor that is involved in various pathological processes in cancer cells [72, 73]. In addition to its role in inflammation, NF-κB regulates important cellular processes such as proliferation, apoptosis, invasion, and angiogenesis [74,75,76,77]. NF-κB acts as a positive regulator of growth factors, controlling cell proliferation [78]. Moreover, NF-κB regulates several chemokines that play crucial roles in metastasis and angiogenesis, including IL-8, VEGF, MMPs, and other small molecules [79, 80]. Consequently, NF-κB activity can promote metastasis and angiogenesis. Furthermore, NF-κB has been shown to regulate Bcl-2, an anti-apoptotic protein, thus inhibiting programmed cell death [81]. Given these roles, NF-κB has been identified as a key therapeutic target in cancer, and agents that can inhibit NF-κB activity are considered important anticarcinogens.
The ability of fisetin to suppress NF-κB activity has been demonstrated in various diseases [82, 83], and in the context of cancer, fisetin exerts multiple biological effects by inhibiting NF-κB activity [84]. Fisetin induces apoptosis in cancer cells by inhibiting NF-κB activity, which leads to increased expression of pro-apoptotic proteins and decreased expression of anti-apoptotic proteins [29]. In prostate cancer cells, fisetin enhances the apoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by inhibiting NF-κB activity [29]. Fisetin also inhibits the invasion of melanoma and cervical cancer cells by interrupting NF-κB activity [29, 85]. Furthermore, fisetin’s inhibitory effects against bladder cancer are associated with the inhibition of NF-κB, ultimately leading to the suppression of cancer cell proliferation [86].
PI3K/Akt/mTOR
The PI3K/AKT pathway is closely related to apoptosis in cancer, and inhibiting this pathway can block proliferation and promote programmed cell death (PCD) of cancer cells [87, 88]. Moreover, PI3K/AKT pathway is associated with chemoresistance [89]. As a critical component of the PI3K/AKT/mTOR pathway, mammalian target of rapamycin (mTOR) is also a key factor in anticancer therapies [88]. AKT is phosphorylated upon activation of PI3K, and the activated AKT regulates the phosphorylation of downstream protein mTOR. Consequently, this signaling pathway regulates various cellular processes, including proliferation, apoptosis, differentiation, autophagy, and migration of cancer cells [90,91,92,93,94]. Additionally, the activation of the PI3K/AKT/mTOR pathway is correlated with poor prognosis in several types of cancer. Hence, the three signaling proteins, PI3K, AKT, and mTOR, represent attractive targets for anticancer therapy.
Fisetin has been shown to be effective against PI3K expression, AKT phosphorylation, and mTOR activation in various cancer cells, including prostate cancer, melanoma, breast cancer and colorectal cancer [38]. By suppressing the PI3K/AKT, fisetin reduces cell survival and proliferation in cancer cells [38]. Fisetin also reduces the formation of mTOR, thereby exhibiting its anticancer biological activities [10]. Furthermore, the role of fisetin in decreasing the invasion and migration of lung cancer cells is dependent on the PI3K/Akt/mTOR pathway, and inhibition of these signaling genes abolishes this effect of fisetin [7]. These studies demonstrate that fisetin has the ability to inhibit the PI3K/Akt/mTOR pathway and may serve as a promising therapeutic agent for anticancer therapies.
Nrf2/HO-1
The activation of the Nrf2/HO-1 pathway plays a significant role in tumor progression and contributes to various aspects of cancer, including cell growth, oxidative stress, angiogenesis, metastasis, chemoresistance, and poor prognosis [95,96,97]. As a result, agents that can inhibit the Nrf2/HO-1 axis are now considered promising approaches for anticancer therapy [98].
