Abstract
This study investigates the effects of Spirulina platensis, Chlorella vulgaris, leaves powder of Salix alba, and ethylenediaminetetraacetic acid treatments on the biochemical and yield traits of Phaseolus plants grown under wastewater irrigation. In addition, to assess the uptake and accumulation of heavy metals into the edible plant part. Water samples were obtained from each irrigation source (fresh tap water and untreated wastewater collected from El-Rahawy drain, Giza, Egypt); the plants were treated with our treatments (3 g per kg soil) at the beginning of the experiment (mixed fully into the soil). The results observed that the irrigation of Phaseolus plants with wastewater markedly stimulated the free proline contents, total phenols, superoxide dismutase, catalase, peroxidase, polyphenol oxidase, lipid peroxidation, and abscisic acid throughout the two growth stages. Indole acetic acid, gibberellic acid, yield parameters, total soluble carbohydrate, and protein in seeds were significantly reduced. The concentrations of nickel (Ni), cadmium (Cd), lead (Pb), and cobalt (Co) in Phaseolus seeds were significantly increased beyond recommended limits set by international organizations. However, our treatments significantly reduced the contents of Ni, Cd, Pb, and Co in seeds; free proline; total phenols; superoxide dismutase; catalase; peroxidase; polyphenol oxidase; lipid peroxidation; and abscisic acid in Phaseolus plants. Moreover, indole acetic acid, gibberellic acid, all yield traits, and seed components were enhanced. This study concluded that Spirulina platensis and salix leaves powder being economically and environmentally friendly can be considered an efficient strategy to mitigate the harmful effects of wastewater on plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The common bean (Phaseolus vulgaris L.), family: Fabaceae, is the most essential international legume for direct human consumption and is a source of dietary fiber, calories, proteins, minerals, and vitamins for millions of people worldwide (Gad 2019). In Egypt, it is regarded as one of the most important leguminous vegetable crops, having a significant economic importance because of its use for local and international trading. It occupies the second grade in export among the legume crops (AbdEl-Baki et al. 2022).
The limitation of freshwater for agriculture irrigation is a global challenge, especially in countries located in the arid region like Egypt. Therefore, many farmers in Egypt had to irrigate their crops using wastewater (USAID 2010). In Egypt, Antar et al. (2012) stated that at least 240,500 feddans in North Delta are irrigated with wastewater from Al-Gharbiyyah Al-Raisi drain because of the shortage of freshwater. Abdelrazek (2019) showed that about 800,000 feddans are irrigated with wastewater from Bahr El-Baqar drain, which is located in the eastern part of the Nile Delta, Egypt.
Plants irrigated using heavy metals (HMs)-contaminated water can carry these metals from their roots and translocate them to various edible parts, where they can accumulate to toxic levels (Nzediegwu et al. 2019). The elevated level of HMs in the edible parts of food crops with continuous wastewater (WW) irrigation resulted in many studies, demonstrating that plants irrigated with WW are generally contaminated with HMs, which poses a serious human health concern (Ahmed and Slima 2018; Hussain et al. 2019). Chronic exposure to the heavy metals, e.g., Ni, Cd, Pb, and Co, can lead to various human disorders, such as kidney dysfunction, liver damage, and other serious human health problems (Al-Swadi et al. 2022).
Kanwal et al. (2020) studied the effect of wastewater irrigation on the yield traits of the wheat plants; they reported that all yielded parameters, e.g., number of grain/plant, grain weight/plant, 1000-grain weight, and harvest index were reduced by 90%, 88%, 44%, and 61%, respectively. Gupta et al. (2008) studied the effects of wastewater irrigation on the accumulation of heavy metals in some vegetables; they found that the concentrations of Pb, Zn, Cd, Cr, and Ni in all the examined vegetables (Lactuca sativa, Mentha viridis, Brassica oleracea, Apium graveolens, Spinacia oleracea, Coriandrum sativum, Carum petroselinum, Allium tuberosum, and Raphanus sativus) were beyond the safe limits. In Egypt, many investigators also studied in detail the effect of wastewater irrigation on different plant species. In this regard, Mussarat et al. (2021) studied the accumulation of heavy metals in the wheat plants irrigated with wastewater; they found that the wheat plants irrigated with wastewater contained high Zn, Mn, Pb, Ni, and Cd in their edible parts (grains) compared to those irrigated by ground water. Similar results were recorded by Elgharably and Mohamed (2016) in wheat, bean, and onion plants.
Removal of HMs from wastewater was conducted using various techniques. Multiple studies have focused on marine algae’s efficiency to chelate heavy metals from contaminated wastes (Ahluwalia and Goyal 2007). Both live and dead algae biomass can be used as adsorbents for heavy metals (Mehta and Gaur 2005), but the recent studies have demonstrated that dead algae may be more effective for metal chelation and more cost-effective than living cells because inactive algae require neither food nor essential elements for biological growth and dead biomass is not affected by the toxicity of metal ions (Pham et al. 2021).
The dried materials of plants were widely used for heavy metal chelation during wastewater treatments, e.g., lettuce leaves, peat and nut shells, coconut shells, rice husk, tea waste, peanut hulls, almond shells, peach stones, citrus peels, wheat bran, and many others (Shartooh et al. 2014; Renu et al. 2017). Salix (Salix alba) dried materials are rich in many bioactive and biostimulant compounds including salicylic acid. Salicylic acid (SA) was investigated by authors and proved to be effective chelating agents for HMs. Popova et al. (2012) showed that salicylic acid application was highly effective in heavy metal chelation, that way reducing the heavy metal bioavailability and toxicity on plants. In addition, as a novel endogenous plant hormone, salicylic acid is considered to have beneficial physiological effects on plants growing under HM stress (Sharma et al. 2022). The results of numerous researches showed that several SA application methods can alleviate the stress effects of many HM elements in varieties of crops (Sharma et al. 2020).
Synthetic chelating agents, such as ethylenediaminetetraacetic acid (EDTA), are the most effective chelating agents used for heavy metal removal because they have a strong chelating ability for different metals (Jean-Soro et al. 2012; Oh and Yoon 2014).
This study aimed to assess the effects of wastewater on some metabolic aspects and yield of common bean (Phaseolus vulgaris L.) plants. Moreover, the study was undertaken to investigate the potential of natural dry algae powder (Spirulina platensis and Chlorella vulgaris), salix plant powder, and EDTA treatments to alleviate the adverse effects of wastewater on metabolism and yield of the study plant. Additionally, a special attention would be paid to evaluating the uptake and accumulation of heavy metals in edible plant parts.
2 Materials and Methods
2.1 Materials
The common bean seeds (Phaseolus vulgaris L.), Giza 6, were obtained from Agricultural Research Center, Ministry of Agriculture, Giza, Egypt. Natural Spirulina platensis and Chlorella vulgaris in the form of algae powder were obtained from the National Research Center, Giza, Egypt. Fresh leaves of the salix plant (Salix alba) were acquired from Botanical Garden, Agriculture Research Center, Giza, Egypt. The leaves were air dried for a few days until a constant dry weight, and then crushed into a fine powder.
2.2 Water Sampling
Water samples were obtained from each irrigation source (fresh tap water and wastewater effluent). Wastewater samples were obtained from El-Rahawy drain (30°11′12.3″N latitude and 31°02′53.3″E longitude), Giza, Egypt, which receives all sewage from El-Giza governorate in addition to agricultural and domestic wastes of El-Rahway village without treatment. The wastewater samples were obtained in plastic bottles and used in irrigation.
3 Methods of Planting, Treatments, and Collection of Plant Samples
A pot experiment was conducted under the field conditions of the Botanical Garden, Botany and Microbiology department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt during the Nile (autumn) season (starting from September 1, 2020). The common bean seeds were surface sterilized by 3.5% sodium hypochlorite for 20 min and washed many times with distilled water. The seeds were planted in pots (pot height, 30 cm; top diameter, 30 cm; base diameter, 20 cm), containing 6.0 kg of clay soil (six seeds were sown in each pot, thinning was carried out after seedling advent, and four plants were kept per pot). The pots were divided into six groups (each group has five replicates), representing the following treatments:
-
I. fresh tap water (control).
-
II. wastewater (WW).
-
III; IV; V; and VI. each contains wastewater + (Spirulina platensis (Sp); Chlorella vulgaris (Ch); leaves of Salix alba (SA); and ethylenediaminetetraacetic acid (EDTA) 3 g kg−1 soil by mixing the materials fully into the soil individually.
The plants of each group were treated with the above-mentioned treatments (as chelate into soil) from the beginning of the experiment. The developed plants were irrigated by fresh tap water (control) and collected wastewater with 1000 mL per pot, every 4 days for 4 weeks. Then, the irrigation intervals were increased to be every 7 days until the end of the experiment (on November 30, 2020). Three fertilizations were done during the experimental period at 14, 28, and 35 days after planting. On the two first fertilizations, each pot awarded 240 mg dm−3 of MgNO3 and 250 mg dm−3 of KNO3. On the last fertilization, 100 mg dm−3 of urea was applied per pot. All pots received the same amount of fertilizers in the form of a nutrient solution.
