Abstract
Several chemical properties of alkaline soils play an important role in dissolving phosphate minerals, which greatly affect the phosphorous availability to plants. The current study was carried out to assess bone char application on the availability and distribution of phosphorus in some alkaline sandy soils. This incubation experiment was performed by selecting some alkaline soils from different locations in Upper Egypt: Arab El-Awamer (Assiut Governorate), West El-Minia (El-Minia governorate), and New Valley Governorate. Bone char was applied at a dose of 4 g kg−1 soil. The incubation periods lasted for 7, 16, 35, 65, and 84 days. Phosphorus availability in Arab El-Awamer soil increased significantly with applying bone char and was greatly influenced by soil chemical properties and incubation periods. Bone char addition caused a relative increase of available phosphorous in the sequence as follows: Arab El-Awamer soil ˃ New Valley soil ˃ West El-Minia soil. Available phosphorous showed a negative correlation with electrical conductivity, soluble calcium, and soluble sulfate. A significant increase of NH4Cl-Pi, NaHCO3-Pi, NaOH-Pi, HCl-Pi, and residual P fractions occurred in some soils with bone char application. Phosphorus fractions distribution in all soils followed: HCl-P ˃ residual P ˃ NaHCO3-P ˃ NaOH-P ˃ NH4Cl-P. The correlation between phosphorus availability and phosphorus fractions was positive. Our results focus on the importance of using bone char as an amendment in P-poor alkaline soils for improving phosphorus availability. So, bone char is an effective technique for sustainable agriculture because it is a clean and renewable resource of phosphate fertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The Russia–Ukraine war led to an unprecedented rise in the prices of all fertilizers, especially phosphate fertilizers (The Fertilizer Institute 2022). Moreover, rising global fertilizer prices have exacerbated concerns about food security in Africa (Gitau 2022). So, it is necessary to find alternatives for these fertilizers. To face the steadily increasing global demand for food, finding renewable resources to substitute chemical phosphate fertilizer became an urgent need (Amin 2023; Cordell et al. 2009). The essential source of chemical phosphate fertilizer is rock phosphate, which is non-renewable and will probably deplete in 50–100 years (Cordell et al. 2009). Excessive application of chemical phosphate fertilizers, as a result of the increasing demand for food, increased pollution with toxic and radioactive elements in the soil and the environment, which is a cause of the deterioration of the ecosystem (Attallah et al. 2019; Khan et al. 2018).
Generally, natural resources must be managed sustainably while minimizing the use of non-renewable energy sources. These measures must be of economic, environmental, and social return (Sarkar et al. 2020). Recycling slaughterhouse waste, especially bone, and converting it into bone char, is one of the processes used to preserve soil fertility, particularly phosphorus. Bone char is considered to be a clean and renewable alternative to chemical phosphate fertilizers as well as helps to protect society and the environment by disposing of waste in a safe manner (Amin 2020; Glæsner et al. 2019). Bone char was a highly efficient phosphate fertilizer from dried bones, possibly attributable to mineralogical changes during the pyrolysis process (Glæsner et al. 2019). Furthermore, bone char contains negligible amounts of toxic elements as well as it is free of radionuclides and organic contaminants such as pharmaceuticals (Siebers and Leinweber 2013; Zimmer et al. 2019). The bone contains calcium phosphate classified as biogenic crystalline apatite which can be differentiated from geological apatite—exists in rock phosphates—by its small crystal size, high carbonate substitution, substantial OH deficiency, vacancies in the lattice, and the resultant increased solubility. All these factors cause high solubility of the biological apatite (Boskey 2007; Wopenka and Pasteris 2005). Several studies observed that the amount of available phosphorus in soils with bone char application was higher than the phosphate rock (Warren et al. 2009; Vassilev et al. 2013). Phosphorus release from bone char into the soil is affected greatly by the chemical properties of the soil (Amin 2021; Warren et al. 2009).
Phosphorus status in the soil is strongly affected by many important mechanisms which are dissolution, leaching, sorption, desorption, mineralization, immobilization, and precipitation (Zhu et al. 2018). It is worth noting that the phosphorous availability in alkaline soil is highly influenced by several factors such as soil texture (Jalali and Jalali 2016; Pizzeghello et al. 2011), calcium carbonate content (Leytem and Mikkelsen 2005), soil salinity (Bai et al. 2017), soil pH, soluble cations (sodium, calcium, and magnesium), soluble anions (chloride, nitrate, and sulfate), and total phosphorus (Li et al. 2019). One of the hypotheses of this study was the significant effect of the chemical properties of the soils on the release of phosphorus from bone char. Most previous studies of bone char were performed in acid soils. However, the current study presents important insights into the application of bone char on the availability and distribution of phosphorus in three agricultural alkaline sandy soils.