The effects of fisetin on the activation of Nrf2 and upregulation of HO-1 have been demonstrated in various diseases [99, 100]. Notably, Nrf2 is essential for fisetin-mediated upregulation of HO-1 levels, and silencing Nrf2 can block the process of fisetin-induced HO-1 upregulation [101]. In the context of cancer cells, fisetin exhibits anticancer properties by modulating the Nrf2/HO-1 axis. For instance, in breast cancer, fisetin can reduce the expression of MMP-2/9 by upregulating Nrf2 expression and promoting HO-1 transcription, leading to a decrease in cancer cell motility and migration [102]. Fisetin has also been found to induce apoptosis and enhance the effects of chemotherapy through Nrf2/HO-1 pathway [68]. Additionally, the Nrf2/HO-1-mediated oxidative stress response plays a role in the growth inhibitory effects of fisetin [31].
Absorption and bioavailability of fisetin
It has been demonstrated that fisetin had anticarcinogenic activity by numerous studies [8, 10]. However, the low water solubility of fisetin poses a significant challenge for its administration, which can limit its biological effects [50]. To address this issue and optimize the delivery of fisetin to cancer cells, researchers have explored the use of nanoemulsion formulations. Nanoemulsion is known for its ability to encapsulate hydrophobic active molecules, offering advantages such as small particle sizes, high drug solubility and loading, good stability, sustained drug release, and low toxicity [103]. It serves as an ideal vehicle for improving the efficacy of various anticancer drugs. Fisetin nanoemulsion has shown remarkable improvements in its anticancer potency through extended release, higher drug loading, and enhanced bioavailability. Compared to free fisetin, fisetin nanoemulsion has demonstrated a 3.9-fold increase in the generation of reactive oxygen species (ROS) and induction of apoptosis, highlighting its enhanced efficacy [104].
Another promising approach for improving the bioavailability and therapeutic efficacy of fisetin is liposomal encapsulation. Liposomes are artificial vesicles composed of phospholipids that can encapsulate fisetin molecules. This encapsulation process enhances the stability and solubility of fisetin, allowing for improved delivery and increased bioavailability. Liposomal encapsulation has shown potential in enhancing the anticancer therapeutic effects of fisetin [105].
Future prospects of fisetin
Autophagy
Recent research indicates that autophagy reduces the resistance to chemotherapy and radiation [106, 107]. However, there is limited research on the effects of fisetin on autophagy in cancer, and the results are inconsistent. Some studies have shown that fisetin induces autophagic programmed cell death in prostate cancer, while in liver cancer, fisetin has been found to inhibit autophagy [10]. Furthermore, the specific signaling pathways involved in fisetin-mediated autophagy are still not well understood [68]. For example, Suh et al. demonstrated that fisetin-induced autophagy occurs through the AMPK/mTOR pathway, whereas another study found that fisetin-induced autophagy in response to ER stress occurs via an AMPK-independent pathway.
Indeed, it is crucial to clarify the effects and mechanisms of fisetin on cancer cells for the development of effective anticancer therapies. Understanding how fisetin influences key cellular processes such as proliferation, apoptosis, angiogenesis, migration, and autophagy can provide valuable insights into its potential as an anticancer agent. By unraveling the underlying molecular pathways and signaling mechanisms through which fisetin exerts its effects, researchers can identify specific targets for therapeutic intervention and design more targeted treatment strategies. Furthermore, gaining a comprehensive understanding of fisetin’s actions in cancer cells will contribute to optimizing its use in anticancer therapy and maximizing its therapeutic benefits.
Conclusion
In this review, we have presented compelling evidence for the biological functions of fisetin in anticancer therapy. Additionally, we have discussed various molecular targets of fisetin in cancer. The above findings prompt that fisetin holds promise as a therapeutic agent in anticancer therapy, particularly when combined with other drugs to overcome chemoresistance and enhance efficacy. However, further studies are needed to fully elucidate the complete range of fisetin’s biological functions and underlying mechanisms. Ultimately, fisetin shows great potential for clinical application in anticancer therapy.
Availability of data and materials
Not applicable.
References
Dey P, Kimmelman AC, DePinho RA. Metabolic codependencies in the tumor microenvironment. Cancer Discov. 2021;11(5):1067–81.
Tajan M, Vousden KH. Dietary approaches to cancer therapy. Cancer Cell. 2020;37(6):767–85.
Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci. 2021;78(8):3817–51.