The plant samples were collected for analysis when the plants were 30 (stage I) and 45 (stage II) days old from planting. At the end of the growth season (90 days), analysis of the yield from the different treatments and the control was conducted.
4 Analysis Technique
4.1 Water Analyses
Electrical conductivity (EC), total dissolved solids (TDS), and pH of fresh tap water and wastewater were measured using pH/electric conductivity meter (914 pH/Conductometer-Metrohm AG). Then, water samples were acidified directly with nitric acid (1-mL HNO3 L−1) for metal analysis (APHA 1999) and measured using a Perkin-Elmer 3100 Atomic Absorption Spectrophotometer. The phosphorus (P) was determined by the molybdenum blue method using a spectrophotometer (UNICO Vis Model 1200, USA) set at 700 nm, and the potassium (K) was determined using Flame Photometer, CORNING M410. All these procedures were according to Allen et al. (1974). Ammonia–nitrogen (NH3-N) was estimated calorimetrically by the nesslerization method described by (Golterman 1991) as following the test solution (20 mL) was mixed; while shaking, with 1 mL of sodium salicylate solution followed by 1-mL Nessler’s reagent then made up to 25 mL, the developed color was measured after 15–30 min at 420 nm. Chemical oxygen demand (COD) was determined by titrimetric analysis (Pittwell 1983), while biochemical oxygen demand (BOD) was estimated by the 5-day BOD method (Delzer and McKenzie 2003). The tolerable limits of some metal ions in drinking water were determined by WHO (2022) and Egypt-decree (2013), Table 1.
4.2 Soil Analyses
The collected soil samples were air-dried and digested using the acid digestion (HNO3/H2SO4/HClO4 (5:1:1, v/v/v) for 8 h at 80 °C) method adopted by (Wade et al. 1992). Digestion was continued until the solution became clear. The transparent digests were filtered using a 0.45-μm pore size cellulose nitrate membrane filter paper (Millipore) and diluted up to 50 mL with distilled water. Soluble cations, anions, and HMs contents were determined using a Perkin-Elmer 3100 Atomic Absorption Spectrophotometer. The total soluble nitrogen (N) was determined by the Kjeldahl method (Pirie 1992) and phosphorus (P) by the molybdenum blue method using a spectrophotometer (UNICO Vis Model 1200, USA) set at 660 nm in case of N and 700 nm in case of P. In addition, potassium (K) was determined using Flame Photometer (CORNING M410). All these procedures were according to Allen et al. (1974). Soil reaction (pH), (1 soil: 5 water), electrical conductivity (EC), (1 soil: 5 water), and total dissolved solids (TDS) were measured according to (Page 1982) using pH/electric conductivity meter (914 pH/Conductometer-Metrohm AG). The maximum tolerable limits of some metals ions in soils as established by WHO (2022) as presented also in Table 2.
4.3 Plant Analyses
4.3.1 Determination of Non-enzymatic Antioxidant Contents
Free Proline
Contents of free proline were estimated according to the method described by (Bates et al. 1973). In this method, 0.5-g dry plant material was homogenized in 10-mL (3%) sulfosalicylic acid. The homogenate was filtered using Whatman No. 2 filter paper. Two milliliters of the filtrate reacted with 2-mL acid ninhydrin (prepared by warming 1.25-g ninhydrin in 30-mL glacial acetic acid and 20 mL 6-M phosphoric acid, with agitation, until dissolved, then kept cool) and 2 mL of glacial acetic acid in a test tube for 1 h in a boiling water bath. The reaction terminated in an ice bath. The reaction mixture was extracted with 4-mL toluene, mixed vigorously by a test tube stirrer for 15–20 s. The chromophore containing toluene was aspirated from the aqueous phase, warmed to room temperature, and the absorbance read to 520 nm using UV-spectrophotometer (UNICO Vis Model 1200, USA). Proline content was expressed as mg g−1 dry mass (DM).
Total Phenol
Total phenolic compounds (mg 100 g−1 DM) were performed according to the method described by (Daniel and Martin 1972) as follows: 1-g dry-defeated ground leaves were extracted in 5- to 10-mL 80% ethanol for at least 24 h at 0 °C; the alcohol was clarified. The remained residue was reextracted with 5- to 10-mL 80% ethanol thrice. In the end, the clarified extract was completed to 50 mL using 80% ethanol. An aliquot of 0.5 mL of the previous extract and 0.5-mL Folin-Denis reagent were well mixed in a dry test tube; the tube was shaken thoroughly for 3 min. About 1.0-mL saturated Na2CO3 solution (35%) was added, mixed well; and 3-mL distilled water was also added. After 1 h, the development color was read at 725 nm by a UV-spectrophotometer (UNICO Vis Model 1200, USA) using 0.5-mL 80% ethanol and reagents only as a blank.
Assays of Enzymatic Antioxidant Activities
Extraction of enzymes was according to Mukherjee and Choudhuri (1983) as follows: 2-g plant terminal buds and the first and second young leaves were homogenized with 10-mL phosphate buffer pH 6.8 (0.1 M), then centrifuged at 2 °C for 20 min at 20,000 rpm in a refrigerated centrifuge. The clear supernatant (containing the enzymes) was taken as the source of the enzyme.
Superoxide Dismutase (SOD)
Superoxide dismutase activities were estimated using the method of Marklund and Marklund (1974), the solution (10 mL) consisting of 3.6-mL distilled water, 0.1-mL enzyme, 5.5-mL of a 50-mM phosphate buffer (pH 7.8), and 0.8 mL of 3-mM pyrogallol (dissolved in 10-mM HCl). The rate of pyrogallol reduction was measured at 325 nm with a UV-spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme that resulted in 50% inhibition of the auto-oxidation rate of pyrogallol at 25 °C.
Catalase (CAT)
The catalase activity was assayed by following the consumption of H2O2 at 240 nm with a UV-spectrophotometer for 60 s (Aebi 1983). The assay mixture contained a 100-mM potassium phosphate buffer (pH 7.0), 15-mM H2O2, and 50-mL sample extract in a 3-mL volume. One unit of enzyme activity was defined as the amount of enzyme that reduced 50% of the H2O2 in 60 s at 25 °C.
Peroxidase (POX)
Peroxidase activities were determined according to the method of Bergmeyer et al. (1974) as follows: The reaction mixture containing 5.8 mL of a 50-mM phosphate buffer at pH 7.0, 0.2-mL enzyme extract, and 2 mL of 20-mM H2O2 after addition of 2 mL of 20-mM pyrogallol; the rate of increase in absorbance as pyrogallol was determined spectrophotometrically using a UV-spectrophotomer within 60 s at 470 nm and 25 °C.
Polyphenol Oxidase (PPO)
Polyphenol oxidase activities were measured according to the methods of Kar and Mishra (1976). The substrate used composed 0.1-M catechol in a sodium acetate buffer (pH 5.0). The reaction occurred at 30 °C for 60 min, and the readings were done at 395 nm (Ultrospec 2000). Polyphenoloxidase activities were expressed as changes in the optical density min−1 g−1 fresh mass (FM).
Lipid Peroxidation Assay
Lipid peroxidation was assayed as malondialdehyde (MDA) content in fresh leaves according to that method described by Zhang et al. (2015), 2 mL of 5% trichloroacetic acid (w/v) was added to fresh leaf samples (0.2 g), which was then grounded, and the resulting homogenate was subsequently centrifuged at 10,000 rpm for 10 min at 4 °C. Afterward, 2 mL of 0.67% thiobarbituric acid (w/v) was added to 2 mL of the resultant supernatant, after which the mixture was kept in boiling water bath for 30 min, and then centrifuged at 10,000 rpm for 10 min at 4 °C. The absorbance of the supernatant was subsequently measured at 450 nm, 532 nm, and 600 nm. The MDA content was then calculated based on the following formula:
The lipid peroxidation levels were expressed as micromoles of MDA per gram of fresh mass (µmol g−1 FM).
5 Determination of Phytohormone Contents
The extraction method of endogenous acidic phytohormones was adopted by Shindy and Smith (1975) and described by Hashem (2016). To estimate the amounts of indole acetic acid (IAA), gibberellic acid (GA3), and abscisic acid (ABA), plant hormone fractions and standard ones were methylated according to Vogel (1975) to be ready for gas chromatography analysis. A flame ionization detector was used for the identification and determination of acidic hormones using Hewlett Packard Gas Chromatography (5890) fitted and equipped with an HP-130-m × 0.32-mm × 0.25-mm capillary column coated with methyl silicone. The column oven temperature was programmed at 10 °C min−1 from 200 (5 min) to 260 °C and kept finally to 10 min. Injector and detector temperatures were 260 and 300 °C, respectively. Gas flow rates were 30, 30, and 300 cm s−1 for N2, H2, and air, respectively, and the flow rate inside the column was adjusted to 2 mL min−1.