2 Materials and Methods
2.1 Soil Collection and Analyses
Three alkaline agricultural soils in Upper Egypt which are widely differing in chemical properties were collected from the surface layer (0–20 cm) of Arab El-Awamer at Assiut Governorate, West El-Minia at El-Minia gGvernorate, and New Valley Governorate. Soil samples were air-dried, ground to pass through a 2-mm sieve, and kept for incubation experiment. These soils are classified as Entisols: Typic Torripsamments (US Soil Taxonomy). The physicochemical properties of the soils under study are presented in Table 1. The bone char used in this experiment was produced at 650 °C for 2 h. Some chemical characteristics of the bone char are given in Table 2 cited by Amin (2020). One hundred grams were taken from the soil under study and put in an airtight plastic jar (330 ml) as well as 0.4 g of bone char was added to each jar and mixed thoroughly with the soil. All treatments were moistened until field capacity by distilled water, arranged in a completely randomized design with three replicates, and incubated at 23 °C in the dark for periods of 7, 16, 35, 65, and 84 days. The jars are opened now and then to maintain the aerobic conditions and moisture content of the soil at field capacity by weighing the jars and adding distilled water equivalent to the loss of the water. At the end of each incubation period, soil samples were taken, air-dried, and crushed to be used for chemical analysis. This experiment and all soil analyses were conducted in the Soil Chemistry Laboratory, Soils and Water Department, Faculty of Agriculture, Assiut University, Assiut, Egypt.
2.2 Soil Chemical Analysis
The soil pH was measured in distilled water suspension (1:1) via a glass electrode. Electrical conductivity (EC) was measured in the soil extracts at 1:2.5 (soil-to-water ratio) using an electrical conductivity meter. Soluble Ca and Mg in the soil extracts were determined by titration using Na2EDTA solution (disodium ethylene diamine tetra-acetic acid); soluble Na was measured by flame photometry method. Soluble bicarbonate (HCO3)+carbonate (CO3) was titrimetrically determined using HCl acid, soluble sulfate was estimated by the turbidimetry method using barium chloride (Baruah and Barthakur 1997), and soluble chloride was determined using silver nitrate solution (Jackson 1973). The available phosphorus (Olsen P) in soil samples was extracted by 0.5 M NaHCO3 at pH 8.5 according to Olsen et al. (1954). This method is preferred in alkaline soils because the bicarbonate solution leads to a decrease in the activity of calcium ions in the solution by precipitation of the calcium carbonate, which in turn causes an increase in the concentration of phosphate ions as a result of the dissolution of calcium phosphate (Sarkar and Haldar 2005). Sequential fractionation of inorganic phosphorus in soil samples has followed the procedure described by Hedley et al. (1982) and modified by Chen et al. (2000), where 2.50 g of the soil was placed in a centrifuge tube of 100 mL for the sequential fractionation. Then, the soil sample in each tube was extracted with 50 ml of 1 M NH4Cl which represents NH4Cl-Pi. The remained soil from the previous step was extracted with 50 ml of 0.5 M NaHCO3 at pH 8.5 which represents NaHCO3-Pi. Soil residue from the previous step was extracted by 0.1 M NaOH which represents NaOH-Pi. Soil remaining from the previous step was extracted by 1 M HCl which represents HCl-Pi; all four previous fractions were shaken for 16 h and then centrifuged for 7 min (4000 rpm). The soil residue from the last fraction was digested with concentrated H2SO4, HNO3, and HClO4; this extract represents the residual P. Phosphorus in the extracts was measured by colorimetric analysis using chlorostannous phosphomolybdic acid method in the sulfuric acid system (Jackson 1973).
2.3 Statistical Analysis
The data obtained from this experiment were analyzed using two-way ANOVA and conducted by the MSTAT-C program (version 2.10). The differences between the treatments were analyzed based on Tukey’s honestly significant difference test (Tukey’s HSD) using the MSTAT-C program. The simple linear correlation analyses were carried out using SPSS 16.0.
3 Results
3.1 Properties of Soils Before Incubation
The studied soils are characterized by sandy texture, and the sand content in all soils is higher than 90 %. Chemical properties are widely varied in the soils under study (Table 1). These soils were identified as alkaline; their pH was 8.33, 8.06, and 8.13 in Arab El-Awamer, West El-Minia, and New Valley, respectively (Table 1). The electrical conductivity (EC) values in the studied soils were 0.22 (Arab El-Awamer), 12.73 (West El-Minia), and 1.11 dS m−1 (New Valley). All soils had a very low content of organic matter, which represents 5.33, 1.65, and 1.16 g kg−1 for Arab El-Awamer, West El-Minia, and New Valley, respectively. Calcium carbonate content in soils was Arab El-Awamer (326.67 g kg−1) ˃ West El-Minia (123.56 g kg−1) ˃ New Valley (46.39 g kg−1). Soluble calcium concentration in the soil extracts was positively correlated with EC. The soluble calcium was ordered as follows: West El-Minia (61.88 mmol kg−1) ˃ New Valley (8.50 mmol kg−1) ˃ Arab El-Awamer (2.63 mmol kg−1). The results showed that the concentrations of soluble cations and anions in the studied soils were in the order West El-Minia ˃ New Valley ˃ Arab El-Awamer (Table 1).