Boija A, Klein IA, Young RA. Biomolecular condensates and cancer. Cancer Cell. 2021;39(2):174–92.
Syed DN, Adhami VM, Khan MI, Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem. 2013;13(7):995–1001.
Khan H, Ullah H, Martorell M, Valdes SE, Belwal T, Tejada S, et al. Flavonoids nanoparticles in cancer: treatment, prevention and clinical prospects. Semin Cancer Biol. 2021;69:200–11.
Khan N, Jajeh F, Eberhardt EL, Miller DD, Albrecht DM, Van Doorn R, et al. Fisetin and 5-fluorouracil: effective combination for PIK3CA-mutant colorectal cancer. Int J Cancer. 2019;145(11):3022–32.
Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–36.
Yamaura K, Nelson AL, Nishimura H, Rutledge JC, Ravuri SK, Bahney C, et al. The effects of fisetin on bone and cartilage: a systematic review. Pharmacol Res. 2022;185: 106504.
Farooqi AA, Naureen H, Zahid R, Youssef L, Attar R, Xu B. Cancer chemopreventive role of fisetin: regulation of cell signaling pathways in different cancers. Pharmacol Res. 2021;172: 105784.
Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020;5(1):90.
Cao Y. Adipocyte and lipid metabolism in cancer drug resistance. J Clin Invest. 2019;129(8):3006–17.
Rahmani AH, Almatroudi A, Allemailem KS, Khan AA, Almatroodi SA. The potential role of fisetin, a flavonoid in cancer prevention and treatment. Molecules. 2022;27(24):9009.
Wang N, Yao F, Li K, Zhang L, Yin G, Du M, et al. Fisetin regulates astrocyte migration and proliferation in vitro. Int J Mol Med. 2017;39(4):783–90.
Nowak-Sliwinska P, Alitalo K, Allen E, Anisimov A, Aplin AC, Auerbach R, et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 2018;21(3):425–532.
Li T, Kang G, Wang T, Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol Lett. 2018;16(1):687–702.
Uemura A, Fruttiger M, D’Amore PA, De Falco S, Joussen AM, Sennlaub F, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84: 100954.
Kubina R, Krzykawski K, Kabala-Dzik A, Wojtyczka RD, Chodurek E, Dziedzic A. Fisetin, a potent anticancer flavonol exhibiting cytotoxic activity against neoplastic malignant cells and cancerous conditions: a scoping, comprehensive review. Nutrients. 2022;14(13):2604.
Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395–417.
Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta P, Wei L, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updates. 2018;41:1–25.
Fathi N, Rashidi G, Khodadadi A, Shahi S, Sharifi S. STAT3 and apoptosis challenges in cancer. Int J Biol Macromol. 2018;117:993–1001.
Kashyap D, Garg VK, Tuli HS, Yerer MB, Sak K, Sharma AK, et al. Fisetin and quercetin: promising flavonoids with chemopreventive potential. Biomolecules. 2019;9(5):174.
Hassan SSU, Samanta S, Dash R, Karpinski TM, Habibi E, Sadiq A, et al. The neuroprotective effects of fisetin, a natural flavonoid in neurodegenerative diseases: focus on the role of oxidative stress. Front Pharmacol. 2022;13:1015835.
Wissler Gerdes EO, Zhu Y, Weigand BM, Tripathi U, Burns TC, Tchkonia T, et al. Cellular senescence in aging and age-related diseases: implications for neurodegenerative diseases. Int Rev Neurobiol. 2020;155:203–34.
Monasterio A, Urdaci MC, Pinchuk IV, Lopez-Moratalla N, Martinez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr Cancer. 2004;50(1):90–100.
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64.
Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA. The role of lipids in cancer progression and metastasis. Cell Metab. 2022;34(11):1675–99.
Luo Y, Ma J, Lu W. The significance of mitochondrial dysfunction in cancer. Int J Mol Sci. 2020;21(16):5598.
Afroze N, Pramodh S, Shafarin J, Bajbouj K, Hamad M, Sundaram MK, et al. Fisetin deters cell proliferation, induces apoptosis, alleviates oxidative stress and inflammation in human cancer cells, HeLa. Int J Mol Sci. 2022;23(3):1707.
