Abstract
Recycling slaughterhouse waste such as bone and converting it into bone char is a promising environmentally friendly, low-cost strategy in a circular economy and an important source of phosphorus. Therefore, this review focused on the impacts of bone char on the availability, dynamics, and transformations of phosphorus in soils as well as plant growth and utilizing bone char in remediating contaminated soils by heavy metals. Bone char is material produced through bone pyrolysis under limited oxygen at 300–1050 °C. Bone char applications to the soils significantly increased phosphorus availability and plant growth. Agricultural practices such as co-applying organic acids or sulfur or nitrogen fertilizers with bone char in some soils played an important role in enhanced phosphorus availability. Also, co-applying bone char with phosphate-solubilizing microorganisms enhanced plant growth and phosphorus availability in the soils. Applying bone char to the soils changed the dynamics and redistribution of phosphorous fractions, enhanced fertility, promoted crop growth and productivity, reduced heavy metals uptake by plants in contaminated soil, and decreased heavy metals bioavailability. Bone char has shown positive performance in remediating soils contaminated by heavy metals. Bone char proved its efficiency in sustainable agriculture and practical applications as an alternative source of phosphate fertilizers, it is safe, cheap and helps in remediating contaminated soils by heavy metals. Using bone char as a slow-release fertilizer is potentially beneficial because it reduces the hazard of excessive fertilizing and nutrient leaching which have negative impacts on the ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, slaughterhouses produce about 130 billion kilograms of animal bone waste annually will become an environmental load if not managed properly (Fayaz et al. 2021). Bone is one slaughterhouse waste that is known for its rich content in phosphorus which is considered promising for sustainability in agriculture and global food security (Sun et al. 2018). Moreover, nutrient recycling in modern agriculture is a promising strategy to meet the challenges of running out of non-renewable resources as well as to diminish environmental pollution resulting from the operations of mining and industrialization (Robles et al. 2020). Recycling animal bones in Ethiopia alone could produce about 28 to 58% of the annual phosphate fertilizers supply (Simons et al. 2014). Animal bones are exposed to thermal decomposition processes, that produce bone char (Amin 2023a; Glæsner et al. 2019). Valorizing and converting these wastes into new materials of economic and environmental value is a safe way, in line with the principles of the circular economy, which aims to eliminate waste and continuous use of resources. The use of bone char as a phosphate fertilizer is a promising strategy in sustainable agriculture because it is a renewable source of phosphorus and its use is also in line with the principles of the circular economy (Piccirillo 2023). Several studies found that bone char can be used as a phosphate fertilizer, which in turn can lead to a reduction in dependence on chemical phosphate fertilizers in sustainable agriculture (Ahmed et al. 2021a; Siebers et al. 2014; Wakweya et al. 2022). However, the concentration of available phosphorus in bone char is usually low due to the low solubility of the main phosphorus compounds in bone, namely apatite (Ahmed et al. 2021a). Moreover, these properties of bone char greatly qualify it for use as potentially slow-release P fertilizers (Leinweber et al. 2019). Some studies showed many other uses for bone char, such as water purification and wastewater treatment processes (Alkurdi et al. 2019a), environmental remediation (Piccirillo 2023), paints, and purifying sugar during its manufacture (Leinweber et al. 2019). Bone char product as a versatile material in many fields is presented in Fig. 1. This review article presents a promising vision for the potential use of bone char (eco-friendly fertilizers) as an alternative to chemical phosphate fertilizers. Many studies have focused on examining bone char's effectiveness by utilizing numerous laboratory and field experiments to enhance its properties. They also focused on the effect of bone char on phosphorus availability and crop yield. The intended objective of this review is to present a thematic review of the latest research results and theoretical and practical developments for bone char applications in soils. The use of bone char as a renewable source of phosphate fertilizers is being reviewed to augment bone char application and reduce environmental and economic impacts. The goals of this review seek to highlight the production conditions and characteristics of bone char and its use as a phosphate fertilizer. The review also provides insight into bone char effects on dynamics and transformations of phosphorus in several soils as well as soil quality and plant growth. Also, it highlights the effects of soil properties and agricultural practices that play a role in improving the dissolving of phosphorus from bone char in soils. Besides, using bone char in the remediation of contaminated soils by heavy metals.
The main source for manufacturing and producing phosphate fertilizers is phosphate rock, which is considered a non-renewable resource. Moreover, approximately 90% of the total phosphorus demand in the world is utilized in the agriculture sector, which is by far the largest consumer of phosphorus (Gupta et al. 2014). As phosphate resources continue to deplete and phosphate prices have recently risen, our current consumption patterns for phosphate require reconsideration. Besides the socioeconomic and geopolitical effects of further reducing phosphate consumption, further reducing the amount of phosphate we use would also be beneficial in terms of the quality of our environment and ecosystem (van Enk et al. 2011). However, it is expected that the global reserve of phosphate rock will remain for long periods depending on growth rates, as it is likely to be between the year 2072 at high growth and after the year 2200 at zero growth (van Enk et al. 2011). The Russia-Ukraine war came at an inopportune time after a series of global disruptions such as the COVID-19 pandemic, strong global demand for food, and low crop productivity in some countries. All these factors led to a significant global increase in fertilizer prices (Hassen and El Bilali 2021). Generally speaking, the fertilizer industry admits that there is a decline in the quality of reserves and an increase in the cost of extraction, processing, and shipping fertilizers (Tirado and Allsopp 2012). African countries were among the countries where food security was most affected as a result of the unrest that led to a rise in global fertilizer prices (FAO 2023). Phosphate fertilizers are one of the sources of pollution in agricultural ecosystems because they contain many heavy and radioactive elements. The accumulation of these elements in the soil with the continued use of phosphate fertilizers can be transferred to food and can have harmful effects on human and animal health (Taylor et al. 2016). Also, soil phosphorus accumulation has become a significant economic and environmental issue because of the continuous excessive use of phosphate fertilizers due to population growth (Gupta et al. 2014). The above reasons prompted us to find sustainable and cost-effective alternatives to chemical phosphate fertilizers like bone char. Hence, we urgently needed to write this review article to fill many knowledge gaps about the effects of using bone char as a renewable fertilizer on soil quality, plant growth, and remediating heavy metals. Also, we aim to suggest recommendations and future perspectives to deal with these gaps, then find solutions and discuss them.
2 Production and Factors Affecting Bone Char Characteristics
2.1 Bone Char Production
Bone char product is produced through the pyrolysis process of de-fatted and de-gelatinized animal bones under an environment of limited oxygen or anoxic conditions at temperatures of 300–1050 °C. The resulting substance after the pyrolysis process is porous black powder rich in calcium and phosphorus, which makes it an excellent source of fertilizer (Amin 2023a; Glæsner et al. 2019). Bone char by itself is not considered biochar due to its low organic carbon content, but a product rich in phosphorus (Glæsner et al. 2019; Zwetsloot et al. 2016). A schematic representation of the process of bone char production is shown in Fig. 2. Many studies found that bone char is produced using several different processes such as low-temperature processes (50–300 °C), pyrolysis, gasification., hydrothermal carbonization, torrefaction, combustion, and ratification processes. The slow pyrolysis process is carried out in the absence of oxygen using slow heating methods and long residence time, ranging from hours to days. Fast pyrolysis is defined as a rapid heating process of the animal bones in the absence of oxygen and then rapid cooling and quenching. The flash pyrolysis process is performed at an even shorter reaction time of about a few seconds than fast pyrolysis and the heating rate is very high (Alkurdi et al. 2019b; Hart et al. 2022; Novotny et al. 2015). The bone char produced from the fast pyrolysis process possesses high surface area and porosity and low O/C and H/C ratios compared to those produced via slow pyrolysis. The char produced from the fast pyrolysis process has a high surface area and porosity and low O/C and H/C ratio compared to char produced from the slow pyrolysis process (Alkurdi et al. 2019b; Novotny et al. 2015).
2.2 Factors Affecting Bone Char Characteristics
There are many factors such as pyrolysis temperature, heating rate, and residence time as well as different animal species bones play an important role in affecting the physical and chemical properties of produced bone char (Ahmed et al. 2021b; Alkurdi et al. 2019a; Amin 2023a; Asgari et al. 2019; Biswas et al. 2021).
2.2.1 Pyrolysis Temperature
The influence of different pyrolysis temperatures on the bone char properties is presented in Table 1. The yields of the bone char decreased markedly with the increase in pyrolysis temperature (Amin 2023a). Augmentation of the concentrations of total elements in bone char is attributed to reducing the yield of bone char due to the loss of moisture and volatilization of organic compounds (van Hoesel et al. 2019). Increasing pyrolysis temperature led to an increase in the hydroxyapatite content in the bone char (Zwetsloot et al. 2015). Releasing phosphorous from bone char is highly influenced by pyrolysis temperatures (Tang et al. 2019). The phosphorus availability in bone char differed highly with pyrolysis temperature (Amin 2023a). Pyrolysis temperatures have a significant effect on the available phosphorous (Olsen-P), as at 300 °C, the Olsen-P decreased from 268.3 mg kg−1 for the bone to 214.7 mg kg−1. At 500 °C, the Olsen-P increased from 268.26 mg kg−1 for the bone to 952.0 mg kg−1. While, at 700 °C, Olsen-P decreased from 268.3 mg kg−1 for the bone to 193.4 mg kg−1. The highest concentration of Olsen-P was observed in a pyrolyzed bone at 500 °C (Amin 2023a). The biological apatite crystals are stable at 200°C. When the pyrolysis temperature is increased from 300 °C to 600 °C, it results in decrystallization. However, as the temperature increases further to 600 °C, the biological apatite recrystallizes and converts to hydroxyapatite at 700°C (Li et al. 2015). The pH of bone char markedly increased with increasing pyrolysis temperature (Alkurdi et al. 2020; Amin 2023a) which may be because of the loss of acidic functional groups and the increase in the alkaline aromatic groups (Alkurdi et al. 2020) as well as the electrical conductivity of bone char increased with increasing pyrolysis temperature (Alkurdi et al. 2020). The total content of carbon and nitrogen in bone char produced by different types of animals decreased with increasing pyrolysis temperatures. Moreover, the total contents of carbon and nitrogen were ranked in the order of chicken bone char ˃ sheep bone char ˃ pig bone char at 300 °C pyrolysis temperature, however, the total contents of carbon and nitrogen at 500 °C pyrolysis temperature were ranked in the order of sheep bone char ˃ chicken bone char ˃ pig bone char (Ahmed et al. 2021b). The total concentration of calcium, phosphorus, and sodium in bone char increased gradually with increasing pyrolysis temperature. The total carbonate content in bone char increased with increasing pyrolysis temperatures from 300 °C to 500 °C. However, as the temperature increases to 700 °C, the total carbonate content is reduced (Amin 2023a).