3.2 Soil Chemical Properties After Incubation
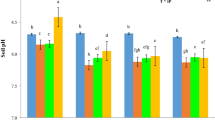
Overall, these soils differed in pH values significantly. The soil pH values significantly decreased with incubation periods increases in West El-Minia and New Valley soils, while the soil pH in Arab El-Awamer decreased non-significantly (Table 3). The soil pH in West El-Minia reduced from 8.06 (before incubation) to 7.97 (at day 84), and in New Valley, it declined from 8.13 before incubation to 7.99 at the end of the 84-day incubation. There are highly significant differences in the electrical conductivity, soluble cations, and soluble anions between these soils. Compared to the initial soil before the incubation, the soluble calcium in West El-Minia and New Valley soil decreased with increasing incubation periods. While the soluble calcium in Arab El-Awamer soil at (7, 15, and 35 days) decreased and increased at 65 and 84 days compared to the initial soil before the incubation (Table 3).
3.3 Phosphorus Availability
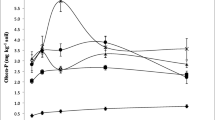
Bone char applications to the soils resulted in a significantly positive availability of phosphorus affected by the soil type and incubation periods in Arab El-Awamer soil. The application of bone char increased the phosphorus availability in all soils (Table 4). However, the increase in available phosphorus for West El-Minia and New Valley was non-significant compared with the initial soil before the start of the incubation experiment. The application of bone char to Arab El-Awamer soil increased the available phosphorus from 4.47 for unamended soil to 6.96 (representing 55.6 % increase), 8.11 (representing 81.5 % increase), 7.37 (representing 64.8 % increase), 7.54 (representing 68.8 % increase), and 7.57 mg kg−1 soil (representing 69.4 % increase) after 7, 16, 35, 65, and 85 days of incubation, respectively. The results revealed that the highest concentration of phosphorus availability was observed during the 16 days of the incubation. In West El-Minia soil, the application of bone char increased the available phosphorus from 4.96 for unamended soil to 5.54 (representing an 11.7 % increase), 4.99 (representing a 0.6 % increase), 5.24 (representing a 7.5 % increase), and 5.27 mg kg−1 soil (representing 6.3 % increase) after 16, 35, 65, and 85 days of incubation, respectively. No significant difference in phosphorus availability was observed after the 7 days of incubation in the soil treated by bone char. In the New Valley soil, bone char increased the concentrations of phosphorus availability from 4.24 for unamended soil to 4.96 (representing a 17.0 % increase), 4.71 (representing an 11.0 % increase), 4.41 (representing a 4.1 % increase), 4.61 (representing 8.7 % increase), and 4.73 mg kg−1 soil (representing 11.6 % increase) after 7, 16, 35, 65, and 85 days of incubation, respectively. The highest concentration of phosphorus availability in New Valley soil was observed at the 7 days of the incubation. The percentage of increase in available phosphorous after adding bone char compared to before incubation was as follows: Arab El-Awamer soil ˃New Valley soil ˃West El-Minia soil (Table 4).
3.4 Inorganic Phosphorus Fractions
The concentrations of NH4Cl-Pi fraction varied significantly in all soil types. The application of bone char resulted in significant increases in NH4Cl-Pi fraction in Arab El-Awamer and New Valley soils compared to the initial soil before the incubation (unamended soil). The bone char application caused significant decreases in NH4Cl-Pi fraction in West El-Minia soil compared to the initial soil before the incubation (unamended soil). In Arab El-Awamer soil, the bone char addition improved NH4Cl-Pi fraction from 6.10 mg kg−1 soil at initial soil before the incubation to 6.120, 6.70, and 6.91 mg kg−1 soil at 35, 65, and 84 days of incubation time, respectively. The amended New Valley soil with bone char enhanced NH4Cl-Pi fraction concentration from 3.530 mg kg−1 soil for initial soil before the incubation (unamended soil) to 4.75, 4.92, 4.81, 4.34, and 4.77 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. Bone char additions to West El-Minia soil caused an increase in the NH4Cl-Pi fraction from 4.59 mg kg−1 soil (unamended soil) to 4.10, 4.08, 3.90, 3.89, and 4.03 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively (Table 4).
The content of the NaHCO3-Pi fraction differed significantly in all soils under study. The NaHCO3-Pi fraction was significantly affected by the bone char applications in all studied soils compared to the initial soil before the incubation (unamended soil). In Arab El-Awamer soil, the application of bone char increased NaHCO3-Pi fraction from 15.25 mg kg−1 soil in the initial soil before the incubation to 28.35, 31.42, 28.26, 28.25, and 27.17 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The results indicated that the highest concentration of NaHCO3-Pi fraction was observed at 16 days of incubation and then tended to reduce with the incubation period. In West El-Minia soil, the amended soil with bone char leads to increasing NaHCO3-Pi fraction from 11.55 mg kg−1 soil at initial soil before the incubation to 25.51, 23.91, 24.91, 25.54, and 24.91 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The effect of incubation periods on NaHCO3-Pi fraction was non-significant. In New Valley soil, bone char addition increased NaHCO3-Pi fraction from 9.12 mg kg−1 soil for the soil without bone char to 26.27, 26.41, 26.69, 27.89, and 25.89 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The effect of incubation periods on NaHCO3-Pi fraction was non-significant (Table 4).