5.1 Determination of Total Soluble Carbohydrate and Protein
Total soluble carbohydrates were estimated and measured UV-spectrophotometrically (UNICO Vis Model 1200, USA) by using anthrone-sulfuric acid according to the method of Umbriet et al. (1959). Contents of total soluble proteins were determined by using Bio-Rad protein assay according to the methods of Lowery et al. (1951) and measured using a UV-spectrophotometer (UNICO Vis Model 1200, USA).
5.2 Determination of Plant Heavy Metal Contents
Heavy metal contents in various samples of plants under study (edible plant parts) were determined according to Parkinson and Allen (1975). The plant samples (a 1-g dry sample) were placed in long digestion tubes followed by the addition of 7.5 mL of concentrated H2SO4. The mixture was allowed to stand for 30 min at room temperature. Approximately 7.5-mL H2O2 (30%) was added to the digestion tubes; the samples were heated on a hot plate for 40 min at 360 °C. Thereafter, 1-mL H2O2 (30%) was added until the digest appeared clear upon cooling. Then, deionized distilled water was added to bring the final sample volume to 50 mL. The solution was filtered through Whatman number 42 filter paper and then determined using a Perkin-Elmer 3100 Atomic Absorption Spectrophotometer.
5.3 Enrichment Factor
The enrichment factor (Barman et al. 2000) has been calculated to determine the degree of heavy metal accumulation in the plants concerning the control according to the equation:
5.4 Statistical Analysis
Statistical calculations were performed using computer programs Microsoft Excel version 365 and the SPSS v.25 (statistical package for the social science version 25.00) statistical program. The differences in the studied variables in the water and soil samples were tested using Student’s t-test. Quantitative data with parametric distribution between the different plant samples were done using analysis of variance for the one-way ANOVA and post hoc Tukey’s test at a 0.05 probability level. The difference between stages I and II values of parameters was also analyzed by Student’s t-test. The artwork and figures were performed using computer programs Microsoft Excel version 365 and the GraphPad Prism program (version 8).
6 Results
6.1 Non-enzymatic Antioxidant Content
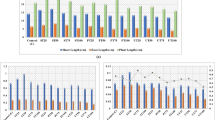
These results (Fig. 1a) indicated that free proline contents were significantly stimulated at stage I and stage II due to irrigation with untreated wastewater compared to control by approximately 179% and 142%, respectively. However, treatments with Spirulina platensis, Chlorella vulgaris, leaves of Salix alba, and EDTA alleviated wastewater-generated toxicity, and the maximum decrease in free proline in stage I was observed by about 64% at WW + Spirulina platensis treatment (except control). In comparison, the maximum decrease in free proline at stage II was observed by about 47% at WW + Chlorella vulgaris treatment (except control) compared to untreated wastewater-irrigated plants (WW).
Effect of different treatments on free proline (a) and total phenols (b) of Phaseolus vulgaris plants. Error bars are means ± standard error (n = 5). Different letters on bars denote that mean values are significant differences (P ≤ 0.05) by Tukey’s test (HSD). * Difference between stages I and II values of parameters is statistically significant (Student’s t-test, p ≤ 0.5). Control is fresh tap water, WW is wastewater, WW + Sp is wastewater + Spirulina platensis, WW + Ch is wastewater + Chlorella vulgaris, WW + SA is wastewater + salix plant powder, and WW + EDTA is wastewater + ethylenediaminetetraacetic acid
Our data in Fig. 1b also indicated that the total phenol content increased in Phaseolus plants irrigated with untreated wastewater throughout the two growth stages. Values were about 107% at stage I and 105% at stage II greater than the control. Conversely, the total phenol contents were considerably decreased by about 44% at stage I and about 49% at stage II when treatment with WW + Chlorella vulgaris (except control) was compared with the plants irrigated with wastewater without any treatment.
6.2 Antioxidant Enzyme Activities
The activity of antioxidant enzymes increased in response to wastewater irrigation throughout the two growth stages (Fig. 2a, b, c, and d). In case of stage I, the rise in the activity of superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and polyphenol oxidase (PPO) enzymes was 367%, 475%, 189%, and 300%, respectively, under the untreated wastewater irrigation compared with control. Likewise, in stage II, the rise in the activity of SOD, CAT, POX, and PPO enzymes was 315%, 222%, 230%, and 242%, respectively, compared with control.
Effect of different treatments on superoxide dismutase (SOD) (a), catalase (CAT) (b), peroxidase (POX), (c) and polyphenol oxidase (PPO) (d) enzymes of Phaseolus vulgaris plants. Error bars are means ± standard error (n = 5). Different letters on bars donate that mean values are significant differences (P ≤ 0.05) by Tukey’s test (HSD). * Difference between stages I and II values of parameters is statistically significant (Student’s t-test, p ≤ 0.5). Control is fresh tap water, WW is wastewater, WW + Sp is wastewater + Spirulina platensis, WW + Ch is wastewater + Chlorella vulgaris, WW + SA is wastewater + salix plant powder, and WW + EDTA is wastewater + ethylenediaminetetraacetic acid
In contrast, the treatment of Phaseolus plants with Spirulina platensis, Chlorella vulgaris, salix plant powder, and EDTA decreased the highest activities of SOD, CAT, POX, and PPO enzymes generated by wastewater toxic stress (Fig. 2a, b, c, and d). In cases of SOD and CAT enzymes, the maximum reduction in activities was recorded in Chlorella vulgaris treatment by about 69% and 78%, respectively, at stage I and by about 66% (in SOD and CAT) at stage II compared with untreated wastewater-irrigated plants. Likewise, in cases of POX enzyme, the maximum reduction in activity was recorded in SA treatments by about 62% at stage I and 64% at stage II as compared with untreated wastewater-irrigated plants. Similarly, in cases of PPO enzyme, the maximum reduction in activity was recorded in Spirulina platensis (stage I) and SA (stage II) treatments by about 59% and 49%, respectively, compared with the untreated wastewater-irrigated plants.
6.3 Lipid Peroxidation Contents
Our results in Fig. 3 indicated that the malondialdehyde (MDA) content significantly increased in the Phaseolus plants irrigated with untreated wastewater by about 163% at stage I and 152% at stage II greater than the control. Conversely, except control, the maximum reduction in MDA contents was recorded in Chlorella vulgaris treatment by about 51% at stage I and by about 54% at stage II in SA treatment as compared with untreated wastewater-irrigated plants.
Effect of different treatments on malondialdehyde (MDA) contents in leaves of Phaseolus vulgaris plants. Error bars are means ± standard error (n = 3). Different letters on bars donate that mean values are significant differences (P ≤ 0.05) by Tukey’s test (HSD). * Difference between stages I and II values of parameters is statistically significant (Student’s t-test, p ≤ 0.5). Control is fresh tap water, WW is wastewater, WW + Sp is wastewater + Spirulina platensis, WW + Ch is wastewater + Chlorella vulgaris, WW + SA is wastewater + salix plant powder, and WW + EDTA is wastewater + ethylenediaminetetraacetic acid
6.4 Phytohormone Contents
Different values in this study in Fig. 4a, b, and c were recorded regarding the acidic phytohormone contents being studied of common bean plants at stages I and II. Irrigation with untreated WW caused reduction in the contents of indole acetic acid (IAA) and gibberellic acid (GA3) by about 59% and 46% at stage I and 51% and 37% at stage II, respectively, compared to control plants, while the contents of abscisic acid (ABA) were significantly increased by about 115% and 114% at stages I and II, respectively, as compared with control plants.
Effect of different treatments on indole acetic acid (IAA) (a), gibberellic acid (GA3) (b), and abscisic acid (ABA) (c) contents of Phaseolus vulgaris plants. Error bars are means ± standard error (n = 5). Different letters on bars donate that mean values are significant differences (P ≤ 0.05) by Tukey’s test (HSD). * Difference between stages I and II values of parameters is statistically significant (Student’s t-test, p ≤ 0.5). Control is fresh tap water, WW is wastewater, WW + Sp is wastewater + Spirulina platensis, WW + Ch is wastewater + Chlorella vulgaris, WW + SA is wastewater + salix plant powder, and WW + EDTA is wastewater + ethylenediaminetetraacetic acid
Alternatively, a positive effect on all acidic phytohormone contents being studied was observed except for ABA content when treated with Spirulina platensis, Chlorella vulgaris, salix plant powder, and EDTA compared to untreated WW-irrigated plants. The highest value of IAA contents was recorded in the Chlorella vulgaris treatment, which increased by about 135% and 171% at stages I and II, respectively; the highest value of GA3 contents was recorded in the Spirulina platensis treatment, which increased by about 73% and 120% at stages I and II, respectively, compared to untreated WW-irrigated plants, while the lowest values of ABA were recorded when the application of Chlorella vulgaris, which decreased by about 53% at stage I and 54% at stage II, was compared to untreated WW-irrigated plants.