2.2.2 Different Animal Species' Bones and their Different Parts
The characteristics of bone char as influenced by different animal species' bones and their different parts are given in Table 1. The total phosphorus was higher in pig bone char than in sheep bone char and chicken bone char irrespective of processing method and temperature. On the other hand, water-soluble P was overall higher in chicken bone char than sheep bone char and pig bone char irrespective of processing method and temperature (Ahmed et al. 2021b). The electrical conductivity was overall higher in sheep bone char ˃ chicken bone char ˃ pig bone char irrespective of processing method and temperature (Ahmed et al. 2021b). The different bone parts taken greatly influence the physical and chemical properties of the resulting bone char (Wang et al. 2020a, b). The total phosphorus content in bone char is derived from legs (178.8 g kg−1) ˃ scapulae (174.1 g kg−1) ˃ ribs (165.4 g kg−1) ˃ vertebrae (137.0 g kg−1). While total calcium content in bone char is derived from legs ˃ scapulae ˃ vertebrae ˃ ribs. Carbon content in bone char derived from ribs ˃ scapulae ˃ vertebrae ˃ legs. The total nitrogen content in bone char is derived from ribs ˃ vertebrae ˃ scapulae ˃ legs (Wang et al. 2020a, b). Bone char derived from ribs was found to have a high specific surface area and basic functional groups (Wang et al. 2020a, b).
3 Bone Char Composition
Many studies showed some important chemical compositions of bones and bone char in Table 1. In dried animal bone the main form of phosphorus was present in poorly crystalline hydroxyapatite (Glæsner et al. 2019). The basic mineral composition of bones is calcium phosphate, often from biological apatite phosphate. Then, there are many variances between biological apatite and geological apatite: formation conditions, the biological apatite has a small crystal size, a high substitution of phosphate (PO4−3) by carbonate (CO3−2), and a substantial deficiency of hydroxide ions (OH); all these characteristics lead to enhancement of the solubility of biological apatite (Boskey 2007; Wopenka and Pasteris 2005). Bone char is a product that mostly contains hydroxyapatite [Ca10(PO4)6(OH)2] 70–76%, carbon 9–11%, and calcium carbonate 7–9% as well as the existence of acid soluble ash < 3%, Calcium sulfate 0.1–0.2%, and iron as Fe2O3 < 0.3%. Moreover, its density is about 0.65 g cm−3 (Mendoza-Castillo et al. 2015). In bone char, the total calcium varied between 247.8–308.2 g kg−1, total phosphorus ranged from 100.9–127.5 g kg−1, Olsen-P ranged between 193.4 to 952.0 g kg−1, changes in these quantities are due to changes in pyrolysis temperatures (Amin 2023c) as well as the low concentrations of potentially harmful elements as total Cd is 0.3 mg kg−1 and total U is below the detection limit (Siebers and Leinweber 2013).
4 Soil Properties and Environmental Factors Affecting the Dissolution of Phosphorus from Bone Char
Phosphorus dynamics in the soils are controlled more by geochemical processes than by biological processes in arid and semi-arid soils. Phosphorus availability in soil was strongly influenced by parent material, topography, and land use. Furthermore, the climate affected the availability of P in soil by affecting the pH dynamics. P availability increased by 21.9% in the most arid environments as pH values increased (Filho et al. 2022). Numerous processes can limit phosphorus availability in terrestrial ecosystems: low content of P in parent materials, running out of phosphorus, sinks, transactional, soil barriers, and anthropogenic activities (Vitousek et al. 2010). Several mechanisms control the availability and transformations of phosphorus in the soils: dissolution, leaching, sorption, desorption, mineralization, immobilization, and precipitation (Zhu et al. 2018). Therefore, many factors contribute to phosphorus availability in soils, including soil particle size distribution (Elbasiouny et al. 2020; Veloso et al. 2023), calcium carbonate (Leytem and Mikkelsen 2005), pH and salinity of soil (Xie et al. 2022), soluble cations and anions (Li et al. 2019). Also, the organic matter of the soil plays a critical role in organizing soil phosphorus dynamics and improving available phosphorus for plants (Jindo et al. 2023). The impact of climate on P availability in the soils of arid and semi-arid regions is associated with the formation of calcium phosphate with low solubility and reduced mobility in the soil profile under high pH conditions, resulting in nutrient deficiency for plants, even when soil P levels are high (Filho et al. 2022). It was the pH and organic matter of the soil that regulated the competition for phosphorus between the plants and the soil's secondary minerals (Hou et al. 2019). Several different soils have shown the amount of phosphorus released from bone char to be significantly impacted by H+, Ca2+, and H2PO4− concentrations in their soil solution, which have a profound impact on their release rate (Amin 2023b; Warren et al. 2009). Also, the solubilization of bone char in the soil depends on pH, the capacity of the soil for phosphorus sorption (Bayata and Mulatu 2024), and sinks for phosphorus in soil (Warren et al. 2009). Soil pH plays an important role in a great effect on the availability of phosphorus resulted from the application of bone char in several soils. High pH soils showed a decrease in available phosphorus resulting from bone char addition, while acidic soils showed an increase in available phosphorus (Glæsner et al. 2019). The phosphorus solubility from bone char was positively correlated with phosphorus sorption capacity in the soils (Siebers and Leinweber 2013). The fate of bone char in soils and its effects on plant growth (Fig. 3). Over time changes in the availability of nutrients especially phosphorus greatly depended on the soil properties more than bone char properties (Száková et al. 2023). It has been shown that the application of bone char led to improving phosphorus availability in the soils, depending greatly on the soil type and incubation period (Amin 2023b). Bone char application to the acidic soil decreased phosphorus adsorption and increased desorption of phosphorus which in turn improved phosphorus availability seven-fold higher than unamended soil. Moreover, amending acidic soil with bone char increased organic matter and total nitrogen under continuous applications for eight years (Wakweya et al. 2022). In one of the studies, the Olsen-P content decreased with increasing electrical conductivity, soluble calcium, and soluble sulfate (Amin 2023b). The availability of nutrients especially phosphorus increased with incubation time under bone char applications in some soils (Száková et al. 2023). Dissolving phosphorus from bone char in many soils is dependent on the ionic composition of the soil solution, the content of organic matter, and the mineralogical composition (Biswas et al. 2021). Soil organic carbon, bone char type, and incubation period have significant effects on the solubilization of phosphorus by phosphate-solubilizing microorganisms (Ahmed et al. 2021a). Many studies found that the amounts of available phosphorus in many different soils amended with bone char were higher than rock phosphate (Vassilev et al. 2013; Warren et al. 2009). The available P dissolved from bone char was two to five times more than rock phosphate, while it was 24% less than triple superphosphate fertilizer (Zwetsloot et al. 2015). In high P-fixing soil, bone char proved its efficiency as an alternative source of phosphate fertilizer as same as triple superphosphate fertilizer (Zwetsloot et al. 2016). The presence of soluble calcium in soil solution is one of the main factors affecting the availability of phosphorus in the soil. The high concentrations of soluble calcium and phosphorus in the soil solution lead to the formation of insoluble calcium phosphate compounds, which rely greatly on calcium sources in the soil such as soluble and exchangeable calcium as well as calcium minerals (Cho 1991; Penn and Camberato 2019). Releasing phosphorus from compounds such as bone depends on supersaturation/undersaturation, pH, ionic strength, and the molar ratio of calcium to phosphate ions (Wang and Nancollas 2008).