The application of bone char to Arab El-Awamer soil caused significant increases in the concentration of NaOH-Pi fraction. Applying bone char to Arab El-Awamer soil increased NaOH-Pi fraction from 9.13 mg kg−1 soil (unamended soil) to 9.77, 9.26, 12.10, 9.34, and 9.84 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The highest content of NaOH-Pi fraction was found at day 35; after that, it tended to decline with incubation time. Adding bone char to West El-Minia and New Valley soils had no significant effect on the content of the NaOH-Pi fraction (Table 4).
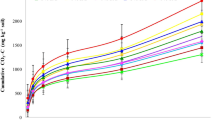
The three soils differ significantly in the HCl-Pi fraction. The application of bone char in all types of soils led to a highly significant increase in HCl-Pi fraction compared to the initial soil before the incubation (unamended soil). The content of the HCl-Pi fraction was higher in Arab El-Awamer soil than in New Valley and West El-Minia soils. Incubation periods did not significantly affect the HCl-Pi fraction in all soils. Application of bone char to Arab El-Awamer soil increased HCl-Pi fraction from 237.4 mg kg−1 soil for the initial soil before the incubation (unamended soil) to 752.3, 745.3, 754.0, 760.5, and 754.5 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The HCl-Pi fraction in West El-Minia soil increased with applying bone char from 58.53 mg kg−1 soil for unamended soil before incubation to 584.8, 583.4, 584.8, 582.3, and 580.2 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The addition of bone char to the New Valley soil increased HCl-Pi fraction concentration from 163.1 mg kg−1 soil (unamended soil) to 660.2, 666.7, 662.6, 668.5, and 663.7 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The soils under study were characterized by the majority of P that mainly exists as the HCl-Pi fraction (Table 4).
The residual P varied greatly between all soil types. Bone char additions to soils under study increased significantly the residual P compared to unamended soil. The concentrations of residual P in Arab El-Awamer soil increased from 33.74 mg kg−1 soil at the initial soil before the incubation (unamended soil) to 73.16, 75.16, 61.08, 62.23, and 68.21 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The concentrations of residual P in West El-Minia soil increased with bone char applications from 12.15 mg kg−1 soil (initial soil before the incubation or unamended soil) to 39.62, 37.59, 38.41, 37.84, and 37.07 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively. The addition of bone char to the New Valley soil increased residual P concentrations from 30.95 mg kg−1 soil (unamended soil) to 77.42, 72.77, 75.06, 69.68, and 72.39 mg kg−1 soil at 7, 16, 35, 65, and 84 days of incubation time, respectively (Table 4). The relative distribution of phosphorus fractions followed the order of HCl-P ˃ residual P ˃ NaHCO3-P ˃ NaOH-P ˃ NH4Cl-P.
3.5 Correlations Between Olsen P and Phosphorus Fractions with Soil Properties
The correlation coefficients between Olsen P and phosphorus fractions with soil properties are outlined in Table 5. Olsen P was positively correlated with soil pH (r=0.663**). Olsen P was negatively correlated with EC (r=−0.288*), soluble Ca (r=−0.306*), and soluble sulfate (r=−0.413*). The concentrations of Olsen P in all soils under study were positively correlated with NH4Cl-Pi fraction (r=0.738**), NaHCO3-Pi fraction (r=0.520**), NaOH-Pi fraction (r=0.456**), HCl-Pi fraction (r=0.547**), and residual P (r=0.339*).