6.5 Yield Characters
Data in Table 3 and Fig. S1 indicated that, compared to control samples, all tested yield parameters were decreased significantly under the effect of WW irrigation. Where the lowest values of the number of pods plant−1, number of seed plant−1, the weight of seeds in gram (g) plant−1, and weight of 100 seeds (g) were recorded when irrigated with wastewater, which reduced by approximately 33%, 13%, 14%, and 19% of the control, respectively.
In contrast, a positive effect on all yield parameters was observed (in most cases) when treated with Spirulina platensis, Chlorella vulgaris, salix plant powder, and EDTA compared to control (Table 3 and Fig. S1). The highest value of the number of pod plant−1 was recorded when treating plants with Chlorella vulgaris, which increased by about 22% of control.
The same trend was observed in a number of seed plant−1 and weight of seed (g) plant−1 (Table 3 and Fig. S1) where the highest values were recorded at Spirulina platensis–treated plants, which increased by about 33% and 27% of control, respectively. Also, the highest value in weight of 100 seeds was recorded in the SA-treated plant, which increased by 10% of control.
6.6 Yield Components
Results in Fig. 5 showed that the total soluble carbohydrates and proteins in seeds of untreated WW-irrigated Phaseolus plants were reduced relative to control plants by about 34% and 13%, respectively. In contrast, the highest value of carbohydrate content in seeds was recorded at SA treatments, which increased by about 54% of control. Similarly, the highest value of protein contents in seeds was recorded at Chlorella vulgaris treatments, which increased by about 17% of control.
Effect of different treatments on total soluble carbohydrate and protein contents of Phaseolus vulgaris seeds. Error bars are means ± standard error (n = 5). Different letters on bars donate that mean values are significant differences (P ≤ 0.05) by Tukey’s test (HSD). Control is fresh tap water, WW is wastewater, WW + Sp is wastewater + Spirulina platensis, WW + Ch is wastewater + Chlorella vulgaris, WW + SA is wastewater + salix plant powder, and WW + EDTA is wastewater + ethylenediaminetetraacetic acid
6.7 Heavy Metal Accumulation in Seeds
The heavy metal contents in Phaseolus seeds (edible part) are indicated in Table 4. The accumulation of Ni, Cd, Pb, and Co is greater in the case of wastewater-irrigated plants than controls. The lowest accumulation of Ni and Co was detected under WW + Chlorella vulgaris treatment (except control). The same trend—the lowest accumulation of Cd and Pb was detected (except control) in treated plants with WW + Sp and WW + SA, respectively.
6.8 The Enrichment Factor
The enrichment of heavy metals in plants irrigated with untreated wastewater is as follows: Cd (251) > Co (164) > Pb (19.5) > Ni (18). Whereas, in the case of WW + Sp treatment, the enrichment of the heavy metals in the plant is as follows: Cd (24) > Pb (9.58) > Ni (4) > Co (3). In the case of WW + Ch treatment, the enrichment of the heavy metals in the plant is as follows: Cd (29) > Pb (9.42) > Ni (3.75) > Co (1.50).
In the case of WW + SA treatment, the enrichment of the heavy metals in the plant is as follows: Cd (27) > Pb (9.25) > Ni (6.25) > Co (2), and, in the case of WW + EDTA treatment, the enrichment of the heavy metals in the plant is as follows: Cd (30) > Pb (9.75) > Ni (5.75) > Co (4).
7 Discussion
Free proline and polyphenols, non-enzymatic antioxidants, support organisms under unfavorable conditions in alleviating the adverse effects of reactive oxygen species (ROS) (Zandi and Schnug 2022). Environmental stresses like heavy metal (HMs) stress have been documented to increase ROS production. Antioxidant molecules and enzymes attain ROS detoxification in plant cells. Antioxidant compounds, including proline and phenols, inhibit oxidation and play vital roles in stress responses (Racchi 2013), which far increased after irrigation of plants with untreated wastewater throughout stage I and stage II, corroborating the finding of Khalilzadeh et al. (2020). Higher proline and phenol levels may be attributed to the strategies adopted by plants to overcome the heavy metal toxicity as proline has many essential functions: osmoticum, scavenger of free radicals, protector role of cytoplasmic enzymes, stabilizer of membranes, and a dip for energy to regulate the redox potential (Hayat et al. 2012). Recently, Pratyusha (2022) reported that the contents of phenolic compounds tend to increase when the plants were under heavy metal stress, as phenolic compounds are reactive oxygen species scavengers and metal chelators. In this regard, Kısa et al. (2016) showed that the application of heavy metal generally increased the total phenolic compounds of the corn plant.
In contrast, the contents of free proline and total phenols significantly decreased in response to our treatments compared to untreated wastewater-irrigated plants throughout the two growth stages. In the case of free proline, the most significant reduction was recorded when the plants were treated with Spirulina platensis in stage I and Chlorella vulgaris in stage II. These results indicate the role of these treatments in alleviating the toxic effects of wastewater on plants. Our results are in harmony with previous studies of Romera et al. (2006); Al-Homaidan et al. (2015) reported that treatment of wastewater or soils with Spirulina sp. and Chlorella sp. significantly reduced the accumulation of toxic metals. In the case of total phenols, the application of WW + Chlorella vulgaris indicated the most significant decrease in stages I and II. The reduction in the contents of total phenols may be attributed to oxidative stress was confronted by Chlorella vulgaris treatment, and further increase in the contents of phenolic compounds (as a non-enzymatic antioxidant) was not needed. These results are in harmony with the recent study of Khodamoradi et al. (2022).
Plant antioxidant enzymes (e.g., SOD, CAT, POX, and PPO) act as the first line of defense to tolerate unfavorable conditions. These enzymes stimulate the detoxification of ROS and decrease the harmful effects caused by abiotic stress (Rai et al. 2022). Enrichments of SOD, CAT, POX, and PPO activities under heavy metal stress were outlined by their roles in constructing a physical barrier against HMs entering the cells (Hegedüs et al. 2001) and in scavenging H2O2 (Yan et al. 2008). Consequently, SOD, CAT, POX, and PPO act as markers of antioxidant activity toward metal toxicity. Therefore, in this study and in others (Kang et al. (2013)), plant irrigation with untreated wastewater stimulated the activities of antioxidant enzymes in stages I and II compared with control. Increased SOD, CAT, POX, and PPO activities with higher proline and phenol content accelerate the remediation of toxic H2O2 to non-toxic H2O, thus reducing oxidative stress. Recently, Nowwar et al. (2022) found that the activities of SOD, CAT, POX, and PPO enzymes were found to be higher in Jute Mallow plants irrigated by wastewater than those irrigated with fresh water.
Alternatively, the application of WW + Sp, WW + Ch, WW + SA, and WW + EDTA alleviated the adverse effects of WW on Phaseolus plant enzymes throughout the various growth stages. This reduction in SOD, CAT, POX, and PPO activities indicated the role of these treatments in alleviating the WW-induced oxidative stress as this fact is supported by the reduction in HMs stress and the ROS level, corroborating the findings of Noriega et al. (2012), which reported that SA decreases the effects of oxidative stress caused by heavy metals by inhibiting the accumulation of these metals in plant cells. Similarly, the use of SA to decrease the harmful effects of some HMs is reported in different plant species (Lu et al. 2018). Recently, Dinesh-Kumar et al. (2020) have indicated that the application of Spirulina platensis can effectively chelate several heavy metals from wastes and mitigate the toxic effects of these metals.
The peroxidation of lipid in biological membranes is considered credible indicators to oxidative stress in plants. When ROS levels exceed the capacity of the plant to scavenge, lipid peroxidation increases (Šoln and Koce 2022). The malondialdehyde is one of the final products of oxidative modification of lipids and is responsible for cell membrane damage including changes to the radical properties of the membrane; these changes finally result in cell death (Sharma et al. 2012). In this study, the MDA contents increased in response to wastewater irrigation; this may be due to the incapacity of oxidative system to reduce the ROS level and so not reducing the damage to the cell membrane. This result also was illustrated by Yildirim et al. (2019) that showed that irrigation with Pb- and Cd- contaminated water generally increased the contents of MDA in rocket plants. Contrariwise, the MDA contents were significantly decreased in response to Spirulina platensis, Chlorella vulgaris, Salix alba, and EDTA treatments as compared to WW-irrigated plants. This may be due to the reduction of ROS production and the extent of the antioxidant system and restoration. These results are supported by the recent study of (Malik et al. 2022).