Iside C, Scafuro M, Nebbioso A, Altucci L. SIRT1 activation by natural phytochemicals: an overview. Front Pharmacol. 2020;11:1225.
Hussain T, Al-Attas OS, Alamery S, Ahmed M, Odeibat HAM, Alrokayan S. The plant flavonoid, fisetin alleviates cigarette smoke-induced oxidative stress, and inflammation in Wistar rat lungs. J Food Biochem. 2019;43(8): e12962.
Sahu BD, Kalvala AK, Koneru M, Mahesh Kumar J, Kuncha M, Rachamalla SS, et al. Ameliorative effect of fisetin on cisplatin-induced nephrotoxicity in rats via modulation of NF-kappaB activation and antioxidant defence. PLoS ONE. 2014;9(9): e105070.
Zhong Y, Yang L, Xiong F, He Y, Tang Y, Shi L, et al. Long non-coding RNA AFAP1-AS1 accelerates lung cancer cells migration and invasion by interacting with SNIP1 to upregulate c-Myc. Signal Transduct Target Ther. 2021;6(1):240.
Mierke CT. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep Prog Phys. 2019;82(6): 064602.
Pape J, Magdeldin T, Stamati K, Nyga A, Loizidou M, Emberton M, et al. Cancer-associated fibroblasts mediate cancer progression and remodel the tumouroid stroma. Br J Cancer. 2020;123(7):1178–90.
de Gooijer MC, Guillen Navarro M, Bernards R, Wurdinger T, van Tellingen O. An experimenter’s guide to glioblastoma invasion pathways. Trends Mol Med. 2018;24(9):763–80.
Alvarez-Elizondo MB, Weihs D. Breast cancer stem cells: mechanobiology reveals highly invasive cancer cell subpopulations. Cell Mol Life Sci. 2022;79(3):134.
Xiao Y, Liu Y, Gao Z, Li X, Weng M, Shi C, et al. Fisetin inhibits the proliferation, migration and invasion of pancreatic cancer by targeting PI3K/AKT/mTOR signaling. Aging (Albany NY). 2021;13(22):24753–67.
Mukhtar E, Adhami VM, Sechi M, Mukhtar H. Dietary flavonoid fisetin binds to beta-tubulin and disrupts microtubule dynamics in prostate cancer cells. Cancer Lett. 2015;367(2):173–83.
do Nascimento RP, Dos Santos BL, Amparo JAO, Soares JRP, da Silva KC, Santana MR, et al. Neuroimmunomodulatory properties of flavonoids and derivates: a potential action as adjuvants for the treatment of glioblastoma. Pharmaceutics. 2022;14(1):116.
Luond F, Sugiyama N, Bill R, Bornes L, Hager C, Tang F, et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev Cell. 2021;56(23):3203-3221 e11.
McCabe EM, Rasmussen TP. lncRNA involvement in cancer stem cell function and epithelial–mesenchymal transitions. Semin Cancer Biol. 2021;75:38–48.
Agraval H, Sharma JR, Prakash N, Yadav UCS. Fisetin suppresses cigarette smoke extract-induced epithelial to mesenchymal transition of airway epithelial cells through regulating COX-2/MMPs/beta-catenin pathway. Chem Biol Interact. 2022;351: 109771.
Son S, Kim JH, Wang X, Zhang C, Yoon SA, Shin J, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–61.
Weingart SN, Zhang L, Sweeney M, Hassett M. Chemotherapy medication errors. Lancet Oncol. 2018;19(4):e191–9.
Chatterjee N, Bivona TG. Polytherapy and targeted cancer drug resistance. Trends Cancer. 2019;5(3):170–82.
Dias MP, Moser SC, Ganesan S, Jonkers J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat Rev Clin Oncol. 2021;18(12):773–91.
Chen YX, Wang CJ, Xiao DS, He BM, Li M, Yi XP, et al. eIF3a R803K mutation mediates chemotherapy resistance by inducing cellular senescence in small cell lung cancer. Pharmacol Res. 2021;174: 105934.