4 Discussion
Alkaline soils are characterized by a pH greater than 7, and they are greatly predominated by alkali (Na+ and K+) and alkaline-earth cations (Ca2+ and Mg2+) in high concentrations (Brady 1984; Tan 2011). Often, alkaline soils prevail in semi-arid and arid regions, as they represent more than 25% of the world’s land. These soils usually have well drainage and porosity as well as contain high concentrations of calcium carbonate (López-Bucio et al. 2000). Dissolved calcium is present in the soil solution in alkaline soils in high concentrations, which reduces the phosphorous solubility due to the formation of insoluble calcium phosphate compounds (López-Bucio et al. 2000; Robinson and Syers 1991). The physical and chemical properties of soils are directly affected by the mineralogy and texture of soils. The concentration of calcium in the soils depends on the pH, CEC, soil type, and mineral composition of the soil (Flis 2019; Haby et al. 1990). Phosphorus availability in the soil is highly affected by many factors which are soil mineralogy, soluble and exchangeable calcium, and not just soil pH (Cho 1991; Penn and Camberato 2019). The concentration of available phosphorus in many different soils amended with bone char was high compared to the rock phosphate (Warren et al. 2009; Vassilev et al. 2013). Many researchers found that bone char application to the soil enhanced the phosphorus released with incubation time, and then a state of equilibrium occurs between calcium and phosphorus in the soil solution, which leads to a decrease in the phosphorous release as well as the formation of insoluble calcium phosphate compounds (Amin 2021, 2023; Morshedizad et al. 2018). Releasing phosphorus from bone char in some soils highly relies on the ionic composition of the soil solution, organic matter content, and mineralogical composition of the soils (Biswas et al. 2021). Soluble calcium in soil solution is one of the most important factors that affect phosphorus availability in soils. Increasing the concentrations of soluble calcium and phosphorous in the soil solution leads to the formation of insoluble calcium phosphate compounds, which depend mainly on calcium sources in the soil such as soluble and exchangeable calcium as well as calcium-bearing minerals (Cho 1991; Penn and Camberato 2019). Furthermore, increased calcium activity and pH encourage the formation of insoluble calcium phosphate compounds (Pizzeghello et al. 2014). At the same pH, the formation of insoluble calcium phosphate is affected greatly by the calcium/phosphate molar ratios (Mekmene et al. 2009). Therefore, the dissolving of phosphorus from bone char in several soils is highly influenced by the concentrations of H+, Ca2+, and H2PO4− in the soil solution (Warren et al. 2009). Recently, using slow-release phosphate fertilizers is one of the mechanisms to enhance phosphorous availability for plants which is more favorable than using soluble phosphate fertilizers in alkaline soils because of increasing phosphorus in soil solution resulting in forming insoluble calcium phosphate (Hopkins and Ellsworth 2005). Consequently, bone char can be used as a slow-release fertilizer.
The fractions of phosphorus in soils are classified into four categories: labile P includes NH4Cl-P and NaHCO3-Pi; moderately labile P contains NaOH-Pi; moderately stable P is HCl-Pi; and stable P or non-labile is residual P (Amin 2018a; Zhang et al. 2004). The type and amount of phosphate fertilizers added to the soils greatly influence the phosphorous forms (Condron and Goh 1989; Pizzeghello et al. 2011). The concentrations of phosphorus fractions in some soils are highly controlled by moisture content, salinity, particle size distribution, exchangeable cations, and nutrient status in soils (Bai et al. 2020). The NH4Cl-P fraction is expressed as a soluble phosphorous and loosely bound into the exchange sites; this phosphorous is considered available for plant uptake (Manojlovic et al. 2007; Sharpley and Smith 1984; Zhang and Kovar 2000). The high concentrations of soluble phosphorus in soil solution at high soil pH values might be attributed to the soluble complexes (ion pairs or complex ions) such as CaHPO2° or CaPO4. But mostly, the soluble complexes of phosphorus in soil solution quickly transformed into orthophosphate ions (Pierzynski et al. 2005). The inorganic phosphorous fraction (NaHCO3-Pi) is adsorbed on the soil surfaces, labile, and available for plants (Cross and Schlesinger 1995; Tian et al. 2019). The NaHCO3-Pi fraction was positively correlated with added P (Condron and Goh 1989; He et al. 2006). NaOH-Pi represents the inorganic phosphorous fraction associated strongly with chemisorption into surfaces of Al and Fe oxides (Costa et al. 2016; Cross and Schlesinger 1995). Some studies found that the addition of phosphate fertilizers did not significantly affect the inorganic phosphorus extracted by NaOH (NaOH-Pi) (Amin and Mihoub 2021; He et al. 2006). The HCl-Pi represents inorganic P associated with Ca, which exists in apatite or octacalcium phosphate (Costa et al. 2016; Cross and Schlesinger 1995). The highest concentrations of P fractions are the insoluble P associated with calcium under bone char applications in soils (Amin and Mihoub 2021; Zimmer et al. 2018). The phosphorus availability for plants is closely related to the concentrations of various P fractions in soils especially the concentration of soluble phosphorus in the soil solution (Zhang et al. 2021). The available phosphorus was positively correlated with the HCl-P fraction (Kolahchi and Jalali 2012). Phosphorus availability is significantly related to inorganic phosphorus fractions except for the residual P (Perrott et al. 1989). High pH and calcium activity lead to an increase in the formation of insoluble calcium phosphate (Pizzeghello et al. 2014). Generally, numerous studies found that the higher pH and calcium content in the soils can interpret the higher concentration of HCl-P which expresses P associated with calcium (Amin 2018b; Weyers et al. 2016). The residual P fraction includes the forms of phosphorous that are chemically stable and recalcitrant (Costa et al. 2016; Cross and Schlesinger 1995). The application of phosphate fertilizer to calcareous sandy loam soil led to a significant increase in the residual P fraction compared to unfertilized soil (Ahmad et al. 2018). However, Amin and Mihoub (2021) found that the application of bone char to calcareous sandy soil resulted in a significant increase in the content of residual P fraction compared to unamended soil.
