Abstract
Phytohormones act as chemical messengers and, under a complex regulation, allow plants to sustain biotic and abiotic stresses. Thus, phytohormones are known for their regulatory role in plant growth and development. Heavy metals (HMs) play an important role in metabolism and have roles in plant growth and development as micronutrients. However, at a level above threshold, these HMs act as contaminants and pose a worldwide environmental threat. Thus, finding eco-friendly and economical deliverables to tackle this problem is a priority. In addition to physicochemical methods, exogenous application of phytohormones, i.e., auxins, cytokinins, and gibberellins, can positively influence the regulation of the ascorbate–glutathione cycle, transpiration rate, cell division, and the activities of nitrogen metabolism and assimilation, which improve plant growth activity. Brassinosteroids, ethylene and salicylic acid have been reported to enhance the level of the anti-oxidant system, decrease levels of ROS, lipid peroxidation and improve photosynthesis in plants, when applied exogenously under a HM effect. There is a crosstalk between phytohormones which is activated upon exogenous application. Research suggests that plants are primed by phytohormones for stress tolerance. Chemical priming has provided good results in plant physiology and stress adaptation, and phytohormone priming is underway. We have reviewed promising phytohormones, which can potentially confer enhanced tolerance when used exogenously. Exogenous application of phytohormones may increase plant performance under HM stress and can be used for agro-ecological benefits under environmental conditions with high HMs level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
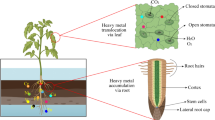
In terms of the degree of danger to the human population across the globe, heavy metals (HMs) are thought to be in second place among pollutants. In the last few decades, HMs have pulled ahead of pesticides and well-known pollutants such as carbon dioxide and sulfur dioxide (Chen et al. 2007). It is predicted that HMs may become the most dangerous contaminant, possibly surpassing solid and nuclear waste (Lajayer et al. 2017). It has been reported that 70% of all HMs and their compounds routed in the human body came from food (Jaishankar et al. 2014). HMs are released into the atmosphere, soil, and water from a variety of sources and from anthropogenic activities (Tchounwou et al. 2012). Later, they are introduced into the food chain, and thus metal toxicity raises the risk and poses concerns for humans and animals. As this review is devoted to the role of phytohormones under HMs contamination in plants, it should be noted that some trace metals are necessary for plant growth and metabolism, but within reasonable limits. HMs in high doses are considered to be harmful for plants (Kumar et al. 2016). Apart from the direct impact of bioactive metals on plants, cell toxicity also occurs by excess production of reactive oxygen species (ROS), which inhibit anti-oxidants and causes oxidative stress (Nanda and Agrawal 2016; Rui et al. 2016). Plants are well adapted, but the resistance depends on the capacity to prevent oxidative stress and tight regulation of anti-oxidant systems, under HMs stress (Sytar et al. 2013; Choudhury et al. 2017; Lajayer et al. 2017). In plants, the permeability of the cell membrane, the synthesis of many biochemical compounds, growth and reproduction are negatively affected by HMs (Munzuroglu and Geckil 2002), thus affecting food quality and yield. This results in lowering of the plant’s maximum genetic potential in terms of growth, development and other related activities (Bücker-Neto et al. 2017).
In recent years, much attention has been given to developing strategies to alleviate HM toxicity in crops and agricultural systems, to fulfil the global food demand. Physicochemical techniques employed for the clean-up of contaminated sites include excavation, stabilization or in situ fixation (i.e., by adding chemicals to change the metal structure so that it does not get absorbed by plants), and washing of soil (physicochemical extraction) (Bradl and Xenidis 2005). However, these physicochemical processes are not cost-effective or efficient (Schnoor 1997). Therefore, the search for eco-friendly, cost-effective and reproducible solutions for the remediation of HM contaminated soils must be a priority.
Environmental and biological scientists work in synergy to develop alternatives and potential strategies to combat adverse effects of HM pollution. Agricultural soils contaminated with HMs pose serious environmental issues. The application of exogenous phytohormones to modulate the biochemical and molecular mechanisms so that plants become equipped to restore land contaminated by pollutants is a promising technique. Thus, the purpose of this review is to summarize the current understanding of the regulation of HM stress through phytohormone priming and its future promise.
Phytohormones and Their Significance for Plants
Phytohormones are small signaling compounds, which act to some degree on virtually every aspect of plant growth and development. The mechanism of the action for different hormones for different processes can be very different. Thus, it has been observed that a single hormone can regulate a diverse array of cellular and developmental processes, whereas, at the same time, multiple hormones may be involved in the regulation of a single process (Gray 2004). Phytohormones such as indole-3-acetic acid or auxin (IAA), cytokinin (CK), ethylene (ET), abscisic acid (ABA), gibberellin (GA), brassinosteroid (BR), salicylic acid (SA), jasmonic acid (JA) and the recently identified strigolactone (SL) are phytohormones that are crucial for the growth and development of plants and also in signaling and crosstalk (Fig. 1). Phytohormones support and manage plants against biotic and abiotic stresses (Bücker-Neto et al. 2017; Nishiyama et al. 2011; Colebrook et al. 2014; Xu et al. 2016). Phytohormones as regulators of HM absorption have been used in agronomical crop management practices to alleviate metal toxicity (Piotrowska-Niczyporuk et al. 2012). It has been found that exogenous application of phytohormones is safe to use and gives promising results for plants under HM toxicity (Zhu et al. 2012, 2013; Agami and Mohamed 2013; Masood et al. 2016). Phytohormones such as CKs, IAAs, BRs, GAs and SA, and so on, have significant roles in signaling, and in biochemical and defence pathways, but the mechanism of providing relief from HM stress is nowadays a topic of global interest (Bücker-Neto et al. 2017). Therefore, phytohormone priming is carried out with the aim of enhancing future research for the management of crop stress.