5 Agricultural Practices Affect the Dissolving Phosphorus from Bone Char in Soils
Generally, the fate of inorganic fertilizers in soil is dependent on soil properties and environmental conditions. Agricultural practices' effects on dissolving phosphorus from bone char in soils are presented in Fig. 2. Phosphorus availability in the soil is greatly affected by different agronomic practices such as applications of nitrogen fertilizer, crop residues, manure, biochar, and water (Jiang et al. 2021), types of nitrogen fertilizer (Amin 2023c; Chien et al. 2011) as well as bone char with sulfur (Amin 2020) and irrigation with saline water (Amin 2021). Moreover, agricultural practices such as fertilization and crop residue incorporation affected the spatio-temporal variability of phosphorus status in the soil (Sun et al. 2015). Nitrogen fertilization is one of the important agronomic practices that have an important role in affecting the chemical properties of the soil, as the addition of nitrogen fertilizer leads to enhancing the activity of microorganisms and enzymes. Moreover, the activity of soil microorganisms and enzymes is closely related to nutrient availability (Sun et al. 2020). The addition of acidic nitrogen fertilizers such as urea, ammonium nitrate, and ammonium sulfate, adding them to the soil in large quantities caused an increase in soil acidification (Chien et al. 2011; Zhou et al. 2014). Decreasing soil pH because of the protons production via the nitrification process resulting from the addition of ammonium nitrogen fertilizers (Wang et al. 2020a, b). The pH greatly affects the solubility of phosphate compounds, which in turn controls the bioavailability of phosphorus in the soil (Penn and Camberato 2019). Our previous study found that the application of different nitrogen fertilizers on the soil pH varied with the type of nitrogen fertilizer in the presence of bone char (Amin 2023c). The fate of bone char in soils and its effects on plant growth (Fig. 3). The incorporation of nitrogen fertilizers (ammonium sulfate, ammonium nitrate, and urea) with bone char resulted in a significant improvement in the availability of phosphorus in calcareous sandy soil compared to the bone char treatment which is soil without any nitrogen fertilizer. The effectiveness of the treatments in this incubation study on the available phosphorus increase was in the order of urea > ammonium sulfate > ammonium nitrate > soil without any nitrogen fertilizer (Amin 2023c). The co-application of organic acids with bone char caused the improved release of phosphorus this is attributed to the dissolution of minerals, exchange of ligands, substitution of phosphate anions by organic anions, as well as formation of metal–organic complexes and phosphorus adsorption sites block (Schütze et al. 2020). Co-application of sulfur with bone char caused a significant increase in the available phosphorus (Olsen-P) in P-poor sandy soil compared to the unamended soil. The concentration of available phosphorus increased from 7.99 mg kg−1 for unamended soil to 10.59 mg kg−1 for bone char + sulfur treatment (Amin 2020). Elemental sulfur application led to increased release of phosphorus from cow bone char because of the increased solubility of calcium phosphates due to oxidizing elemental sulfur producing H+ and SO42− ions, which decline soil pH (Zhi-Hui et al. 2010). Bone char modification with sulfur not only increased the solubility of phosphorus but also supplied more phosphorus availability for plants compared to bone char, causing excess fertilizer P to accumulate in a bioavailable form like triple superphosphate (Jia et al. 2023). The presence of sulfate anion led to a faster phosphorus release from nano-bone char than that which would have been produced by the chloride and nitrate anions (El Refaey et al. 2022). Phosphorus release has resulted from desorption from exchange sites by sulfate ions by applying sulfur in calcareous soil (Jaggi et al. 2005). The application of sulfur-enriched bone char to the soil led to increased availability of phosphorus compared to more than bone char because of sulfur oxidation produced from biological activity (Zimmer et al. 2018). Saline irrigation water has an important role in affecting nutrient availability and the physicochemical and biological properties of soil (Chen et al. 2019). Adding saline water in the presence of bone char significantly increased the Olsen-P in calcareous sandy soil in comparison with the unamended soil and bone char with distilled water treatment. Soil pH values declined with the addition of bone char combined with saline water (Amin 2021). Releasing phosphorus from bone char in the presence of saline water possibly due to the reduction of soil pH resulted from augmentation of the soluble sulfate in soil solution and desorption of phosphorus from exchange sites by sulfate ions (Jaggi et al. 2005). Moreover, the anions in saline water may compete with phosphate ions for binding sites on the surface of soil particles; this influence may be important in inhibiting phosphorus sorption by sediments in a saline medium (Bai et al. 2017; Bruland and DeMent 2009). The dissolution of phosphorus from bone char in several different soils relies on concentrations of ions such as H+, Ca2+, and H2PO4− in the soil solution (Warren et al. 2009). Particle size of bone char had a great effect on phosphorus release in the soil. The optimum particle size of bone char for maximum phosphorus release with relatively small concentrations of cadmium in the soil solution was 0.5–1 mm. Particles of this size are acceptable for use in commercial fertilizer machines (Morshedizad and Leinweber 2017). Many studies found that the co-application of bone char with phosphate-solubilizing microorganisms plays a vital role in promoting the growth and health of plants and increasing phosphorus availability in soils which is attributed to applying microorganisms leading to solubilizing bone char (Postma et al. 2010; Vassilev et al. 2013; Zwetsloot et al. 2016). Numerous different types of bacteria in soils have the capability of phosphorus dissolution from insoluble phosphorus compounds such as bone char and apatite (Postma et al. 2010). Through the combination of bone char with beneficial bacteria, crop production systems can become more sustainable by recycling phosphorus from the wastes and reducing the addition of pesticides (Postma et al. 2010).
5.1 Integration of Bone Char with Other Sustainable Agricultural Practices
Inoculations of seeds, crops, and soil by phosphate-solubilizing microorganisms represent promising strategies to enhance world food production without causing any environmental hazards (Alori et al. 2017). Bone char combined with phosphate-solubilizing microorganisms increased phosphorus solubilization in the soil. Moreover, possibly the co-application of sulfur-oxidizing bacteria with bone char to soil caused acidification of the soil, which in turn leads to an increase in the dissolved phosphorus (Piccirillo 2023). Solubilization of inorganic phosphate in soil by phosphate-solubilizing microorganisms is due to the excretion of organic acids, siderophores, protons, hydroxyl ions, and carbon dioxide. Organic acids with their carboxyl and hydroxyl ions chelate cations or decrease pH to release phosphorus. Also, microorganisms produced inorganic acids such as sulfuric, nitric, and carbonic acids as well as chelating substances led to the solubilization of phosphorus in soil. Solubilization of inorganic phosphate in the soil increases the bioavailability of phosphorus for plants through phosphate-solubilizing microorganisms, which improves sustainable agriculture, soil fertility, and crop productivity (Alori et al. 2017).
6 Bone Char Effects on Soil Quality and Plant Growth
Bone char applications to soil improve the physical properties such as porosity and water-holding capacity. This in turn plays an important role in improving the properties of sandy soils that suffer from drought conditions (Leinweber et al. 2019). Applying bone char prepared at 500 and 800 °C to the alkaline soil significantly increased the pH, electrical conductivity, organic matter content, total nitrogen, and total phosphorus compared to the control treatment. The content of organic matter in soil amended with bone char prepared at 500 °C was higher than bone char prepared at 800 °C. However, applying bone char prepared at 500 °C to the soil significantly increased the dissolved organic carbon compared to the control treatment. Lower content of dissolved organic carbon in the soil was found with the application of bone char prepared at 800 °C (Azeem et. al 2021). Compared to the control treatment, bone char applications to the alkaline soil showed significant differences in the activity of enzymes. The higher activity of β-glucosidase was observed at applying 10% bone char was prepared at 500 °C. On the other hand, higher activities of urease and phosphatase were noticed at 10% bone char prepared at 800 °C. However, the dehydrogenase and β-glucosidase activities were reduced after applying 10% bone char prepared at 800 °C (Azeem et. al 2021). The significant contents of carbon, nitrogen, and phosphorus in bone char significantly enhanced the urease, β-glucosidase, and phosphatase. The low activity of dehydrogenase and β-glucosidase after the applications of bone char prepared at 800 °C may attributed to its low content of dissolved organic carbon (Azeem et. al 2021).
The rhizosphere is a dynamic zone where all interactions between plants, soil, and microorganisms occur. Plant roots in the soil can significantly modify the root environment through their different physiological activities, especially the secretion of organic compounds such as mucilage, organic acids, phosphatases, and some specific signaling substances, which are the main drivers of different root processes. The amount of phosphorus taken up by the plants and the extent to which it is utilized by them play crucial roles in determining the yield of the crop (Shen et al. 2011). The doses of bone char applied can be modified to be useful to the plants without damaging the environment. Also, the co-application of bone char with organic manures is a promising amendment and a substitute for chemical phosphate fertilizer (Pinheiro and Nair 2021). Recently, bone char has been used as a promising strategy for an alternative source of chemical phosphate fertilizer in modern agriculture. Compared with unamended soil, diammonium phosphate, and triple superphosphate, adding bone char in highly Cd-contaminated soil with sufficient P supply improved the yield of potato tuber and wheat heads as well as decreased total dry matter of the lettuce plant. However, applying bone char in moderately Cd-contaminated and P-deficient soil led to increases in the total dry matter of lettuce, wheat, and potato plants compared to the unamended soil (Siebers et al. 2014). Bone char applications increased in potato dry matter only, while wheat plants showed no response to all doses of bone char additions, and reduced dry matter yield of onions (Siebers et al. 2012). The grain yield of maize and soybean increased significantly with the applications of bone char into acidic soil. Moreover, bone char addition increased grain yield by 1.7 Mg ha−1 for maize and 1.8 Mg ha−1 for soya bean compared to the unamended soil. The high increase in yield is due to the high concentrations of nutrients such as available phosphorus, calcium, and magnesium as a result of the addition of bone char (Wakweya et al. 2022). Co-application of bone char with arbuscular mycorrhizae inoculation led to improved maize yield in P-fixing soil (Zwetsloot et al. 2016). Bone char application into contaminated smelter soil improved the fresh and dry weight of maize plants and decreased the concentrations of Zn and Cd in the roots and shoots of maize. Moreover, the fresh and dry weight of maize plants under applying bone char prepared at 800 °C was higher than bone char prepared at 500 °C (Azeem et al. 2021). Sulfur-modified bone char enhanced available soil phosphorus and it contributed to improving phosphorus uptake of grain in P-poor soil (Panten and Leinweber 2020). These are because of the bone char richness in nutrient content which leads it to be an important organic fertilizer that helps boost soil fertility and soil enzyme levels, thus improving the growth of maize plants as well as Zn and Cd retention mechanisms such as complexation and ion exchange with the bone char applications, which leads to a decrease in the uptake of these elements by maize plants (Azeem et al. 2021). Applying bone char in Pb-contaminated soil promotes the shoot of Chinese cabbage and decreases concentrations of Pb in the shoot (Chen et al. 2006). The applications of bone char improved the growth and yield of ridge gourd compared to the unamended soil (Um-e-Laila et al. 2021). Bone char addition into contaminated soil led to enhanced biomass and yield of rice, because it improved the uptake of phosphorus by rice plants, as well as, decreased the contents of rare earth elements in the roots, shoots, and grains of rice in comparison with the control treatment this is attributed to increasing adsorption of rare earth elements, higher concentrations of P led to precipitate rare earth elements because the formation of insoluble compounds phosphate form decreasing rare earth elements concentration in the soil solution (Jin et al. 2018). Adding bone char to soil decreased the genotoxicity of heavy metals, which resulted in reduced DNA damage in plants, which is proportionate with changes in the chemical forms of lead, cadmium, copper, and zinc. Accordingly, soil's reduced genotoxicity may be due to changes in heavy metal chemical forms (Lin et al. 2007). Bone char applications to the soil significantly decreased the stress of heavy metals on plant leaves, suggesting that bone char played a great role in promoting plant adaptation to soil contamination with heavy metals because bone charcoal changes the environment of plant rhizosphere by decreasing heavy metals availability, decreasing toxicity, and alleviating stress (Xiao et al. 2023).