5 Conclusions
This study provides a future vision that is very promising for the use of bone char as a clean, cheap, and renewable alternative to phosphate chemical fertilizers, to face the increase in the prices of phosphate fertilizers resulting from the repercussions of the Russian–Ukrainian crisis. Therefore, the chemical properties of alkaline sandy soils are considered important factors that control the bone char effect on the availability and distribution of phosphorus. The amount of phosphorous dissolved from bone char increased in soil with low calcium content and electrical conductivity. Overall results show that the soluble calcium was a significant factor in P availability with incubation periods. Most phosphorus fractions increased with applying bone char. It can be concluded that the bone char amendment affected the availability and distribution of phosphorus in alkaline agricultural sandy soils. Nowadays, the use of bone char can be increased due to its availability and lower cost compared to chemical phosphate fertilizers.
References
Ahmad M, Ahmad M, El-Naggar AH, Usman ARA, Abduljabbar A, Vithanage M, Elfaki J, Al-Faraj A, Al-Wabel MI (2018) Aging effects of organic and inorganic fertilizers on phosphorus fractionation in a calcareous sandy loam soil. Pedosphere 28:873–883. https://doi.org/10.1016/S1002-0160(17)60363-1
Amin AA (2018a) Phosphorus dynamics and corn growth under applications of corn stalks biochar in a clay soil. Arab J Geosci 11:379. https://doi.org/10.1007/s12517-018-3719-8
Amin AA (2018b) Availability and transformations of phosphorus in calcareous sandy soil as affected by farmyard manure and elemental sulfur applications. Alexandria Sci Exchange J 39:98–111. https://doi.org/10.21608/ASEJAIQJSAE.2018.5795
Amin AA (2020) Sulfur, Na2-EDTA and their mixture effects on phosphorus release from cow bone char in P-poor sandy soil. Environ Technol Innov 17:100636. https://doi.org/10.1016/j.eti.2020.100636
Amin AA (2021) Enhancement of releasing phosphorus from bone char in calcareous sandy soil under applying different levels of water salinity. J Soil Sci Plant Nutr 21:476–486. https://doi.org/10.1007/s42729-020-00376-x
Amin AA (2023) Effects of pyrolysis temperatures on bone char characterization and its releasing phosphorus in sandy soil. Arch Agron Soil Sci 69:304–313. https://doi.org/10.1080/03650340.2021.1988940
Amin AA, Mihoub A (2021) Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus spp.) on release and distribution of phosphorus in high calcareous P-fixing soils. J Soil Sci Plant Nutr 21:2041–2047. https://doi.org/10.1007/s42729-021-00500-5
Attallah MF, Metwally SS, Moussa SI, Soliman MA (2019) Environmental impact assessment of phosphate fertilizers and phosphogypsum waste: elemental and radiological effects. Microchem J 146:789–797. https://doi.org/10.1016/j.microc.2019.02.001
Bai J, Ye X, Jia J, Zhang G, Zhao Q, Cui B, Liu X (2017) Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 188:677–688. https://doi.org/10.1016/j.chemosphere.2017.08.117
Bai J, Yu L, Ye X, Yu Z, Wang D, Guan Y, Cui B, Liu X (2020) Dynamics of phosphorus fractions in surface soils of different flooding wetlands before and after flow-sediment regulation in the Yellow River Estuary, China. J Hydrol 580:124256. https://doi.org/10.1016/j.jhydrol.2019.124256
Baruah TC, Barthakur HP (1997) A textbook of soil analysis. Vikas Publishing House PVT LTD, New Delhi, India
Biswas PP, Liang B, Turner-Walker G, Rathod J, Lee Y, Wang C, Chang C (2021) Systematic changes of bone hydroxyapatite along a charring temperature gradient: an integrative study with dissolution behavior. Sci Total Environ 766:142601. https://doi.org/10.1016/j.scitotenv.2020.142601
Boskey AL (2007) Mineralization of bones and teeth. Elements 3:385–391. https://doi.org/10.2113/GSELEMENTS.3.6.385
Brady NC (1984) The nature and properties of soils, 9th edn. Macmillan Publishing Company, New York
Chen CR, Condron LM, Davis MR, Sherlock RR (2000) Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 220:151–163. https://doi.org/10.1023/A:1004712401721
Cho CM (1991) Phosphate transport in calcium-saturated systems: I. Theory. Soil Sci Soc Am J 55:1275–1281. https://doi.org/10.2136/sssaj1991.03615995005500050013x
Condron LM, Goh KM (1989) Effects of long-term phosphatic fertilizer applications on amounts and forms of phosphorus in soils under irrigated pasture in New Zealand. Eur J Soil Sci 40:383–395. https://doi.org/10.1111/j.1365-2389.1989.tb01282.x
Cordell D, Drangert JF, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Costa MG, Gama-Rodrigues AC, Gonçalves JLM, Gama-Rodrigues EF, Sales MVS, Aleixo S (2016) Labile and non-labile fractions of phosphorus and its transformations in soil under Eucalyptus plantations, Brazil. Forests 7:1–15. https://doi.org/10.3390/f7010015
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64:197–214. https://doi.org/10.1016/0016-7061(94)00023-4
Flis S (2019) Calcium: Improved plant health and nutrition. Crops & Soils Magazine 52(4):28–30. https://doi.org/10.2134/cs2019.52.0401
Gitau M (2022) Surging fertilizer prices set to exacerbate African food crisis. Bloomberg: January 29. https://www.bloomberg.com/news/articles/2022-01-29/surging-fertilizer-prices-set-to-exacerbate-african-food-crisis.