The endogenous acidic phytohormones (IAA, GA3, and ABA) are found in plant cells at either low or high concentrations, regulating plant growth and acting as signaling regulators to alleviate abiotic stresses such as HM stress (Sytar et al. 2019). HMs have negative effects on IAA, and GA3 is reported to be related to an increase in ABA content in plant cells, indicating the potential of this hormone mediating a part of the metal-enforced phytotoxicity (Sharma and Kumar 2002). Plant exposure to HMs stress induces the expression of ABA biosynthetic genes, which improves ABA’s endogenous contents (Bücker-Neto et al. 2017). Also, the accumulation of phenols was related to a significant decreased IAA. Denaxa et al. (2022) reported that increased phenolic content might stimulate IAA-oxidase and thus decline IAA contents. Our results clearly indicated that the irrigation of the common bean with untreated WW significantly reduced the contents of IAA and GA3, but increased ABA content. These results were illustrated by Atici et al. (2005) in chickpea and Kim et al. (2014) in rice plants. Nowwar et al. (2022) showed that irrigation of Jute mallow plants with heavy metals–contaminated water caused reduction in the contents of GA3, IAA, but ABA contents were increased. In contrast, the application of the different tested treatments showed marked increases in the contents of IAA and GA3 but decreased in the ABA’s contents. These results show the role of these treatments in alleviating the negative effect of WW on common bean. This may be due to the role of IAA and GA3 to regulate plant responses to wastewater stresses by modulating their biosynthesis, transport, signaling, conjugation, and degradation processes (Saini et al. 2021); also, via reducing the effects of plant growth inhibitors by reducing the contents of abscisic acid as inhibitory phytohormones (Emamverdian et al. 2020). Our result agreement with the recent study of Torun et al. (2022) recorded that the treatments of Hordeum vulgare L. with SA resulted in a significant increase in the auxin (IAA) levels and reduction in ABA contents under abiotic stress.
All yield characters of Phaseolus plants decreased upon irrigation with untreated wastewater, which agrees with the recent findings of Han-jie et al. (2022). The reduction in yield parameters in response to wastewater irrigation could be due to a high level of toxic compounds in wastewater (Kanwal et al. 2020), higher accumulation of heavy metals in plants (Bhat et al. 2019), high wastewater ammonia-N level (Liu and Wirén 2017), and high salinity of wastewater (Ungureanu et al. 2020). Recently, Nowwar et al. (2022) have reported that the growth and yield (number of pods and seeds per plant, weight of seeds per plant, and weight of 1000 seeds) parameters of Jute mallow plants significantly decreased when irrigated with untreated wastewater as compared to that irrigated by fresh water as a control.
Alternatively, all yield characters were enhanced in response to applying the different tested treatments. The most significant increase in yield parameters was recorded in plants treated with WW + Sp, WW + Ch, and WW + SA. Similar observations were indicated by Ammar et al. (2022) that found that the application of algae treatments (e.g., Spirulina platensis, Chlorella vulgaris, Nostoc sp., Scenedesmus sp., Chlorococcum sp., Chroococcus sp., Phormidium sp., Anabaena sp., Fischerella sp., and Spirogyra sp.) for some plants (wheat, pearl millet, pea, maize, and rice) increased the economic yield, the average increase in seeds and grain yield, and quality parameters. Gonçalves (2021) stated that dry micro and macro algae contain high amount of macro and micronutrients, polysaccharides, hormone-like substances, amino acids, and other bio-stimulants, which improved plant growth and yield production. Safi et al. (2014) concluded that a quantity of 2- and 3-g dry Chlorella vulgaris algae per kg soil (as soil additives) increased yield quality and productivity and alleviated the environmental pollution. Gitau et al. (2022) indicated that treatment of Solanum lycopersicum plants with algae extract (Chlorella vulgaris) significantly increased yield productivity and its components and quality. Elansary et al. (2016) stated that the application of algae extracts increased the yield in cereals and vegetables. In the same way, the biostimulant effects of salix dried materials on yield may be related to the rich bioactive and biostimulant substance contents in salix, such as salicylates and phenolic compounds, which are considered to have beneficial physiological effects on plant growth (Mutlu-Durak and Kutman 2021). SA as a phytohormone also has promoting effects on bud formation and flowering regulation (Vicente and Plasencia 2011). Recently, Hasanuzzaman et al. (2019) reported that the application of SA improved the yield of Brassica campestris plants and resulted in the lowering of oxidative damage under heavy metal stress.
The total soluble carbohydrates and proteins in seeds were significantly decreased in response to irrigation with untreated wastewater compared to control. These results are in accordance with the results obtained by other investigators (Farahat et al. (2017); Hajihashemi et al. (2020). The reduction in the total soluble carbohydrate and protein content after irrigation with untreated WW probably corresponded with heavy metal stress (Banadka and Nagella 2022), possible interaction of heavy metals with the reactive center of ribulosebisphosphate carboxylase, inhibited the photosynthetic production or stimulated the respiration rate (John et al. 2008), the cycles of carbohydrate catabolism and related enzymatic reactions (Rabie et al. 1992), lowered synthesis or diversion of the metabolites to other synthesis processes (Aldoobie and Beltagi 2013), enhanced the degradation process (Palma et al. 2002), different structural and functional modifications by the denaturation and fragmentation (Monteiro et al. 2009), modification in gene expression (Kovalchuk et al. 2005), or due to the interaction with thiol residues of proteins and replacement of them with heavy metals in metallo-proteins (Pál et al. 2006). But, alternatively, the total soluble carbohydrates and proteins in seeds were enhanced in response to the application of the different tested treatments. The most significant increases in total soluble carbohydrates and proteins in yield were recorded due to the application of salix plant powder and Chlorella vulgaris, respectively. This study’s results agree with those of Luo et al. (2014). Duan et al. (2022) suggested that the application of SA in stressed plants could induce their tolerance by accelerating their carbohydrate metabolism. El-Tayeb et al. (2006) reported that SA treatments increased the level of soluble carbohydrates, which provided some HMs tolerance in Helianthus annuus. Wasti et al. (2012) concluded that the application of SA enhanced the accumulation of soluble carbohydrates in tomato plants and offered stress avoidance. In the same way, the effect of algae extracts on increasing seed carbohydrates and protein contents was reported by Shedeed et al. (2022) on Lupinus luteus plants.
The application of untreated wastewater in irrigation generally led to a significantly high accumulation of all studded heavy metals in wastewater-irrigated common bean seeds (edible part) than freshwater-irrigated plants. Similar results were also obtained by Gupta et al. (2010). Also, Parashar and Prasad (2013) reported that the use of wastewater for irrigation increased the accumulation of metals Fe, Cd, Cu, Zn, and Pb in the edible parts of the studded plants (Spinach, Cabbage, Beetroot, Reddish, Okra, Tomato, and Cucumber). Recently, Tariq (2021) has found that the irrigation with wastewater caused a significant increases in the contents of Ni, Cd, Cr, Cu, Pb, and Zn in the edible parts of chard, celery, cress, and leek plants.
Alternatively, the application of WW + Spirulina platensis, WW + Chlorella vulgaris, and WW + salix plant powder showed the lowest accumulation of Ni, Cd, Pb, and Co in common bean seeds. Our results are in harmony with the recent study by Almomani and Bhosale (2021) that found that Spirulina platensis and Chlorella vulgaris can chelate some heavy metals from wastewater up to 95% and 87% efficacy, respectively. Piccini et al. (2019) reported that both Spirulina and Chlorella sp. cell wall contain a range of numerous active sites that can bind to metals and act as chelating agents; these include acetamide chitin, structural polysaccharides, phosphate and amino groups of nucleic acids, amino and carboxyl groups of proteins, and hydroxyl groups of polysaccharides. Similarly, Doyurum and Çelik (2006) reported that plant-dried materials are mainly consisted of polysaccharides, proteins, and lipids, functional groups such as carboxyl, hydroxyl, sulphate, phosphate, and amino groups that can be used for metals adsorbent and chelating agents. Recently, Lu et al. (2018) have concluded that the application of SA significantly reduced Cd accumulation on Lemna minor plants.
Among the metals studied, higher rates of plant uptake and enrichment in common bean seeds with respect to control samples were cadmium and lead, which indicated that these metals are mainly accumulated due to irrigation with wastewater. Anyway, all heavy metal concentrations being studied after application of our treatments are still within the safe limit, as it is recommended below the safe limit for some food crops by FAO/WHO (2019, 2020).