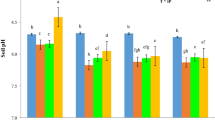
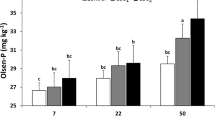
7 Bone Char Effects on Dynamics and Transformations of Phosphorus in Soils
Soil phosphorus dynamics play an important role in soil P bioavailability, but quantifying the dynamics is difficult, whether in the field, in the lab, or the greenhouse. Phosphorus dynamics in the soils consist mainly of sorption/desorption, precipitation/dissolution, mineralization/immobilization, weathering, and solid-phase P transformations, including diffusion, penetration, and recrystallization (Hou et al. 2019). The soil phosphorus is found in various forms such as inorganic phosphorus and organic phosphorus, which are both present in various quantities. The behavior of these phosphorus forms in the soil is different, as are their fates in it (Shen et al. 2011). In the soils, the dynamics of phosphorus depend largely on several different factors that are biotic and abiotic such as soil texture, structure, mineralogical composition, soil pH, oxidation–reduction conditions, salinity, organic matter, high content of toxic elements, interaction with micronutrients, runoff, leaching, soluble phosphorus, content of total phosphorus, activity of enzymes, phosphate solubilizing microorganisms, and mycorrhizas (Lizcano-Toledo et al. 2021). The dynamics of phosphorus fractions in the soil are affected by different types of tillage and cropping systems under long-term conservation agriculture (Anil et al. 2022). Soil properties such as texture, content of moisture, organic matter, pH, calcium carbonate, anions competing, and oxidation–reduction reactions greatly influence the distribution of inorganic phosphorus fractions (Shao et al. 2019). There is a significant relationship between the chemical properties of the alkaline sandy soil and the phosphorus fractions upon application of bone char (Amin 2023b). In most soils, the inorganic phosphorus has lower mobility than other nutrients, as well as being a major factor that limits the growth of plants in terrestrial ecosystems and organisms in aquatic ecosystems. Bioavailable phosphorus differs from inorganic phosphorus and can be explained due to many complex reactions between physical, chemical, and biological processes. The mentioned processes are greatly affected by several features of soil solution: pH, ionic strength, cations content, and anions concentrations (Devau et al. 2009). The fate of bone char in soils and its effects on phosphorus forms (Fig. 3). The content of most P fractions in soil increased with decreasing aggregate size under applications of bone char and sulfur-modified bone char in acidic soil (Jia et al. 2023). The effects of bone char and incubation periods on changes of inorganic phosphorus fractions in alkaline soil were presented in Fig. 4 (Amin 2023b). The NH4Cl-Pi fraction differed significantly with soil type. Adding bone char caused a significant increase in NH4Cl-Pi fraction in some alkaline soils, but, significantly decreased NH4Cl-Pi fraction in another alkaline soil compared to the unamended soil. Bone char application increased the NH4Cl-Pi fraction from 6.10 mg kg−1 for unamended soil to 6.91 mg kg−1 at 84 days of incubation time (Amin 2023b). High soluble phosphorus at high pH levels of soils has resulted from soluble complexes (ion pairs or complex ions) such as CaHPO2° or CaPO4. Mostly, soluble phosphorus compounds in soil solution rapidly convert into orthophosphate ions (Pierzynski et al. 2005). However, the resin-P fraction in acidic soil after ryegrass cropping increased significantly with applications of sulfur-enriched bone char, resin-P increased from 8 mg kg−1 for control to 11 mg kg−1 (Morshedizad et al. 2018). With the addition of bone char to some alkaline soils, a significant change was observed as far as the concentrations of NaHCO3-Pi fraction were concerned. Applying bone char increased NaHCO3-Pi fraction from 15.25 mg kg−1 for unamended soil to 27.17 mg kg−1 at 84 days of incubation time (Amin 2023b). The changes in phosphorus fractions in acidic soil after cultivating ryegrass are shown in Fig. 5 (Morshedizad et al. 2018). The additions of bone char and sulfur-enriched bone char in acidic soil caused an increased non-significant NaHCO3-Pi and NaHCO3-Po fractions (Morshedizad et al. 2018). These changes in phosphorus fraction are attributed to the pH and content of organic carbon, calcium, and potassium in the soil were closely related to labile-P fraction (Guera and da Fonseca 2022). Applying bone char to the soil doesn't cause changes in the labile P proportion because of its low solubility (Siebers et al. 2013). The applications of sulfur-modified bone char to the acidic soil contributed to increases in labile-P that are similar to those that existed in triple superphosphate. Bone char modified with sulfur has a similar fate to triple superphosphate in soil. This encourages utilizing bone char as a potential alternative to phosphate fertilizers leading to more sustainability for agriculture with more closed P cycles (Jia et al. 2023). Salinity and sodicity of the soils play an important role in influencing phosphorus dynamics as a result of changes in the sorbent surfaces because of the increment in poorly crystalline Fe oxides and changes in silicate clays, the ratio of Na/Ca and concentrations of sulfate (Dominguez et al. 2001; Meena et al. 2018). Bone char applications into alkaline soil resulted in a significantly increased NaOH-Pi fraction, while, some alkaline soils had no significant effect on the NaOH-Pi fraction. Applying bone char to calcareous sandy soil increased NaOH-Pi fraction from 9.13 mg kg−1 (unamended soil) to 12.10 mg kg−1 35 84 days of incubation time (Amin 2023b). In acidic soil, adding bone char led to an increased non-significant NaOH-Pi and NaOH-Po fractions, however, sulfur-enriched bone char led to an increased significant NaOH-Pi fraction and increased non-significant NaOH-Po fraction. Generally, the applications of bone char and sulfur-enriched bone char in acidic soil increased non-significantly all organic phosphorus fractions (Morshedizad et al. 2018). The low concentrations of NaOH-Po and NaHCO3-Po in the soil with the addition of bone char are due to the low amount of accumulated organic matter (Kruse et al. 2022). It is observed that increasing moderately labile phosphorus in soil happened with increasing organic carbon and Ca concentrations (Guera and da Fonseca 2022). Bone char additions in many alkaline soils caused a highly significant increment of HCl-Pi and residual P fractions compared to unamended soil (Amin 2023b; Amin and Mihoub 2021). Adding bone char to the calcareous sandy soil increased the HCl-Pi fraction from 237.4 mg kg−1 (unamended soil) to 754.5 mg kg−1 at 84 days of incubation time. The residual P content in soil increased from 33.74 mg kg−1 (unamended soil) to 68.21 mg kg−1 for bone char at 84 days of incubation time (Amin 2023b). In long-term fertilizer experiments, it was found that hydroxyapatite accounted for more than half of the total inorganic phosphorus in calcareous soils (Shen et al. 2011). However, adding bone char and sulfur-enriched bone char in acidic soil resulted in increased non-significant HCl-Pi and HCl-Po fractions (Morshedizad et al. 2018). By comparing alkaline and acidic soils, it was found that the phosphorus fractions resulting from applying bone char differed greatly, as the HCl–P fraction was predominant in alkaline soil, while the NaOH-P fraction was predominant in acidic soil. This indicates that phosphorus transformations are greatly affected by soil properties. In alkaline soil, the distribution of phosphorus fractions after adding bone char followed the order of HCl-P ˃ residual P ˃ NaHCO3-P ˃ NaOH-P ˃ NH4Cl-P (Amin 2023b). However, adding bone char and sulfur-enriched bone char in acidic soil led to the distribution of phosphorus fractions in the order of NaOH– P > NaHCO3–P > HCl–P > resin–P after ryegrass cropping (Morshedizad et al. 2018). Increasing soil salinity caused an increase in the HCl-P fraction (Ca-bound P) and reduced NaOH-P occluded Fe + Al-bound P fraction (Yun et al. 2010). Soil salinity led to a rapid transformation of inorganic phosphorus fractions and the concentration of any fraction of the transformations of inorganic phosphorus rely on the other forms of phosphorus. Generally, soil salinity reduces the availability of inorganic phosphorus to plants through the process of sorption and reducing the solubility of phosphorus-fixing minerals (Nasrin et al. 2016). The highest contents of P fractions are the insoluble P associated with calcium during adding bone char in soils (Amin and Mihoub 2021; Zimmer et al. 2018). But, in acidic soil, the highest concentration of P exists in the NaOH-Pi fraction including inorganic P adsorbed and bound to Al- and Fe-oxide minerals (Morshedizad et al. 2018). Inorganic and organic phosphorus fractions in the soil are greatly affected as a result of the applications of long-term nitrogen fertilizers in cultivation (Mahmood et al. 2021). The results of co-applying bone char with nitrogen fertilizer type and incubation periods on changes of phosphorus (P) fractions in calcareous sandy soil were presented in Fig. 6 (Amin 2023c). Water-soluble inorganic phosphorus (Pi–H2O) showed a significant decline after adding nitrogen fertilizers with the presence of bone char in comparison with the bone char alone, only the treatment of applying urea at 3 days of incubation increased significantly H2O-P fraction, the interaction between nitrogen fertilizer treatments and incubation periods significantly affects the H2O-P fraction. Applying nitrogen fertilizer to calcareous sandy soil decreased the concentration of soluble phosphorus from 5.46 mg kg−1 soil for the bone char treatment to 2.48, 3.24, and 3.16 mg kg−1 for bone char + ammonium sulfate, bone char + ammonium nitrate, and bone char + urea, respectively at the end of the incubation (Amin 2023c). The applications of nitrogen to the soils greatly influence phosphorus transformations (Chen et al. 2018; Jing et al. 2021). Moreover, land management and fertilizer addition control H2O-P and NaHCO3-P fractions amount in the soil (Tian et al. 2020). Other researchers reported that when the amounts of soluble calcium and phosphorus increase in soil solution, insoluble Ca-phosphates are formed that reduce soluble phosphorus (Penn and Camberato 2019; Tunesi et al. 1999). Applying nitrogen fertilizers to the soil showed a significant increment in NaHCO3-Pi content, on the other hand, declined HCl-Pi and Res-P fractions in the presence of bone char with an increasing incubation period. This is due to the dissolution of the insoluble calcium phosphate that was present in the bone char because of soil acidification resulting from the applied nitrogen fertilizer. At the end of incubation, the addition of nitrogen fertilizers to alkaline soil increased NaHCO3-Pi fraction from 20.47 mg kg−1 for bone char treatment to 28.04, 27.47, and 23.58 mg kg−1 for bone char + ammonium sulfate, bone char + ammonium nitrate, and bone char + urea treatments, respectively (Amin 2023c). However, the applications of bone char to the soil caused non-significant differences in NaOH-Pi fraction, but the application of sulfur-enriched bone char caused a significant increment in NaOH-Pi fraction compared to unfertilized soil because of the dissolving insoluble calcium phosphate in bone char (Morshedizad et al. 2018). Furthermore, applying ammonium nitrate into the soil reduced the NaHCO3-Pi fraction significantly and significant increment of NaOH-Pi fraction these are attributed to changes in soil pH, while non-significant changes in the H2O-Pi, HCl-Pi, and residual-P fractions (Yang et al. 2015). The total inorganic phosphorus increased with the application of bone char and sulfur-modified bone char to the acidic soil (Jia et al. 2023). Significant increase in stable P fraction after the addition of bone char in the soil compared with the control treatment (Kruse et al. 2022). The addition of bone char led to increases significant in the content of insoluble P (H2SO4-P) fraction in soil (Siebers et al. 2013). The high concentration of relatively non-labile phosphorus in bone char serves as a reservoir for many nutrients in some soils (Száková et al. 2023). After five years, applying bone char increased significantly the proportions of H2SO4-P. This may be attributed to the sequestrating decomposed bone char particles and not as a result of the dissolution of phosphorus from the bone char and its subsequent fixation of the released phosphorus in moderately labile and stable phosphorus pools (Jia et al. 2023).