Glæsner N, Hansen HCB, Hu Y, Bekiaris G, Bruun S (2019) Low crystalline apatite in bone char produced at low temperature ameliorates phosphorus-deficient soils. Chemosphere 223:723–730. https://doi.org/10.1016/j.chemosphere.2019.02.048
Haby VA, Russelle MP, Skogley EO (1990) Testing soils for potassium, calcium, and magnesium. In: Westerman RL (ed) Soil testing and plant analysis, 3rd edn. Soil Science Society of America, Inc. Madison, Wisconsin, pp 181–227. https://doi.org/10.2136/sssabookser3.3ed.c8
He Z, Griffin TS, Honeycutt CW (2006) Soil phosphorus dynamics in response to dairy manure and fertilizer applications. Soil Sci 171:598–609. https://doi.org/10.1097/01.ss.0000228039.65023.20
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Hopkins BG, Ellsworth JW (2005) Phosphorus availability with alkaline/ calcareous soil. Western Nutr Manag Conf 6:88–93
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India Private Limited, New Delhi
Jalali M, Jalali M (2016) Relation between various soil phosphorus extraction methods and sorption parameters in calcareous soils with different texture. Sci Total Environ 566:1080–1093. https://doi.org/10.1016/j.scitotenv.2016.05.133
Khan MN, Mobin M, Abbas ZK, Alamri SA (2018) Fertilizers and their contaminants in soils, surface and groundwater. In: DellaSala DA, Goldstein MI (eds) The encyclopedia of the Anthropocene. Elsevier, Oxford, pp 225–240. https://doi.org/10.1016/B978-0-12-809665-9.09888-8
Kolahchi Z, Jalali M (2012) Speciation of phosphorus in phosphorus-amended and leached calcareous soils using chemical fractionation. Pol J Environ Stud 21:395–400
Leytem AB, Mikkelsen RL (2005) The nature of phosphorus in calcareous soils. Better Crops 89:11–13 https://eprints.nwisrl.ars.usda.gov/id/eprint/14
Li F, Liu L, Liu J, Yang K (2019) Abiotic and biotic controls on dynamics of labile phosphorus fractions in calcareous soils under agricultural cultivation. Sci Total Environ 681:163–174. https://doi.org/10.1016/j.scitotenv.2019.05.091
López-Bucio J, Guevara-García A, Ramírez-Rodríguez V, Nieto MF, de la Fuente JM, Herrera-Estrella L (2000) Agriculture for marginal lands: transgenic plants towards the third millennium. Dev Plant Genet Breed 5:159–165. https://doi.org/10.1016/S0168-7972(00)80025-0
Manojlovic D, Todorovica M, Jovicic J, Krsmanovic VD, Pfendt PA, Golubovic R (2007) Preservation of water quality in accumulation Lake Rovni: the estimate of the emission of phosphorus from inundation area. Desalination 213:104–109. https://doi.org/10.1016/j.desal.2006.05.058
Mekmene O, Quillard S, Rouillon T, Bouler J, Piot M, Gaucheron F (2009) Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci Technol 89:301–316. https://doi.org/10.1051/dst/2009019
Morshedizad M, Panten K, Klysubun W, Leinweber P (2018) Bone char effects on soil: sequential fractionations and XANES spectroscopy. Soil 4:23–35. https://doi.org/10.5194/soil-4-23-2018
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular/United States Department of Agriculture (no. 939)
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9(120):1–18. https://doi.org/10.3390/agriculture9060120
Perrott KW, Maher FW, Thorrold BS (1989) Accumulation of phosphorus fractions in yellow-brown pumice soils with development. NZ J Agric Res 32:53–62. https://doi.org/10.1080/00288233.1989.10423477
Pierzynski GM, McDowell RW, Sims JT (2005) Chemistry, cycling, and potential movement of inorganic phosphorus in soils. In: Sims J, Sharpley A (eds) Phosphorus: Agriculture and the environment. ASA, CSSA, SSSA, Madison, pp 53–86. https://doi.org/10.2134/agronmonogr46.c3
Pizzeghello D, Berti A, Nardi S, Morari F (2011) Phosphorus forms and P sorption in three alkaline soils after long-term mineral and manure applications. Agric Ecosyst Environ 141:58–66. https://doi.org/10.1016/j.agee.2011.02.011
Pizzeghello D, Berti A, Nardi S, Morari F (2014) Phosphorus-related properties in the profiles of three Italian soils after long-term mineral and manure applications. Agric Ecosyst Environ 189:216–228. https://doi.org/10.1016/j.agee.2014.03.047