8 Conclusions
Irrigation of common bean plants using untreated wastewater significantly increased the contents of free proline, total phenols, superoxide dismutase, catalase, peroxidase, polyphenol oxidase, malondialdehyde (MDA), and abscisic acid (ABA) throughout the two growth stages compared with control. Indole acetic acid (IAA), gibberellic acid (GA3), all yield parameters, and total soluble carbohydrate and protein contents in seeds significantly decreased. In all cases, cadmium and lead exhibit the highest rate of plant uptake and enrichment in common bean seeds. Moreover, the concentrations of nickel (Ni), cadmium (Cd), lead (Pb), and cobalt (Co) were significantly increased and above toxic concentrations in seeds (edible part) of the untreated wastewater-irrigated plants. On the other hand, application of Spirulina platensis, Chlorella vulgaris, salix plant powder, and ethylenediaminetetraacetic acid (EDTA) alleviated the adverse effects of wastewater irrigation via decreasing the oxidative damage. As a result, the contents of free proline, total phenols, superoxide dismutase, catalase, peroxidase, polyphenol oxidase, MDA, and ABA were significantly decreased. Moreover, IAA, GA3, all tested yield parameters, and biochemical contents of seeds were significantly increased than control. Further protection under irrigation with wastewater was achieved by applying our treatments, which reduce the accumulation of Ni, Cd, Pb, and Co in seeds (edible part) of common bean plants. Finally, Spirulina platensis and leaves powder of Salix alba being economically and environmentally friendly can be recommended for farmers to use in their fields to alleviate the harmful effects of wastewater on plants.
References
AbdEl-Baki GK, Rafat A, Mostafa D (2022) Differential responses of common bean, (Phaseolus vulgaris L.) to the interactive effects of ascorbic acid and Trichoderma harzianum under salinity stress. Egypt Acad J Biol Sci H Bot 13:11–29. https://doi.org/10.21608/eajbsh.2022.249901
Abdelrazek S (2019) Monitoring irrigation water pollution of Nile Delta of Egypt with heavy metals. Alexandria Sci Exch J 40:441–450. https://doi.org/10.21608/asejaiqjsae.2019.50350
Aebi HE (1983) Catalase. Methods Enzym Anal 105:121–126
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257. https://doi.org/10.1016/j.biortech.2005.12.006
Ahmed DA, Slima DF (2018) Heavy metal accumulation by Corchorus olitorius L. irrigated with wastewater. Environ Sci Pollut Res 25:14996–15005. https://doi.org/10.1007/s11356-018-1675-1
Aldoobie NF, Beltagi MS (2013) Physiological, biochemical and molecular responses of common bean (Phaseolus vulgaris L.) plants to heavy metals stress. African J Biotechnol 12:4614–4622. https://doi.org/10.5897/AJB2013.12387
Al-Homaidan AA, Alabdullatif JA, Al-Hazzani AA, Al-Ghanayem AA, Alabbad AF (2015) Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J Biol Sci 22:795–800. https://doi.org/10.1016/j.sjbs.2015.06.010
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974) Chemical analysis of ecological materials., 2nd Ed. Blackwell Scientific Publications.
Almomani F, Bhosale RR (2021) Bio-sorption of toxic metals from industrial wastewater by algae strains Spirulina platensis and Chlorella vulgaris: application of isotherm, kinetic models and process optimization. Sci Total Environ 755:142654. https://doi.org/10.1016/j.scitotenv.2020.142654
Al-Swadi HA, Usman ARA, Al-Farraj AS, Al-Wabel MI, Ahmad M, Al-Faraj A (2022) Sources, toxicity potential, and human health risk assessment of heavy metals-laden soil and dust of urban and suburban areas as affected by industrial and mining activities. Sci Rep 12:8972. https://doi.org/10.1038/s41598-022-12345-8
Ammar EE, Aioub AAA, Elesawy AE, Karkour AM, Mouhamed MS, Amer AA, EL-Shershaby NA (2022) Algae as bio-fertilizers: between current situation and future prospective: the role of algae as a bio-fertilizer in serving of ecosystem. Saudi J Biol Sci 29:3083–3096. https://doi.org/10.1016/j.sjbs.2022.03.020
Antar AS, Gendy AAS, El-Sanat GMA (2012) Study on some agrochemical pollutants in drains water at North Delta. Egypt. J Soil Sci Agric Eng 3:237–247. https://doi.org/10.21608/jssae.2012.53847
APHA (1999) Standard methods for the examination of water and wastewater. Am Public Heal Assoc Washington, DC, USA p, p 541
Atici Ö, Ağar G, Battal P (2005) Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol Plant 49:215–222. https://doi.org/10.1007/s10535-005-5222-9
Banadka A, Nagella P (2022) Effect of heavy metals on germination, biochemical, and L-DOPA content in Mucuna pruriens (L.) DC. J Appl Biol Biotechnol 10:117–126. https://doi.org/10.7324/JABB.2022.100613
Barman SC, Sahu RK, Bhargava SK, Chaterjee C (2000) Distribution of heavy metals in wheat, mustard, and weed grown in field irrigated with industrial effluents. Bull Environ Contam Toxicol 64:489–496. https://doi.org/10.1007/s001280000030
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bergmeyer HU, Gawehn K, Grassl M (1974) Methods of enzymatic analysis. Bergmeter HU, ed. Acad Press, New York 3:1205–1215
Bhat JA, Shivaraj SM, Singh P, Navadagi DB, Tripathi DK, Dash PK, Solanke AU, Sonah H, Deshmukh R (2019) Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 8:71. https://doi.org/10.3390/plants8030071
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386. https://doi.org/10.1590/1678-4685-gmb-2016-0087
Daniel HD, Martin GC (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances1. J Am Soc Hortic Sci 97:651–654. https://doi.org/10.21273/JASHS.97.5.651
Delzer GC, McKenzie SW (2003) Five-day biochemical oxygen demand. US Geol Surv Tech Water-Resources Investig Book 9,:chap. A7, section 7.0. https://doi.org/10.3133/twri09A7.0
Denaxa N-K, Tsafouros A, Roussos PA (2022) Role of phenolic compounds in adventitious root formation. In: Environmental, physiological and chemical controls of adventitious rooting in cuttings. Elsevier, 251–288. https://doi.org/10.1016/B978-0-323-90636-4.00013-1
Dinesh-Kumar M, Gopikumar S, Uan DK, Adishkumar S, Rajesh Banu J (2020) Constructed wetlands: an emerging green technology for the treatment of industrial wastewaters. In: Emerging eco-friendly green technologies for wastewater treatment. Springer, 21–44
Doyurum S, Çelik A (2006) Pb(II) and Cd(II) removal from aqueous solutions by olive cake. J Hazard Mater 138:22–28. https://doi.org/10.1016/j.jhazmat.2006.03.071
Duan X, Zhu Z, Yang Y, Duan J, Jia Z, Chen F, Sang Z, Ma L (2022) Salicylic acid regulates sugar metabolism that confers freezing tolerance in Magnolia wufengensis during natural cold acclimation. J Plant Growth Regul 41:227–235. https://doi.org/10.1007/s00344-020-10281-3
Egypt-decree (2013) For the protection of the Nile River and its waterways from pollution. Decree of Minister of Water Resources and Irrigation no. 92 for year 2013 for the Executive Regulation of Law 48/1982, 92/2013. (in Arabic). https://www.mwri.gov.eg/index.php/ministry/ministry-17/12-1984
Elansary HO, Skalicka-Woźniak K, King IW (2016) Enhancing stress growth traits as well as phytochemical and antioxidant contents of Spiraea and Pittosporum under seaweed extract treatments. Plant Physiol Biochem 105:310–320. https://doi.org/10.1016/j.plaphy.2016.05.024
Elgharably A, Mohamed HM (2016) Heavy metals uptake by wheat, bean and onion and characterization of microorganisms in a long-term sewage wastewater treated soil. Egypt J Soil Sci 56:605–620. https://doi.org/10.21608/ejss.2016.3334
El-Tayeb MA, El-Enany AE, Ahmed NL (2006) Salicylic acid alleviates the copper toxicity in sunflower seedlings. Int J Bot 2:380–387. https://doi.org/10.3923/ijb.2006.380.387
Emamverdian A, Ding Y, Mokhberdoran F, Ahmad Z (2020) Mechanisms of selected plant hormones under heavy metal stress. Polish J Environ Stud 30:497–507. https://doi.org/10.15244/pjoes/122809
FAO/WHO (2019) Food Standards Programme Codex Alimentarius Commission: Report of the 11th session of the codex committee on contaminants in foods. April 2017. Rio de Janeiro, Accessed 7 November 2019.Brazil 3–7. http://www.fao.org/fao-who-codexalimentarius/en/