Effects of bone char and incubation periods on changes of inorganic phosphorus (P) fractions in alkaline soil. (The data in this Figure is taken from Amin 2023b). NH4Cl-Pi: inorganic phosphorus in soil extracted by 1 molar ammonium chloride solution; NaHCO3-Pi: inorganic phosphorus in soil extracted by 0.5 molar sodium bicarbonate solution; NaOH-Pi: inorganic phosphorus in soil extracted by 0.1 molar sodium hydroxide solution; HCl-Pi: inorganic phosphorus in soil extracted by 1 molar hydrochloric acid solution; Residual phosphorus: The soil residue from the last fraction was digested with a mixture of concentrated acids. The NH4Cl-P fraction indicates a soluble phosphorous and loosely bound into the exchange sites; NaHCO3-Pi fraction expressed on inorganic phosphorous adsorbed on the soil surfaces, labile, and available for plants; NaOH-Pi fraction includes inorganic phosphorous associated strongly with chemisorption into surfaces of Al and Fe oxides; HCl-Pi fraction represents inorganic P is associated with calcium, which exists in apatite or octacalcium phosphate; residual P fraction represents the phosphorous forms that are chemically stable and recalcitrant
Effects of bone char and bone char plus on the transformations of phosphorus (P) fractions in acidic soil after growing ryegrass. (The data in this Figure is taken from Morshedizad et al. 2018). BC: bone char; BCplus: bone char enriched with reduced sulfur compounds; Resin-P: phosphorus in soil extracted by resin strips; NaHCO3–P: phosphorus in soil extracted by 0.5 molar sodium bicarbonate solution; NaOH–P: phosphorus in soil extracted by 0.1 molar sodium hydroxide solution; HCl–P: phosphorus in soil extracted by 1 molar hydrochloric acid solution. Resin-P fraction represents mobile and readily available P; NaHCO3-P fraction includes labile inorganic and organic fractions weakly absorbed to mineral surfaces and some microbial P; NaOH-P fraction indicates inorganic P adsorbed and bound to aluminum and iron oxide minerals and organic P from humic substances; HCl-P fraction includes a relatively insoluble fraction of P bound to calcium and magnesium minerals and apatite
Effects of co-applying bone char with nitrogen fertilizer type and incubation periods on the changes of phosphorus (P) fractions in calcareous sandy soil. Labile P: H2O-Pi + NaHCO3-Pi; Moderately labile P: NaOH-Pi; Moderately stable P: HCl-Pi; Non-labile P: Residual P. (The data in this Figure is taken from Amin 2023c). H2O-Pi: inorganic phosphorus in soil extracted by distilled water; NaHCO3-Pi: inorganic phosphorus in soil extracted by 0.5 molar sodium bicarbonate solution; NaOH-Pi: inorganic phosphorus in soil extracted by 0.1 molar sodium hydroxide solution; HCl-Pi: inorganic phosphorus in soil extracted by 1 molar hydrochloric acid solution; Residual phosphorus: The soil residue from the last fraction was digested with a mixture of concentrated acids. The H2O-Pi fraction indicates a soluble phosphorous; NaHCO3-Pi fraction expressed on inorganic phosphorous adsorbed on the soil surfaces, labile, and available for plants; NaOH-Pi fraction includes inorganic phosphorous associated strongly with chemisorption into surfaces of Al and Fe oxides; HCl-Pi fraction represents inorganic P is associated with calcium, which exists in apatite or octacalcium phosphate; residual P fraction represents the phosphorous forms that are chemically stable and recalcitrant
8 Using Bone Char in Remediation of Contaminated Soils by Heavy Metals
Several studies suggested that the use of bone char as an adsorbent is a promising strategy in the remediation of contaminated soils by heavy metals because of its cheap price and may not require any prior chemical treatments (Mei et al. 2022; Wang et al. 2020a, b; Xiao-Wei et al. 2010). The sorption characteristics of bone char for remediation of heavy metals rely heavily on the inorganic composition of this sorbent, especially calcium phosphate (Mendoza-Castillo et al. 2015). Bone char adsorbs pollutants from the soil largely based on its main component hydroxyapatite, and the adsorption sites of hydroxyapatite are formed by protonation and deprotonation of the surface affected by the pH point of zero charge and the pH of the solution (Hart et al. 2023). Bone char produced from different bone parts had different concentrations of carbon and calcium phosphate as well as different specific surface areas. The rib-derived bone char had the highest specific surface area and thus showed the highest copper removal performance (Wang et al. 2020a, b). In comparison with the control, the application of bone char to the soil decreased leaching Cu, Zn, Pb, and Cd by 91.2%, 38.6%, 67.6%, and 54.3%, respectively as well as improved pea growth and decline concentrations of heavy metals in shoots (Mei et al. 2022). Bone char addition to contaminated soil decreased the availability of Pb and Zn, these are attributed to the application of bone char converted the Pb and Zn from non-residual fractions into a residual fraction (Xiao-Wei et al. 2010). The Pb uptake by Chinese cabbage decreased with increasing bone char doses in Pb-contaminated soil because adding bone char to a Pb-contaminated soil led to decreased availability of Pb (Chen et al. 2006). The mechanisms of remediating heavy metals from the soils by the bone char include surface complexation, exchange of heavy metal cations with Ca2+ in bone char, precipitation, immobilization, and electrostatic interaction (Azeem et al. 2022; Mei et al. 2022). Increasing doses of added bone char result in a gradual decrease of Pb, Cr, and Cd mobility in contaminated soil because these metals react with phosphate and precipitate in insoluble phosphate forms (Gruden et al. 2017). Several factors affect bone char's immobilization of heavy metals in soil, including pyrolysis temperature, levels of application, treatment time, soil type, type of metal, and soil pH (Azeem et al. 2022; Mei et al. 2022). In the presence of phosphate-solubilizing bacteria, bone char can serve as an effective Pb immobilization material. Therefore, it is expected that bone char will be used to treat Pb-contaminated soils (Li et al. 2023). Bone char applications in aqueous solutions resulted in the adsorption of 60 to 92% of heavy metals, so it is used as a promising strategy in water purification (Mendoza-Castillo et al. 2015). Applying bone char to the soil reduced the concentrations of Cd, Cu, and Zn in water-soluble, exchangeable, carbonate-bound fractions, but increased the concentrations of these metals in the organic fraction or residual fraction (Lin et al. 2007). Adding bone char to contaminated soil by rare earth elements decreased significantly the concentrations of rare earth elements in the soil solution this is attributed to the phosphate ions produced by bone char can lead to the formation of a resistant mineral phosphate precipitate with ions of rare earth elements (Jin et al. 2018). There are several mechanisms responsible for the removal of heavy metal ions by bone char such as cation exchange, cation-π bonding, electrostatic interaction, surface complexation, and chemical precipitation. Also, bone char contains various functional groups, such as carboxyl, carbonyl, pyridyl, hydroxyl, ester, and pyrrole groups, which play a major role in the reaction with heavy metals (Xiao et al. 2020). Bone char application has the ability to convert fractions of Cd, Pb, Cu, and Mn in the soil from labile fractions to a stable fraction. The stabilization of heavy metals in the soil increases with increasing doses of bone char applications, which in turn leads to a decrease in the available concentrations of heavy metals in the soil (Xiao et al. 2023).
9 Using Bone Char for Water Purification
Water contamination naturally and anthropogenically has a significant effect on human health and economic development across the globe (Castillo et al. 2023). Bone char can be used to treat groundwater in regions with high fluoride concentrations (Shahid et al. 2019). Pyrolysis of animal bones to produce bone char has proven to be a promising strategy for removing heavy metals due to the integration of components such as hydroxyapatite and carbon into the char. Mechanisms of adsorption of some heavy and radioactive elements and organic compounds on bone char surfaces include electrostatic attraction, surface complexation, precipitation, cation exchange, cation-π coordination, and π–π interaction (Castillo et al. 2023; Yang et al. 2022). Using bone char as a treatment method for manganese contamination of groundwater is an efficient, eco-friendly, and effective solution that is economical and sustainable (Michael et al. 2022). Several studies have found that using bone char as a green sorbent is a promising strategy more reliable and sustainable for removing fluoride and arsenic from drinking water (Alkurdi et al. 2019a, b).