Robinson JS, Syers JK (1991) Effects of solution calcium concentration and calcium sink size on the dissolution of Gafsa phosphate rock in soils. Eur J Soil Sci 42:389–397. https://doi.org/10.1111/j.1365-2389.1991.tb00417.x
Sarkar D, Haldar A (2005) Physical and chemical methods of soil analysis. New Age International Publishers, India
Sarkar D, Kar SK, Chattopadhyay A, Shikha RA, Tripathi VK, Dubey PK, Abhilash PC (2020) Low input sustainable agriculture: a viable climate-smart option for boosting food production in a warming world. Ecol Indic 115:106412. https://doi.org/10.1016/j.ecolind.2020.106412
Sharpley AN, Smith SJ (1984) Fractionation of inorganic and organic phosphorus in virgin and cultivated soils. Soil Sci Soc Am J 49:127–130. https://doi.org/10.2136/sssaj1985.03615995004900010025x
Siebers N, Leinweber P (2013) Bone char-a clean and renewable fertilizer with cadmium immobilizing capability. J Environ Qual 42:405–411. https://doi.org/10.2134/jeq2012.0363
Tan KH (2011) The principles of soil chemistry, 4th edn. CRC Press, Boca Raton
The Fertilizer Institute (2022) Russia-Ukraine conflict will impact ‘already-tight’ global market for fertilizer. AgriBusiness Global. https://www.agribusinessglobal.com/plant-health/npk/tfi-russia-ukraine-conflict-will-impact-already-tight-global-market-for-fertilizer/.
Tian H, Chen X, Han H, Jing H, Liu X, Li Z (2019) Seasonal variations and thinning effects on soil phosphorus fractions in Larix principis-rupprechtii Mayr. plantations. Forests 10:172. https://doi.org/10.3390/f10020172
Vassilev N, Martos E, Mendes G, Martos V, Vassileva M (2013) Biochar of animal origin: a sustainable solution to the global problem of high-grade rock phosphate scarcity?. J Sci Food Agric 93:1799–1804. https://doi.org/10.1002/jsfa.6130
Warren GP, Robinson JS, Someus E (2009) Dissolution of phosphorus from animal bone char in 12 soils. Nutr Cycl Agroecosys 84:167–178. https://doi.org/10.1007/s10705-008-9235-6
Weyers E, Strawn DG, Peak D, Moore A, Baker L, Cade–Menun B (2016) Phosphorus speciation in calcareous soils following annual dairy manure amendments. Soil Sci Soc Am J 80:1531–1542. https://doi.org/10.2136/sssaj2016.09.0280
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143. https://doi.org/10.1016/j.msec.2005.01.008
Zhang H, Kovar JL (2000) Phosphorus fractionation. In: Pierzynski GM (ed) Methods of phosphorus analysis for soils, sediments, residuals, and waters, Southern cooperative series, Bulletin No 396 pp 50–59. http://www.soil.ncsu.edu/sera17/publications/sera17-2/pm_cover.htm
Zhang TQ, MacKenzie AF, Liang BC, Drury CF (2004) Soil test phosphorus and phosphorus fractions with long-term phosphorus addition and depletion. Soil Sci Soc Am J 68:519–528. https://doi.org/10.2136/sssaj2004.5190
Zhang Y, Dalal RC, Bhattacharyya R, Meyer G, Wang P, Menzies NW, Kopittke PM (2021) Effect of long-term no-tillage and nitrogen fertilization on phosphorus distribution in bulk soil and aggregates of a Vertisol. Soil Tillage Res 205:104760. https://doi.org/10.1016/j.still.2020.104760
Zhu J, Li M, Whelan M (2018) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537. https://doi.org/10.1016/j.scitotenv.2017.08.095
Zimmer D, Kruse J, Siebers N, Panten K, Oelschläger C, Warkentin M, Hu Y, Zuin L, Leinweber P (2018) Bone char vs. S-enriched bone char: multi–method characterization of bone chars and their transformation in soil. Sci Total Environ 643:145–156. https://doi.org/10.1016/j.scitotenv.2018.06.076
Zimmer D, Panten K, Frank M, Springer A, Leinweber P (2019) Sulfur-enriched bone char as alternative P fertilizer: spectroscopic, wet chemical, and yield response evaluation. Agriculture 9:1–22. https://doi.org/10.3390/agriculture9010021
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amin, A.EE.A.Z. Chemical Properties of Some Alkaline Sandy Soils and Their Effects on Phosphorus Dynamics with Bone Char Application as a Renewable Resource of Phosphate Fertilizer. J Soil Sci Plant Nutr 23, 1589–1598 (2023). https://doi.org/10.1007/s42729-023-01199-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01199-2