FAO/WHO (2020) Codex Alimentarius International Food Standards: General standard for contaminants and toxins in food and feed (CXS 193–1995). 2019. Accessed 26 January 2020. https://www.fao.org/fao-who-codexalimentarius/thematic-areas/contaminants/en/
Farahat EA, Galal TM, Elawa OE, Hassan LM (2017) Health risk assessment and growth characteristics of wheat and maize crops irrigated with contaminated wastewater. Environ Monit Assess 189:535. https://doi.org/10.1007/s10661-017-6259-x
Gad HA (2019) First report on the susceptibility of certain dry Egyptian common bean (Phaseolus vulgaris L.) (Fabaceae) varieties to infestation by Acanthoscelides obtectus (Say, 1831) (Coleoptera: Chrysomelidae: Bruchinae). Polish J Entomol 88:149–161. https://doi.org/10.2478/pjen-2019-0012
Gitau MM, Farkas A, Ördög V, Maróti G (2022) Evaluation of the biostimulant effects of two Chlorophyta microalgae on tomato (Solanum lycopersicum). J Clean Prod 364:132689. https://doi.org/10.1016/j.jclepro.2022.132689
Golterman HL (1991) Direct nesslerization of ammonia and nitrate in fresh-water. Ann Limnol - Int J Limnol 27:99–101. https://doi.org/10.1051/limn/1991007
Gonçalves AL (2021) The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating and biopesticide roles. Appl Sci 11:1–21. https://doi.org/10.3390/app11020871
Gupta N, Khan DK, Santra SC (2008) An assessment of heavy metal contamination in vegetables grown in wastewater-irrigated areas of Titagarh, West Bengal, India. Bull Environ Contam Toxicol 80:115–118. https://doi.org/10.1007/s00128-007-9327-z
Gupta S, Satpati S, Nayek S, Garai D (2010) Effect of wastewater irrigation on vegetables in relation to bioaccumulation of heavy metals and biochemical changes. Environ Monit Assess 165:169–177. https://doi.org/10.1007/s10661-009-0936-3
Hajihashemi S, Mbarki S, Skalicky M, Noedoost F, Raeisi M, Brestic M (2020) Effect of wastewater irrigation on photosynthesis, growth, and anatomical features of two wheat cultivars (Triticum aestivum L.). Water 12:591–607. https://doi.org/10.3390/w12020607
Han-jie W, Wang J, Yu X (2022) Wastewater irrigation and crop yield: a meta-analysis. J Integr Agric 21:1215–1224. https://doi.org/10.1016/S2095-3119(21)63853-4
Hasanuzzaman M, Matin MA, Fardus J, Hasanuzzaman M, Hossain MS, Parvin K (2019) Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot 72:1–16. https://doi.org/10.5586/aa.1765
Hashem HA (2016) Physiological and molecular actions of jasmonic acid on soybean plant (Doctoral dissertation, Ph. D Thesis, Fac. of Sci., Ain Shams Univ., Cairo, Egypt)
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1456–1466. https://doi.org/10.4161/psb.21949
Hegedüs A, Erdei S, Horváth G (2001) Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci 160:1085–1093. https://doi.org/10.1016/S0168-9452(01)00330-2
Hussain A, Priyadarshi M, Dubey S (2019) Experimental study on accumulation of heavy metals in vegetables irrigated with treated wastewater. Appl Water Sci 9:1–11. https://doi.org/10.1007/s13201-019-0999-4
Jean-Soro L, Bordas F, Bollinger J-C (2012) Column leaching of chromium and nickel from a contaminated soil using EDTA and citric acid. Environ Pollut 164:175–181. https://doi.org/10.1016/j.envpol.2012.01.022
John R, Ahmad P, Gadgil K, Sharma S (2008) Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemna polyrrhiza L. Plant, Soil Environ 54:262–270. https://doi.org/10.17221/2787-PSE
Kang GZ, Li GZ, Liu GQ, Xu W, Peng XQ, Wang CY, Zhu YJ, Guo TC (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol Plant 57:718–724. https://doi.org/10.1007/s10535-013-0335-z
Kanwal A, Farhan M, Sharif F, Hayyat MU, Shahzad L, Ghafoor GZ (2020) Effect of industrial wastewater on wheat germination, growth, yield, nutrients and bioaccumulation of lead. Sci Rep 10:11352–11361. https://doi.org/10.1038/s41598-020-68208-7
Kar M, Mishra D (1976) Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol 57:315–319. https://doi.org/10.1104/pp.57.2.315
Khalilzadeh R, Pirzad A, Sepehr E, Khan S, Anwar S (2020) Long-term effect of heavy metal–polluted wastewater irrigation on physiological and ecological parameters of Salicornia europaea L. J Soil Sci Plant Nutr 20:1574–1587. https://doi.org/10.1007/s42729-020-00299-7
Khodamoradi S, Sagharyan M, Samari E, Sharifi M (2022) Changes in phenolic compounds production as a defensive mechanism against hydrogen sulfide pollution in Scrophularia striata. Plant Physiol Biochem 177:23–31. https://doi.org/10.1016/j.plaphy.2022.02.013
Kim Y-H, Khan AL, Kim D-H, Lee S-Y, Kim K-M, Waqas M, Jung H-Y, Shin J-H, Kim J-G, Lee I-J (2014) Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa L. silicon genes, and endogenous phytohormones. BMC Plant Biol 14:13. https://doi.org/10.1186/1471-2229-14-13
Kısa D, Elmastaş M, Öztürk L, Kayır Ö (2016) Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59:813–820. https://doi.org/10.1007/s13765-016-0229-9
Kovalchuk I, Titov V, Hohn B, Kovalchuk O (2005) Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat Res Mol Mech Mutagen 570:149–161. https://doi.org/10.1016/j.mrfmmm.2004.10.004
Liu Y, Wirén NV (2017) Ammonium as a signal for physiological and morphological responses in plants. J Exp Bot 68:2581–2592. https://doi.org/10.1093/jxb/erx086
Lowery OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q (2018) Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf 147:500–508. https://doi.org/10.1016/j.ecoenv.2017.09.015
Luo YL, Su ZL, Bi TJ, Cui XL, Lan QY (2014) Salicylic acid improves chilling tolerance by affecting antioxidant enzymes and osmoregulators in sacha inchi (Plukenetia volubilis). Brazilian J Bot 37:357–363. https://doi.org/10.1007/s40415-014-0067-0
Malik Z, Afzal S, Dawood M, Abbasi GH, Khan MI, Kamran M, Zhran M, Hayat MT, Aslam MN, Rafay M (2022) Exogenous melatonin mitigates chromium toxicity in maize seedlings by modulating antioxidant system and suppresses chromium uptake and oxidative stress. Environ Geochem Health 44:1451–1469. https://doi.org/10.1007/s10653-021-00908-z
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Mehta SK, Gaur JP (2005) Use of algae for removing heavy metal ions from wastewater: progress and prospects. Crit Rev Biotechnol 25:113–152. https://doi.org/10.1080/07388550500248571
Monteiro MS, Santos C, Soares A, Mann RM (2009) Assessment of biomarkers of cadmium stress in lettuce. Ecotoxicol Environ Saf 72:811–818. https://doi.org/10.1016/j.ecoenv.2008.08.002
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Mussarat M, Jamal WA, Muhammad D, Ahmad M, Saleem A, Khan S, Aman F, Bibi H, Shah WA, Dawar K, Ul Akbar N, Mian IA, Waheed M, Ali I, Zia A, Malik W (2021) Risk of heavy metals accumulation in soil and wheat grains with waste water irrigation under different NPK levels in alkaline calcareous soil. PLoS One 16:e0258724. https://doi.org/10.1371/journal.pone.0258724
Mutlu-Durak H, Kutman BY (2021) Seed treatment with biostimulants extracted from weeping willow (Salix babylonica) enhances early maize growth. Plants 10:1–20. https://doi.org/10.3390/plants10071449
Noriega G, Caggiano E, Lecube ML, Cruz DS, Batlle A, Tomaro M, Balestrasse KB (2012) The role of salicylic acid in the prevention of oxidative stress elicited by cadmium in soybean plants. Biometals 25:1155–1165. https://doi.org/10.1007/s10534-012-9577-z
Nowwar A, Farghal I, Ismail M, Amin M (2022) Biochemical changes on jute mallow plant irrigated with wastewater and its remediation. Egypt J Chem 65:0–0. https://doi.org/10.21608/ejchem.2022.109007.4972
Nzediegwu C, Prasher S, Elsayed E, Dhiman J, Mawof A, Patel R (2019) Effect of biochar on heavy metal accumulation in potatoes from wastewater irrigation. J Environ Manage 232:153–164. https://doi.org/10.1016/j.jenvman.2018.11.013
Oh S-Y, Yoon M-K (2014) Chemical extraction of arsenic from contaminated soils in the vicinity of abandoned mines and a smelting plant under subcritical conditions. Soil Sediment Contam an Int J 23:166–179. https://doi.org/10.1080/15320383.2014.808169