10 Conclusions and Future Perspectives
Excessive utilization of chemical phosphate fertilizers in agricultural production has resulted in increasing the pollution of soil, water bodies, and groundwater to a level harmful to the ecosystems as well as the global high cost of chemical phosphate fertilizers has led to finding novel and effective solutions to search for renewable sources of phosphorus, such as the production of bone char. This review article showed important information about the addition of bone char as a novel and potentially sustainable agricultural practice for the fertilization of the soils and improving crop production. Yet, the growing interest in bone char for agricultural applications motivated us to produce such a review. The novelty of this review article is the effect of applying bone char on the release, transformations, and fractions of phosphorus in soils as well as the effects of agricultural practices and soil properties on the enhancement of dissolving phosphorus from bone char in soils. Additionally, it assessed using bone char in the remediation of contaminated soils by heavy metals. The applications of bone char to acidic and alkaline soils improved phosphorus availability. The changes in availability and dynamics of phosphorus in different soils under bone char applications are dependent on bone char characteristics and soil properties. Agricultural practices, such as co-applying sulfur, organic acids, and nitrogen fertilizers (ammonium sulfate, ammonium nitrate, and urea) with bone char, showed a significant effect on enhancing available phosphorus in some soils. Also, Co-applications of bone char with phosphate-solubilizing microorganisms could represent a promising strategy for improving soil phosphorus availability. The yield of crops increased as a function of applying bone char to several soils. The co-application of bone char with phosphate-solubilizing microorganisms led to improving the plants growth and increasing phosphorus availability in soils. The applications of bone char to the soil enhance the health and quality of the soil. Bone char application to contaminated soils led to higher immobilization of heavy metals and promoted the growth and yield of plants. Bone char has shown positive performance in the remediation of soils contaminated by heavy metals. Moreover, there are future prospects for developing a method to improve the quality of fertilizers produced from bone char through some treatments. Combining bone char with humic acid and composting bone char with green manure are examples of these treatments which in turn increase the release of phosphorus in soils from bone char and promote plant growth. Furthermore, more studies on the effectiveness of bone char application are urgently needed to evaluate these results through long-term field conditions. Using bone char as an alternative to chemical phosphate fertilizers has become one of the novel and effective solutions in sustainable agriculture. Additionally, using bone char as a green sorbent is a promising strategy for drinking water purification.
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
References
Ahmed M, Nigussie A, Addisu S, Belay B, Sato S (2021b) Valorization of animal bone into phosphorus biofertilizer: effects of animal species, thermal processing method, and production temperature on phosphorus availability. Soil Sci Plant Nutr 67:471–481. https://doi.org/10.1080/00380768.2021.1945403
Ahmed M, Nigussie A, Addisu S, Belay B, Lehmann J, Sato S (2021a) Valorization of animal bone waste for agricultural use through biomass co-pyrolysis and bioaugmentation. Biomass Convers Biorefin 1–10. https://doi.org/10.1007/s13399-021-02100-w
Alkurdi SSA, Al-Juboori RA, Bundschuh J, Hamawand I (2019a) Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ Int 127:704–719. https://doi.org/10.1016/j.envint.2019.03.065
Alkurdi SSA, Herath I, Bundschuh J, Al-Juboori RA, Vithanage M, Mohan D (2019b) Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: What needs to be done in future research? Environ Int 127:52–69. https://doi.org/10.1016/j.envint.2019.03.012
Alkurdi SSA, Al-Juboori RA, Bundschuh J, Bowtell L, McKnight S (2020) Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ Pollut 262:114221. https://doi.org/10.1016/j.envpol.2020.114221
Alkurdi SSA, Al-Juboori RA, Bundschuh J, Bowtell L, Marchuk A (2021) Inorganic arsenic species removal from water using bone char: a detailed study on adsorption kinetic and isotherm models using error functions analysis. J Hazard Mater 405:124112. https://doi.org/10.1016/j.jhazmat.2020.124112
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971. https://doi.org/10.3389/fmicb.2017.00971
Amin AA (2020) Sulfur, Na2-EDTA and their mixture effects on phosphorus release from cow bone char in P-poor sandy soil. Environ Technol Innov 17:100636. https://doi.org/10.1016/j.eti.2020.100636
Amin AA (2021) Enhancement of releasing phosphorus from bone char in calcareous sandy soil under applying different levels of water salinity. J Soil Sci Plant Nutr 21:476–486. https://doi.org/10.1007/s42729-020-00376-x
Amin AA (2023a) Effects of pyrolysis temperatures on bone char characterization and its releasing phosphorus in sandy soil. Arch Agron Soil Sci 69:304–313. https://doi.org/10.1080/03650340.2021.1988940
Amin AA (2023b) Chemical properties of some alkaline sandy soils and their effects on phosphorus dynamics with bone char application as a renewable resource of phosphate fertilizer. J Soil Sci Plant Nutr 23:1589–1598. https://doi.org/10.1007/s42729-023-01199-2
Amin AA (2023c) Effect of co-applying different nitrogen fertilizers with bone char on enhancing phosphorus release in calcium carbonate-rich soil: an incubation study. J Soil Sci Plant Nutr 23:1565–1575. https://doi.org/10.1007/s42729-023-01217-3
Amin AA, Mihoub A (2021) Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus spp.) on release and distribution of phosphorus in high calcareous P-fixing soils. J Soil Sci Plant Nutr 21:2041–2047. https://doi.org/10.1007/s42729-021-00500-5
Anil AS, Sharma VK, Parihar CM, Datta SP, Barman M, Chobhe KA, Kumawat C, Patra A, Jatav SS (2022) Impact of long-term conservation agriculture practices on phosphorus dynamics under maize-based cropping systems in a sub-tropical soil. Land 11:1488. https://doi.org/10.3390/land11091488
Asgari G, Dayari A, Ghasemi M et al (2019) Efficient fluoride removal by preparation, characterization of pyrolysis bone: Mixed level design experiment and Taguchi L8 orthogonal array optimization. J Mol Liq 275:251–264. https://doi.org/10.1016/j.molliq.2018.10.137
Azeem M, Ali A, Jeyasundar PGSA et al (2021) Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil. Chemosphere 282:131016. https://doi.org/10.1016/j.chemosphere.2021.131016
Azeem M, Shaheen SM, Ali A, Jeyasundar PG et al (2022) Removal of potentially toxic elements from contaminated soil and water using bone char compared to plant- and bone-derived biochars: A review. J Hazard Mater 427:128131. https://doi.org/10.1016/j.jhazmat.2021.128131
Bai J, Ye X, Jia J, Zhang G, Zhao Q, Cui B, Liu X (2017) Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 188:677–688. https://doi.org/10.1016/j.chemosphere.2017.08.117
Bayata A, Mulatu G (2024) Effect of bone char application on soil quality, soil enzyme and in enhancing crop yield in agriculture a review. Am J Chem Eng 12:13–28. https://doi.org/10.11648/j.ajche.20241202.11
Biswas PP, Liang B, Turner-Walker G, Rathod J, Lee Y, Wang C, Chang C (2021) Systematic changes of bone hydroxyapatite along a charring temperature gradient: an integrative study with dissolution behavior. Sci Total Environ 766:142601. https://doi.org/10.1016/j.scitotenv.2020.142601
Boskey AL (2007) Mineralization of bones and teeth. Elements 3:385–391. https://doi.org/10.2113/GSELEMENTS.3.6.385
Bruland GL, DeMent G (2009) Phosphorus sorption dynamics of Hawaii’s coastal wetlands. Estuar Coasts 32:844–854. https://doi.org/10.1007/s12237-009-9201-9
Castillo NAM, González Fernández LA et al (2023) Bone char for water treatment and environmental applications: a review. J Anal Appl Pyrol 175:106161. https://doi.org/10.1016/j.jaap.2023.106161
Chen S, Zhu Y, Ma Y, McKay G (2006) Effect of bone char application on Pb bioavailability in a Pb-contaminated soil. Environ Pollut 139:433–439. https://doi.org/10.1016/j.envpol.2005.06.007
Chen H, Chen M, Li D, Mao Q, Zhang W, Mo J (2018) Responses of soil phosphorus bioavailability to nitrogen addition in a legume and a non-legume plantation. Geoderma 322:12–18. https://doi.org/10.1016/j.geoderma.2018.02.017
Chen L, Li C, Feng Q, Wei Y, Zhao Y, Zhu M, Deo RC (2019) Direct and indirect impacts of ionic components of saline water on irrigated soil chemical and microbial processes. CATENA 172:581–589. https://doi.org/10.1016/j.catena.2018.09.030
Chien SH, Gearhart MM, Villagarc S (2011) Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: a review. Soil Sci 176:327–335. https://doi.org/10.1097/SS.0b013e31821f0816
Cho CM (1991) Phosphate transport in calcium-saturated systems: I Theory. Soil Sci Soc Am J 55:1275–1281. https://doi.org/10.2136/sssaj1991.03615995005500050013
Devau N, Cadre EL, Hinsinger P, Jaillard B, Gérard F (2009) Soil pH controls the environmental availability of phosphorus: Experimental and mechanistic modelling approaches. Appl Geochemistry 24:2163–2174. https://doi.org/10.1016/j.apgeochem.2009.09.020
Dominguez R, Del Campillo C, Pena F, Delgado A (2001) Effect of soil properties and reclamation practices on phosphorus dynamics in reclaimed calcareous marsh soils from the Guadalquivir Valley, SW Spain. Arid Land Res Manag 15:203–221. https://doi.org/10.1080/15324980152119775
El Refaey AA, Mohamed NA, Mostafa HE, Gouda NA (2022) Performance of fast and slow phosphorus release from nano-bone char. Egypt J Soil Sci 62:223–235. https://doi.org/10.21608/ejss.2022.151212.1519