Khozooei S, Lettau K, Barletta F, Jost T, Rebholz S, Veerappan S, et al. Fisetin induces DNA double-strand break and interferes with the repair of radiation-induced damage to radiosensitize triple negative breast cancer cells. J Exp Clin Cancer Res. 2022;41(1):256.
Sabarwal A, van Rooyen JC, Caburet J, Avgenikos M, Dheeraj A, Ali M, et al. A novel 4’-brominated derivative of fisetin induces cell cycle arrest and apoptosis and inhibits EGFR/ERK1/2/STAT3 pathways in non-small-cell lung cancer without any adverse effects in mice. FASEB J. 2022;36(12): e22654.
Tabasum S, Singh RP. Fisetin suppresses migration, invasion and stem-cell-like phenotype of human non-small cell lung carcinoma cells via attenuation of epithelial to mesenchymal transition. Chem Biol Interact. 2019;303:14–21.
Li S, Livingston MJ, Ma Z, Hu X, Wen L, Ding HF, et al. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight. 2023;8: e166643.
Ferreira de Oliveira JMP, Pacheco AR, Coutinho L, Oliveira H, Pinho S, Almeida L, et al. Combination of etoposide and fisetin results in anti-cancer efficiency against osteosarcoma cell models. Arch Toxicol. 2018;92(3):1205–14.
Shi YS, Li CB, Li XY, Wu J, Li Y, Fu X, et al. Fisetin attenuates metabolic dysfunction in mice challenged with a high-fructose diet. J Agric Food Chem. 2018;66(31):8291–8.
Chen YP, Sivalingam K, Shibu MA, Peramaiyan R, Day CH, Shen CY, et al. Protective effect of Fisetin against angiotensin II-induced apoptosis by activation of IGF-IR-PI3K-Akt signaling in H9c2 cells and spontaneous hypertension rats. Phytomedicine. 2019;57:1–8.
Sobierajska K, Ciszewski WM, Sacewicz-Hofman I, Niewiarowska J. Endothelial cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1234:71–86.
Pulkkinen HH, Kiema M, Lappalainen JP, Toropainen A, Beter M, Tirronen A, et al. BMP6/TAZ-Hippo signaling modulates angiogenesis and endothelial cell response to VEGF. Angiogenesis. 2021;24(1):129–44.
Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int J Mol Sci. 2018;19(4):1232.
Ribatti D, Annese T, Tamma R. Controversial role of mast cells in breast cancer tumor progression and angiogenesis. Clin Breast Cancer. 2021;21(6):486–91.
Trouillas J, Delgrange E, Wierinckx A, Vasiljevic A, Jouanneau E, Burman P, et al. Clinical, pathological, and molecular factors of aggressiveness in lactotroph tumours. Neuroendocrinology. 2019;109(1):70–6.
Takahashi S, Bhattacharjee S, Ghosh S, Sugimoto N, Bhowmik S. Preferential targeting cancer-related i-motif DNAs by the plant flavonol fisetin for theranostics applications. Sci Rep. 2020;10(1):2504.
Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13(1):113.
Zhang M, Zhang S. Mitogen-activated protein kinase cascades in plant signaling. J Integr Plant Biol. 2022;64(2):301–41.
Drosten M, Barbacid M. Targeting the MAPK pathway in KRAS-driven tumors. Cancer Cell. 2020;37(4):543–50.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007.
Fan J, Ren D, Wang J, Liu X, Zhang H, Wu M, et al. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020;11(2):126.
Lu H, Wang H, Huang G, Wang X, Bu X. Therapeutic targeting of mechanical stretch-induced FAK/ERK signaling by fisetin in hypertrophic scars. Eur J Pharmacol. 2022;932: 175228.
Prem PN, Sivakumar B, Boovarahan SR, Kurian GA. Recent advances in potential of Fisetin in the management of myocardial ischemia-reperfusion injury-A systematic review. Phytomedicine. 2022;101: 154123.