Page A (1982) Methods of soil analysis, part 2: chemical and microbiological properties, 2nd Ed Am. Soc. at Agron. Inc Soil Sci Soc Am Inc, Madison, Wisconsin, VSA
Pál M, Horváth E, Janda T, Páldi E, Szalai G (2006) Physiological changes and defense mechanisms induced by cadmium stress in maize. J Plant Nutr Soil Sci 169:239–246. https://doi.org/10.1002/jpln.200520573
Palma JM, Sandalio LM, Javier Corpas F, Romero-Puertas MC, McCarthy I, del Río LA (2002) Plant proteases, protein degradation, and oxidative stress: role of peroxisomes. Plant Physiol Biochem 40:521–530. https://doi.org/10.1016/S0981-9428(02)01404-3
Parashar P, Prasad FM (2013) Study of heavy metal accumulation in sewage irrigated vegetables in different regions of Agra District, India. Open J Soil Sci 03:1–8. https://doi.org/10.4236/ojss.2013.31001
Parkinson JA, Allen SE (1975) A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun Soil Sci Plant Anal 6:1–11. https://doi.org/10.1080/00103627509366539
Pham BN, Kang J, Lee C, Park S (2021) Removal of heavy metals (Cd2+, Cu2+, Ni2+, Pb2+) from aqueous solution using Hizikia fusiformis as an algae-based bioadsorbent. Appl Sci 11:8604. https://doi.org/10.3390/app11188604
Piccini M, Raikova S, Allen MJ, Chuck CJ (2019) A synergistic use of microalgae and macroalgae for heavy metal bioremediation and bioenergy production through hydrothermal liquefaction. Sustain Energy Fuels 3:292–301. https://doi.org/10.1039/c8se00408k
Pirie NW (1992) Plant Toxin Analysis 13:23. https://doi.org/10.1007/978-3-662-02783-7
Pittwell LR (1983) STANDARD COD. Chem Br 19:907
Popova LP, Maslenkova LT, Ivanova A (2012) Role of salicylic acid in alleviating heavy metal stress. Environ Stress Toler Plants Era Clim Chang Springer-Verlag 447–466. https://doi.org/10.1007/978-1-4614-0815-4
Pratyusha S (2022) Phenolic compounds in the plant development and defense: an overview. In: Plant stress physiology-perspectives in agriculture. Plant Stress Physiology-Perspectives in Agriculture., 125–185
Rabie MH, Eleiwa ME, Aboseoud MA, Khalil KM (1992) Effect of nickel on the content of carbohydrate and some mineral in corn and broad bean plant. JKAU Sci 4:37–43
Racchi M (2013) Antioxidant defenses in plants with attention to prunus and citrus spp. Antioxidants 2:340–369. https://doi.org/10.3390/antiox2040340
Rai GK, Mushtaq M, Bhat BA, Kumar RR, Singh M, Rai PK (2022) Reactive oxygen species: friend or foe. In: Thermotolerance in crop plants. Springer Nature Singapore, Singapore, 129–162. https://doi.org/10.1007/978-981-19-3800-9_6
Renu MA, Singh K, Upadhyaya S, Dohare RK (2017) Removal of heavy metals from wastewater using modified agricultural adsorbents. Mater Today Proc 4:10534–10538. https://doi.org/10.1016/j.matpr.2017.06.415
Romera E, González F, Ballester A, Blázquez ML, Muñoz JA (2006) Biosorption with algae: a statistical review. Crit Rev Biotechnol 26:223–235. https://doi.org/10.1080/07388550600972153
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Saini S, Kaur N, Pati PK (2021) Phytohormones: key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf 223:112578. https://doi.org/10.1016/j.ecoenv.2021.112578
Sharma SS, Kumar V (2002) Responses of wild type and abscisic acid mutants of Arabidopsis thaliana to cadmium. J Plant Physiol 159:1323–1327. https://doi.org/10.1078/0176-1617-00601
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Sharma A, Sidhu GPS, Araniti F, Bali AS, Shahzad B, Tripathi DK, Brestic M, Skalicky M, Landi M (2020) The role of salicylic acid in plants exposed to heavy metals. Molecules 25:540. https://doi.org/10.3390/molecules25030540
Sharma A, Kapoor D, Gautam S, Landi M, Kandhol N, Araniti F, Ramakrishnan M, Satish L, Singh VP, Sharma P, Bhardwaj R, Tripathi DK, Zheng B (2022) Heavy metal induced regulation of plant biology: recent insights. Physiol Plant 174:1–12. https://doi.org/10.1111/ppl.13688
Shartooh SM, Kasim SA, Obaid RH, Hadi AA, Abdulmajeed AA (2014) Lettuce leaves as biosorbent material to remove heavy metal ions from industerial wastewater. Baghdad Sci J 11:1164–1170. https://doi.org/10.21123/bsj.11.3.1164-1170
Shedeed ZA, Gheda S, Elsanadily S, Alharbi K, Osman MEH (2022) Spirulina platensis biofertilization for enhancing growth, photosynthetic capacity and yield of Lupinus luteus. Agriculture 12:781. https://doi.org/10.3390/agriculture12060781
Shindy WW, Smith OE (1975) Identification of plant hormones from cotton ovules. Plant Physiol 55:550–554. https://doi.org/10.1104/pp.55.3.550
Šoln K, Koce JD (2022) Oxidative stress in roots: Detection of lipid peroxidation and total antioxidative capacity. In: Klemenčič M, Stael S, Huesgen PF (eds) Plant proteases and plant cell death: methods and protocols. Springer US, New York, NY, 221–231. https://doi.org/10.1007/978-1-0716-2079-3_18
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2019) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38:739–752. https://doi.org/10.1007/s00344-018-9886-8
Tariq FS (2021) Heavy metals concentration in vegetables irrigated with municipal wastewater and their human daily intake in Erbil city. Environ Nanotechnology, Monit Manag 16:100475. https://doi.org/10.1016/j.enmm.2021.100475
Torun H, Novák O, Mikulík J, Strnad M, Ayaz FA (2022) The effects of exogenous salicylic acid on endogenous phytohormone status in Hordeum vulgare L. under salt stress. Plants 11:618. https://doi.org/10.3390/plants11050618
Umbriet WW, Burris RH, Stauffer JF, Cohen PP, Johnse WJ, Leepage GA, Potten VR, Schneider WC (1959) Manometric techniques. A manual describing methods applicable to the study of tissue metabolism. Burgess, Minneap 239
Ungureanu N, Vlăduț V, Voicu G (2020) Water scarcity and wastewater reuse in crop irrigation. Sustainability 12:9055. https://doi.org/10.3390/su12219055
USAID (2010) Integrated water resources management feasibility of wastewater reuse. Report No. 14, Egypt. Contact No. Epp-1–04–00024
Vicente R-SM, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338. https://doi.org/10.1093/jxb/err031
Vogel AJ (1975) A text book of practical organic chemistry. 3 [sup] rd ed. London English Lang B Soc Longmans Gr Ltd 969–971
Wade TL, Brooks JM, Kennicutt MC, McDonald TJ, Sericano JL, Jackson TL (1992) Trace metals and organic contaminants analytical techniques 5. Sampl Anal Methods Natl Status Trend Progr 121–139
Wasti S, Mimouni H, Smiti S, Zid E, Ben Ahmed H (2012) Enhanced salt tolerance of tomatoes by exogenous salicylic acid applied through rooting medium. Omi A J Integr Biol 16:200–207. https://doi.org/10.1089/omi.2011.0071
WHO (2022) Guidelines for drinking-water quality: fourth edition incorporating the first and second addenda, World Health Organization. https://www.who.int/publications/i/item/9789240045064
Yan R, Gao S, Yang W, Cao M, Wang S, Chen F (2008) Nickel toxicity induced antioxidant enzyme and phenylalanine ammonia-lyase activities in Jatropha curcas L cotyledons. Plant, Soil Environ 54:294–300. https://doi.org/10.17221/423-PSE
Yildirim E, Ekinci M, Turan M, Agar G, Örs S, Dursun A, Kul R, Balcl T (2019) Impact of cadmium and lead heavy metal stress on plant growth and physiology of rocket (Eruca sativa L). J Agric Nat 22:843–850. https://doi.org/10.18016/ksutarimdoga.vi.548626
Zandi P, Schnug E (2022) Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology (basel) 11:1–30. https://doi.org/10.3390/biology11020155
Zhang Z, Huber DJ, Qu H, Yun Z, Wang H, Huang Z, Huang H, Jiang Y (2015) Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem 171:191–199. https://doi.org/10.1016/j.foodchem.2014.09.001
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nowwar, A.I., Farghal, I.I., Ismail, M.A. et al. Impact of Irrigation with Wastewater on Accumulation of Heavy Metals in Phaseolus vulgaris L. and Its Remediation. J Soil Sci Plant Nutr 23, 761–777 (2023). https://doi.org/10.1007/s42729-022-01080-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-01080-8