Elbasiouny H, Elbehiry F, El-Ramady H, Brevik EC (2020) Phosphorus availability and potential environmental risk assessment in alkaline soils. Agriculture 10:172. https://doi.org/10.3390/agriculture10050172
FAO (2023) Rising global food insecurity: Assessing policy responses. https://www.fao.org/3/cc5392en/cc5392en.pdf
Fayaz H, Alshahrani S, Abbas MM et al (2021) Waste animal bones as catalysts for biodiesel production. A Mini Review Catalysts 11:630. https://doi.org/10.3390/catal11050630
Filho JDSO, Barrozo MVDS, Pereira MG (2022) Environmental factors and land use changes controlling the availability of phosphorus in dryland soils. J Arid Environ 202:104770. https://doi.org/10.1016/j.jaridenv.2022.104770
Gamage A, Gangahagedara R, Gamage J, Jayasinghe N, Kodikara N, Suraweera P, Merah O (2023) Role of organic farming for achieving sustainability in agriculture. Farming System 1:100005. https://doi.org/10.1016/j.farsys.2023.100005
Glæsner N, Hansen HCB, Hu Y, Bekiaris G, Bruun S (2019) Low crystalline apatite in bone char produced at low temperature ameliorates phosphorus-deficient soils. Chemosphere 223:723–730. https://doi.org/10.1016/j.chemosphere.2019.02.048
Gruden E, Bukovec P, Zupancic M (2017) Preliminary evaluation of animal bone char as potential metal stabilization agent in metal contaminated soil. Acta Chim Slov 64:577–581. https://doi.org/10.17344/acsi.2016.2889
Guera KCS, da Fonseca AF (2022) Phosphorus fractions and their relationships with soil chemical attributes in an integrated crop-livestock system under annual phosphates fertilization. Front Sustain Food Syst 6:893525. https://doi.org/10.3389/fsufs.2022.893525
Gupta DK, Chatterjee S, Datta S, Veer V, Walther C (2014) Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 108:134–144. https://doi.org/10.1016/j.chemosphere.2014.01.030
Hart A, Ebiundu K, Peretomode E et al (2022) Value-added materials recovered from waste bone biomass: technologies and applications. RSC Adv 12:22302–22330. https://doi.org/10.1039/D2RA03557J
Hart A, Porbeni DW, Omonmhenle S, Peretomode E (2023) Waste bone char-derived adsorbents characteristics adsorption mechanism and model approach. Environ Technol Rev 12:175–204. https://doi.org/10.1080/21622515.2023.2197128
Hassen BT, El Bilali H (2021) Impacts of the Russia-Ukraine war on global food security: Towards more sustainable and resilient food systems? Foods 11:2301. https://doi.org/10.3390/foods11152301
Hou E, Lu X, Jiang L, Wen D, Luo Y (2019) Quantifying Soil Phosphorus Dynamics: A Data Assimilation Approach. JGR Biogeosciences 124:2159–2173. https://doi.org/10.1029/2018JG004903
Jaggi RC, Aulakh MS, Sharma AR (2005) Impacts of elemental S applied under various temperature and moisture regimes on pH and available P in acidic, neutral and alkaline soils. Biol Fertil Soils 41:52–58. https://doi.org/10.1007/s00374-004-0792-9
Jia Y, Siebers N, Panten K, Kruse J (2023) Fate and availability of phosphorus from bone char with and without sulfur modification in soil size fractions after five-year field fertilizations. Soil Tillage Res 231:105720. https://doi.org/10.1016/j.still.2023.105720
Jiang B, Shen J, Sun M, Hu Y, Jiang W, Wang J, Li Y, Wu J (2021) Soil phosphorus availability and rice phosphorus uptake in paddy fields under various agronomic practices. Pedosphere 31:103–115. https://doi.org/10.1016/S1002-0160(20)60053-4
Jin S, Hu Z, Huang Y, Pan H, Hu Y (2018) Effects of rice straw, rice straw ash, and bone charcoal on uptake and accumulation of rare earth elements in rice plants. BioRes 13:8593–8613. https://doi.org/10.15376/biores.13.4.8593-8613
Jindo K, Audette Y, Olivares FL, Canellas LP, Smith DS, Voroney RO (2023) Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: a review. Chem Biol Technol Agric 10:29. https://doi.org/10.1186/s40538-023-00401-y
Koul B, Yakoob M, Shah MP (2022) Agricultural waste management strategies for environmental sustainability. Environ Res 206:112285. https://doi.org/10.1016/j.envres.2021.112285
Kruse J, Panten K, Siebers N (2022) The fate of phosphorus from bone char-based fertilizers in soil pools in a 5-year crop rotation. Nutr Cycl Agroecosyst 124:263–277. https://doi.org/10.1007/s10705-022-10228-y
Leinweber P, Hagemann P, Kebelmann L, Kebelmann K, Morshedizad M (2019) Bone char as a novel phosphorus fertilizer. In: Ohtake H, Tsuneda S (eds) Phosphorus recovery and recycling. Springer, Singapore. https://doi.org/10.1007/978-981-10-8031-9_29
Leytem AB, Mikkelsen RL (2005) The nature of phosphorus in calcareous soils. Better Crops 89:11–13. https://eprints.nwisrl.ars.usda.gov/id/eprint/14/1/1159.pdf
Li Z, Wu SP, Ye CL (2015) Temperature-related changes of bioapatite based on hypermineralized dolphin’s bulla. J Raman Spectrosc 46:964–968. https://doi.org/10.1002/jrs.4653
Li F, Liu L, Liu J, Yang K (2019) Abiotic and biotic controls on dynamics of labile phosphorus fractions in calcareous soils under agricultural cultivation. Sci Total Environ 681:163–174. https://doi.org/10.1016/j.scitotenv.2019.05.091
Li J, Bai R, Chen W, Ren C, Yang F, Tian X, Xiao X, Zhao F (2023) Efficient lead immobilization by bio-beads containing Pseudomonas rhodesiae and bone char. J Hazard Mater 447:130772. https://doi.org/10.1016/j.jhazmat.2023.130772
Lin AJ, Zhang XH, Su YH, Hu Y, Cao Q, Zhu YG (2007) Chemical fixation of metals in soil using bone char and assessment of the soil genotoxicity. Environmental Science 28:232–237
Lizcano-Toledo R, Reyes-Martín MP, Celi L, Fernández-Ondoño E (2021) Phosphorus dynamics in the soil–plant–environment relationship in cropping systems: A Review. Appl Sci 11:11133. https://doi.org/10.3390/app112311133
Mahmood M, Tian Y, Ma Q, Hui X, Elrys AS, Ahmed W, Mehmood S, Wang Z (2021) Changes in phosphorus fractions in response to long-term nitrogen fertilization in loess plateau of China. Field Crops Res 270:108207. https://doi.org/10.1016/j.fcr.2021.108207
McLaughlin MJ, McBeath TM, Smernik SSP, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils—implications for fertiliser management and design: an Australian perspective. Plant Soil 349:69–87. https://doi.org/10.1007/s11104-011-0907-7
Meena M, Narjary B, Sheoran P, Jat H, Joshi P, Chinchmalatpure A, Yadav G, Yadav R, Meena M (2018) Changes of phosphorus fractions in saline soil amended with municipal solid waste compost and mineral fertilizers in a mustard-pearl millet cropping system. CATENA 160:32–40. https://doi.org/10.1016/j.catena.2017.09.002
Mei H, Huang W, Wang Y, Xu T, Zhao L, Zhang D, Luo Y, Pan X (2022) One stone two birds: Bone char as a cost-efective material for stabilizing multiple heavy metals in soil and promoting crop growth. Sci Total Environ 840:156163. https://doi.org/10.1016/j.scitotenv.2022.156163
Mendoza-Castillo DI, Bonilla-Petriciolet A, Jáuregui-Rincón J (2015) On the importance of surface chemistry and composition of bone char for the sorption of heavy metals from aqueous solution. Desalin Water Treat 54:1651–1662. https://doi.org/10.1080/19443994.2014.888684
Michael K, Wilson AW, Govender PP (2022) Modelling of manganese-contaminated groundwater through batch experiments: Implications for bone char remediation. Environ Adv 10:100323. https://doi.org/10.1016/j.envadv.2022.100323
Morshedizad M, Panten K, Klysubun W, Leinweber P (2018) Bone char effects on soil: sequential fractionations and XANES spectroscopy. Soil 4:23–35. https://doi.org/10.5194/soil-4-23-2018
Morshedizad M, Leinweber P (2017) Leaching of phosphorus and cadmium in soils amended with different bone chars. CLEAN – Soil, Air, Water 45:1600635. https://doi.org/10.1002/clen.201600635
Nasrin S, Biswas TK, Amin MS, Khatun M (2016) Study of salinity effects on the inorganic phosphorus transformation in three different soil series of Ganges River Floodplain. Jahangirnagar Univ J Biol Sci 5:71–79. https://doi.org/10.3329/jujbs.v5i1.29745
Novotny EH, Maia CMB, Carvalho MT, Madari BE (2015) Biochar: pyrogenic carbon for agricultural use-a critical review. Rev Bras Cienc Solo 39:321–344. https://doi.org/10.1590/01000683rbcs20140818
Panten K, Leinweber P (2020) Agronomic evaluation of bone char as phosphorus fertiliser after five years of consecutive application. J Kulturpflanzen 72:561–576. https://doi.org/10.5073/JfK.2020.12.02
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agric 9:120. https://doi.org/10.3390/agriculture9060120
Piccirillo C (2023) Preparation, characterisation and applications of bone char, a food waste-derived sustainable material: A review. J Environ Manage 339:117896. https://doi.org/10.1016/j.jenvman.2023.117896
Pierzynski GM, McDowell RW, Sims JT (2005) Chemistry, cycling, and potential movement of inorganic phosphorus in soils. In: Sims J, Sharpley A (eds) Phosphorus: Agriculture and the environment. ASA, CSSA, SSSA, Madison, pp 53–86
Pinheiro FM, Nair VD (2021) Characterization of bone char as a soil amendment in tropical soils. Horticult Int J 5:74–76. https://doi.org/10.15406/hij.2021.05.00206
Postma J, Nijhuis E, Someus E (2010) Selection of phosphorus solubilizing bacteria with biocontrol potential for growth in phosphorus rich animal bone charcoal. Appl Soil Ecol 46:464–469. https://doi.org/10.1016/j.apsoil.2010.08.016
Postma J, Clematis F, Nijhuis EH, Someus E (2013) Efficacy of four phosphate-mobilizing bacteria applied with an animal bone charcoal formulation in controlling Pythium aphanidermatum and Fusarium oxysporum f.Sp. Radicis Lycopersici in Tomato Biol Control 67:284–291. https://doi.org/10.1016/j.biocontrol.2013.07.002
Robles Á, Aguado D, Barat R, Borrás L, Bouzas A, Giménez JB, Martí N, Ribes J, Ruano MV, Serralta J, Ferrer J, Seco A (2020) New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour Technol 300:122673. https://doi.org/10.1016/j.biortech.2019.122673