Yan W, Chen S, Zhao Y, Ye X. Fisetin inhibits the proliferation of gastric cancer cells and induces apoptosis through suppression of ERK 1/2 activation. Oncol Lett. 2018;15(6):8442–6.
Kammerud SC, Metge BJ, Elhamamsy AR, Weeks SE, Alsheikh HA, Mattheyses AL, et al. Novel role of the dietary flavonoid fisetin in suppressing rRNA biogenesis. Lab Invest. 2021;101(11):1439–48.
Adan A, Baran Y. The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumour Biol. 2015;36(11):8973–84.
Taniguchi K, Karin M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18(5):309–24.
Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y, et al. Ubiquitination of NF-kappaB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance. Cell Death Differ. 2022;29(2):381–92.
Patel M, Horgan PG, McMillan DC, Edwards J. NF-kappaB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43–56.
Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, et al. Icariin-induced inhibition of SIRT6/NF-kappaB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020;111(11):4242–56.
Wang Y, Zhang J, Li YJ, Yu NN, Liu WT, Liang JZ, et al. MEST promotes lung cancer invasion and metastasis by interacting with VCP to activate NF-kappaB signaling. J Exp Clin Cancer Res. 2021;40(1):301.
Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, et al. B7–H3 promotes colorectal cancer angiogenesis through activating the NF-kappaB pathway to induce VEGFA expression. Cell Death Dis. 2020;11(1):55.
Gu P, Zhang M, Zhu J, He X, Yang D. Suppression of CDCA3 inhibits prostate cancer progression via NF-kappaB/cyclin D1 signaling inactivation and p21 accumulation. Oncol Rep. 2022;47(2):1–10.
Zhang M, Liu ZZ, Aoshima K, Cai WL, Sun H, Xu T, et al. CECR2 drives breast cancer metastasis by promoting NF-kappaB signaling and macrophage-mediated immune suppression. Sci Transl Med. 2022;14(630):eabf5473.
Wan X, Guan S, Hou Y, Qin Y, Zeng H, Yang L, et al. FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts. Theranostics. 2021;11(10):4975–91.
Bai Y, Lam HC, Lei X. Dissecting programmed cell death with small molecules. Acc Chem Res. 2020;53(5):1034–45.
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother. 2020;122: 109772.
Molagoda IMN, Jayasingha J, Choi YH, Jayasooriya R, Kang CH, Kim GY. Fisetin inhibits lipopolysaccharide-induced inflammatory response by activating beta-catenin, leading to a decrease in endotoxic shock. Sci Rep. 2021;11(1):8377.
Khan H, Ullah H, Castilho P, Gomila AS, D’Onofrio G, Filosa R, et al. Targeting NF-kappaB signaling pathway in cancer by dietary polyphenols. Crit Rev Food Sci Nutr. 2020;60(16):2790–800.
Pal HC, Sharma S, Strickland LR, Katiyar SK, Ballestas ME, Athar M, et al. Fisetin inhibits human melanoma cell invasion through promotion of mesenchymal to epithelial transition and by targeting MAPK and NFkappaB signaling pathways. PLoS ONE. 2014;9(1): e86338.
Li J, Qu W, Cheng Y, Sun Y, Jiang Y, Zou T, et al. The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NF-kappa B pathways in a rat bladder carcinogenesis model. Basic Clin Pharmacol Toxicol. 2014;115(4):321–9.
Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–32.
Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–60.
Qian J, LeSavage BL, Hubka KM, Ma C, Natarajan S, Eggold JT, et al. Cancer-associated mesothelial cells promote ovarian cancer chemoresistance through paracrine osteopontin signaling. J Clin Invest. 2021;131(16): e146186.
Stefani C, Miricescu D, Stanescu S II, Nica RI, Greabu M, Totan AR, et al. Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: where are we now? Int J Mol Sci. 2021;22(19):10260.
Zhou J, Jiang YY, Chen H, Wu YC, Zhang L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020;53(2): e12739.
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5(1):8.
Ma Z, Lou S, Jiang Z. PHLDA2 regulates EMT and autophagy in colorectal cancer via the PI3K/AKT signaling pathway. Aging (Albany NY). 2020;12(9):7985–8000.