Saleh ME., Khaled IAM, El-Gamal EH, Abdelsalam AA., Kamh MA (2023) Available phosphorous and organic carbon as an indication for the evaluation of bone char and bone ash applied to calcareous soil. Alex Sci Exch J 44:175–192. https://asejaiqjsae.journals.ekb.eg/article_305025.html#:~:text=10.21608/ASEJAIQJSAE.2023.305025
Schütze E, Gypser S, Freese D (2020) Kinetics of phosphorus release from vivianite, hydroxyapatite, and bone char influenced by organic and inorganic compounds. Soil Syst 4:15. https://doi.org/10.3390/soilsystems4010015
Shahid MK, Kim JY, Choi Y (2019) Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Groundw Sustain Dev 8:324–331. https://doi.org/10.1016/j.gsd.2018.12.003
Shao W, Zhu J, Teng Z, Zhang K, Liu S, Li M (2019) Distribution of inorganic phosphorus and its response to the physicochemical characteristics of soil in Yeyahu Wetland, China. Process Saf Environ Prot 125:1–8. https://doi.org/10.1016/j.psep.2019.02.025
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus Dynamics: From Soil to Plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Siebers N, Leinweber P (2013) Bone char: a clean and renewable phosphorus fertilizer with cadmium immobilization capability. J Environ Qual 42:405–411. https://doi.org/10.2134/jeq2012.0363
Siebers N, Kruse J, Leinweber P (2013) Speciation of phosphorus and cadmium in a contaminated soil amended with bone char: sequential fractionations and XANES spectroscopy. Water Air Soil Pollut 224:1564. https://doi.org/10.1007/s11270-013-1564-7
Siebers N, Godlinski F, Leinweber P (2014) Bone char as phosphorus fertilizer involved in cadmium immobilization in lettuce, wheat, and potato cropping. J Plant Nutr Soil Sci 177:75–83. https://doi.org/10.1002/jpln.201300113
Siebers N, Godlinski F, Leinweber P (2012) The phosphorus fertilizer value of bone char for potatoes, wheat, and onions: First results. Landbauforsch vTI Agric For Res 62:59–64. https://d-nb.info/1023890631/34
Simons A, Solomon D, Chibssa W, Blalock G, Lehmann J (2014) Filling the phosphorus fertilizer gap in developing countries. Nat Geosci 7:3. https://doi.org/10.1038/ngeo2049
Sun W, Huang B, Qu M, Tian K, Yao L, Fu M, Yin L (2015) Effect of farming practices on the variability of phosphorus status in intensively managed soils. Pedosphere 25:438–449. https://doi.org/10.1016/S1002-0160(15)30011-4
Sun D, Hale L, Kar G, Soolanayakanahally R, Adl S (2018) Phosphorus recovery and reuse by pyrolysis: applications for agriculture and environment. Chemosphere 194:682–691. https://doi.org/10.1016/j.chemosphere.2017.12.035
Sun J, Li W, Li C, Chang W, Zhang S, Zeng Y, Zeng C, Peng M (2020) Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front Plant Sci 11:613760. https://doi.org/10.3389/fpls.2020.613760
Száková J, Stiborová H, Mercl F, Hailegnaw NS, Lhotka M, Derevyankina T, Paul CS, Taisheva A, Brabec M, Tlustoš P (2023) Woodchips biochar versus bone char in a one-year model soil incubation experiment: the importance of soil/char pH alteration on nutrient availability in soil. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.7421
Tang L, Shen Z, Duan X, Wang Z, Wu Y, Shao X, Song X, Hu S, Li Z (2019) Evaluating the potential of charred bone as P hotspot assisted by phosphate-solubilizing bacteria. Sci Total Environ 696:133965. https://doi.org/10.1016/j.scitotenv.2019.133965
Taylor M, Kim N, Smidt G et al. (2016) Trace element contaminants and radioactivity from phosphate fertiliser. In: Schnug E, De Kok L (eds) Phosphorus in agriculture: 100 % Zero. Springer, Dordrecht, pp 231–266 https://doi.org/10.1007/978-94-017-7612-7_12
Tian L, Guo Q, Yu G, Zhu Y, Lang Y, Wei R, Hu J, Yang X, Ge T (2020) Phosphorus fractions and oxygen isotope composition of inorganic phosphate in typical agricultural soils. Chemosphere 239:124622. https://doi.org/10.1016/j.chemosphere.2019.124622
Tirado R, Allsop M (2012) Phosphorus in agriculture: problems and solutions. Greenpeace Research Laboratories Technical Report (Review). Greenpeace International, Ottho Heldringstraat 5, 1066 AZ Amsterdam
Tunesi S, Poggi V, Gessa C (1999) Phosphate adsorption and precipitation in calcareous soils: the role of calcium ions in solution and carbonate minerals. Nutr Cycling Agroecosyst 53:219–227. https://doi.org/10.1023/A:1009709005147
Um-e-Laila HA, Nazir A, Shafiq M, Firdaus-e-Bareen (2021) Potential application of biochar composite derived from rice straw and animal bones to improve plant growth. Sustainability 13:11104. https://doi.org/10.3390/su131911104
van Enk RJ, van der Vee G, Acera LK, Schuiling R, Ehlert PAI (2011) The phosphate balance : current developments and future outlook. (Report / InnovationNetwork; No. no. 10.2.232E). InnovationNetwork. https://edepot.wur.nl/165195
van Hoesel A, Reidsma FH, van Os BJH, Megens L, Braadbaart F (2019) Combusted bone: physical and chemical changes of bone during laboratory simulated heating under oxidising conditions and their relevance for the study of ancient fire use. J Archaeol Sci Rep 28:102033. https://doi.org/10.1016/j.jasrep.2019.102033
Vassilev N, Martos E, Mendes G, Martos V, Vassileva M (2013) Biochar of animal origin: a sustainable solution to the global problem of high-grade rock phosphate scarcity? J Sci Food Agric 93:1799–1804. https://doi.org/10.1002/jsfa.6130
Veloso FR, Marques DJ, de Melo EI, Bianchini HC, Maciel GM, de Melo AC (2023) Different soil textures can interfere with phosphorus availability and acid phosphatase activity in soybean. Soil Tillage Res 234:105842. https://doi.org/10.1016/j.still.2023.105842
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Wakweya T, Nigussie A, Worku G, Biresaw A, Aticho A, Hirko O, Ambaw G, Mamuye M, Dume B, Ahmed M (2022) Long-term effects of bone char and lignocellulosic biochar-based soil amendments on phosphorus adsorption–desorption and crop yield in low-input acidic soils. Soil Use Manage 38:703–713. https://doi.org/10.1111/sum.12757
Wang L, Nancollas GH (2008) Calcium orthophosphates: Crystallization and dissolution. Chem Rev 108:4628–4669. https://doi.org/10.1021/cr0782574
Wang J, Tu X, Zhang H, Cui J, Ni K, Chen J, Cheng Y, Zhang J, Chang SX (2020a) Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci Total Environ 747:141340. https://doi.org/10.1016/j.scitotenv.2020.141340
Wang M, Liu Y, Yao Y, Han L, Liu X (2020b) Comparative evaluation of bone chars derived from bovine parts: Physicochemical properties and copper sorption behavior. Sci Total Environ 700:134470. https://doi.org/10.1016/j.scitotenv.2019.134470
Warren GP, Robinson JS, Someus E (2009) Dissolution of phosphorus from animal bone char in 12 soils. Nutr Cycl Agroecosys 84:167–178. https://doi.org/10.1007/s10705-008-9235-6
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143. https://doi.org/10.1016/j.msec.2005.01.008
Xiao J, Hu R, Chen G (2020) Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J Hazard Mater 387:121980. https://doi.org/10.1016/j.jhazmat.2019.121980
Xiao J, Li X, Cao Y et al (2023) Does micro/nano biochar always good to phytoremediation? A case study from multiple metals contaminated acidic soil using Salix jiangsuensis “172.” Carbon Res 2:21. https://doi.org/10.1007/s44246-023-00053-5
Xiao-Wei H, Yi-Zong H, Yan-Shan C (2010) Effect of bone char addition on the fractionation and bio-accessibility of Pb and Zn in combined contaminated soil. Acta Ecol Sin 30:118–122. https://doi.org/10.1016/j.chnaes.2010.03.012
Xie W, Yang J, Gao S, Yao R, Wang X (2022) The effect and influence mechanism of soil salinity on phosphorus availability in coastal salt-affected soils. Water 14:2804. https://doi.org/10.3390/w14182804
Yang K, Zhu J, Gu J, Yu L, Wang Z (2015) Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann for Sci 72:435–442. https://doi.org/10.1007/s13595-014-0444-7
Yang Z, Dong Y, Meng X, Yang X, Hu R, Liu Y, Wu J (2022) Nitrogen-functionalized bone chars with developed surface area for efficient adsorption of multiple aquatic pollutants. Colloids Surf a: Physicochem Eng Asp 647:129061. https://doi.org/10.1016/j.colsurfa.2022.129061
Yun EY, Ro HM, Lee GT, Choi WJ (2010) Salinity effects on chlorpyrifos degradation and phosphorus fractionation in reclaimed coastal tideland soils. Geosci J 14:371–378. https://doi.org/10.1007/s12303-010-0032-2
Zhi-Hui Y, Stoven K, Haneklaus S, Singh BR, Schnug E (2010) Elemental sulfur oxidation by Thiobacillus spp. and aerobic heterotrophic sulfur-oxidizing bacteria. Pedosphere 20:71–79. https://doi.org/10.1016/S1002-0160(09)60284-8
Zhou J, Xia F, Liu X, He Y, Xu J, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J Soils Sediments 14:415–422. https://doi.org/10.1007/s11368-013-0695-1
Zhu J, Li M, Whelan M (2018) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537. https://doi.org/10.1016/j.scitotenv.2017.08.095
Zimmer D, Kruse J, Siebers N, Panten K, Oelschläger C, Warkentin M, Hu Y, Zuin L, Leinweber P (2018) Bone char vs. S-enriched bone char: multi–method characterization of bone chars and their transformation in soil. Sci Total Environ 643:145–156. https://doi.org/10.1016/j.scitotenv.2018.06.076
Zwetsloot MJ, Lehmann J, Solomon D (2015) Recycling slaughterhouse waste into fertilizer: how do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry? J Sci Food Agricul 95:281–288. https://doi.org/10.1002/jsfa.6716
Zwetsloot MJ, Lehmann J, Bauerle T, Vanek S, Hestrin R, Nigussie A (2016) Phosphorus availability from bone char in a P-fixing soil influenced by root mycorrhizae-biochar interactions. Plant Soil 408:95–105. https://doi.org/10.1007/s11104-016-2905-2
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amin, A.EE.A.Z. Using Bone Char as a Renewable Resource of Phosphate Fertilizers in Sustainable Agriculture and its Effects on Phosphorus Transformations and Remediation of Contaminated Soils as well as the Growth of Plants. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-02018-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-02018-y