Chen K, Zhu P, Chen W, Luo K, Shi XJ, Zhai W. Melatonin inhibits proliferation, migration, and invasion by inducing ROS-mediated apoptosis via suppression of the PI3K/Akt/mTOR signaling pathway in gallbladder cancer cells. Aging (Albany NY). 2021;13(18):22502–15.
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12(11):1079.
Chakraborty S, Balan M, Flynn E, Zurakowski D, Choueiri TK, Pal S. Activation of c-Met in cancer cells mediates growth-promoting signals against oxidative stress through Nrf2-HO-1. Oncogenesis. 2019;8(2):7.
Zhang YY, Yao YD, Chen F, Guo X, Kang JL, Huang YF, et al. (9S,13R)-12-oxo-phytodienoic acid attenuates inflammation by inhibiting mPGES-1 and modulating macrophage polarization via NF-kappaB and Nrf2/HO-1 pathways. Pharmacol Res. 2022;182: 106310.
Lou Y, Guo Z, Zhu Y, Kong M, Zhang R, Lu L, et al. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J Exp Clin Cancer Res. 2019;38(1):242.
Li D, Liu X, Pi W, Zhang Y, Yu L, Xu C, et al. Fisetin attenuates doxorubicin-induced cardiomyopathy in vivo and in vitro by inhibiting ferroptosis through SIRT1/Nrf2 signaling pathway activation. Front Pharmacol. 2021;12: 808480.
Zhang L, Wang H, Zhou Y, Zhu Y, Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018;118:304–13.
Park C, Noh JS, Jung Y, Leem SH, Hyun JW, Chang YC, et al. Fisetin attenuated oxidative stress-induced cellular damage in ARPE-19 human retinal pigment epithelial cells through Nrf2-mediated activation of heme oxygenase-1. Front Pharmacol. 2022;13: 927898.
Tsai CF, Chen JH, Chang CN, Lu DY, Chang PC, Wang SL, et al. Fisetin inhibits cell migration via inducing HO-1 and reducing MMPs expression in breast cancer cell lines. Food Chem Toxicol. 2018;120:528–35.
Moghassemi S, Dadashzadeh A, Azevedo RB, Amorim CA. Nanoemulsion applications in photodynamic therapy. J Control Release. 2022;351:164–73.
Vishwas S, Singh SK, Gulati M, Awasthi A, Khursheed R, Corrie L, et al. Harnessing the therapeutic potential of fisetin and its nanoparticles: journey so far and road ahead. Chem Biol Interact. 2022;356: 109869.
Dutta S, Moses JA, Anandharamakrishnan C. Encapsulation of nutraceutical ingredients in liposomes and their potential for cancer treatment. Nutr Cancer. 2018;70(8):1184–98.
Onorati AV, Dyczynski M, Ojha R, Amaravadi RK. Targeting autophagy in cancer. Cancer. 2018;124(16):3307–18.
Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12.
Acknowledgements
Xiang Gao provided financial support for the research (Ningbo Health Branding Subject Fund, PPXK2018-04). Chenhui Zhou provided financial support for the research (the National Natural Science Foundation of China, 82101354 and the Medicine and Health Science and Technology Projects of Zhejiang Province, 2023KY265). Partly funded by Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, China (2022E10026).
Funding
Ningbo Health Branding Subject Fund, PPXK2018-04. The National Natural Science Foundation of China, 82101354. The Medicine and Health Science and Technology Projects of Zhejiang Province, 2023KY265.
Author information
Authors and Affiliations
Contributions
Chenhui Zhou designed the studies and wrote the manuscript. Yi Huang contributed to English language editing. Sheng Nie prepared figures. Shengjun Zhou contributed to edit the references. Xiang Gao and Gao Chen contributed to the design of the study and wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
All the authors confirm that there are no known conflicts of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, C., Huang, Y., Nie, S. et al. Biological effects and mechanisms of fisetin in cancer: a promising anti-cancer agent. Eur J Med Res 28, 297 (2023). https://doi.org/10.1186/s40001-023-01271-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01271-8