Behavior of Phytohormones During HM Stress Response
Phytohormones are the most important endogenous molecules for modifying physiological and molecular reactions, and are critically required by the plant for its survival under HM stress (Fahad et al. 2015). Phytohormones in very low concentrations regulate cell membrane permeability, enzyme activity and secondary metabolites, growth, and reproduction (Wani et al. 2016). HM toxicity in plants is manifested as the retardation of plant growth and development, which results because of its accumulation in the aboveground and underground organs of a plant. As a rule, a heavy dose of HMs leads to a decrease in the root and shoot biomass in many plant species, which is normally regulated by phytohormones. Several HMs in high concentrations have been shown to cause a delay in seed germination. Cadmium (Cd), copper (Cu), lead (Pb), and nickel (Ni) are considered highly toxic for the plant germination process, and seeds may vary in their stress response to different HMs (Wang et al. 2011; Sethy and Ghosh 2013). Ni modulated the enzymatic activity of amylase, ribonuclease and protease enzymes, thus reducing seed germination and growth activity (Ahmad and Ashraf 2011). The oxidizing ability of roots, the activity of peroxidases and polyphenol oxidases, the activity of enzymes of carbohydrate metabolism—α-amylases, β-amylases, acid phosphatases and acid invertases—were severely inhibited under Pb exposure (Singh et al. 2011). Protein oxidative damage was due to oxidative stress under Cu stress and catalase activity was also observed to be decreased (Pena et al. 2011). Cd toxicity stimulates the expression of the glutathione peroxidase enzyme and glutathione reductase activity (Smiri et al. 2011). The reaction of plants to HM stress depends on the concentration and kind of HMs and on complex interactions with other stresses. The effect of phytohormones on HM stress has been evaluated on model plants or plants having the potential for phytoextraction (Bulak et al. 2014).
Phytohormones and HM Toxicity
ABA is designated as an essential phytohormone having a role in abiotic stresses. In response to environmental stresses, a rapid increase in endogenous ABA levels has been observed, which activates the specific signaling pathways and modulates gene expression levels in the plant (Brien and Benkova 2013). Exposure to HMs such as cadmium (Cd), mercury (Hg), copper (Cu), arsenic (As) and so on induces expression of ABA biosynthetic genes and in turn increases the endogenous levels of ABA (Hollenbach et al. 1997; Bücker-Neto et al. 2017). ABA transcriptionally regulates up to 10% of protein-encoding genes (Nemhauser et al. 2006; Wani et al. 2016). The mechanism behind the role of induced ABA in response to HMs needs to be explored.
Promising results have been observed by the utilization of ABA-deficient and ABA-insensitive mutants towards Cd sensitivity and have shown that Cd-induced growth inhibition is not a result of ABA signaling, but instead the increased ABA level upon HM stress might be regulating stomata closure to regulate water balance in plants (Sharma and Kumar 2002). How exogenous application of ABA can regulate the HM response is still an area to be explored. ABA might act as a trade-off between plant responses to the stress induced by HMs, thereby triggering a balance between survival and growth.
IAA is one of the most multi-functional phytohormones and is responsible not only for the development of plants under normal conditions but also for plant growth under stress conditions (Kazan 2013). IAA plays a basic role in the adaptation of plants under salt stress, and is noted to increase under salt or HMs stresses. The elevated IAA level has been connected with growth reduction, which can be a result of changed hormonal balance under stress conditions (Fahad et al. 2015). The hormone interaction and miRNAs expression in the regulation of HM (As) response has been suggested under exogenous supply of IAA. It was shown to improve growth of plants under HM (As) stress (Srivastava et al. 2013). IAA, ROS, and ET crosstalk and cooperate during the signaling pathway with alteration in the root system of plants (Camacho-Cristóbal et al. 2015). It has been reported that PINFORMED1 (PIN1) protein participates in IAA distribution under conditions of HM stress. For example, under boron (B) starvation, IAA distribution is altered, which leads to lower regulation of the PIN1 protein and limitation of root elongation (Li et al. 2015).
The anti-oxidative system of plants involves glutathione reductase (GR), glutathione sulfotransferase (GST), superoxide (SOD), ascorbate peroxidase (APX), catalase (CAT) and peroxidase (POD) enzymes. These enzymes take part in stress response and can be regulated by BRs under different environmental stresses (Cao et al. 2005). Seventy BRs have been identified among plant steroids, which are able to coordinate phytomorphogenesis, germination of seeds, cell division and elongation, flowering, vascular differentiation, formation of stomata, male fertility, and plant senescence (Mandava 1988; Vardhini 2014). Endogenous BRs are present in roots, leaves, shoots, vascular cambium, flower buds, fruits, pollen, seeds (Bajguz and Hayat 2009) and can be responsible for the support of plant growth under HM stress. 24-Epibrassinolide (EBL) is an important plant growth hormone that has the potential to reduce HMs (Shahzad et al. 2018).
The GAs are a broad class of tetracyclic diterpenoid carboxylic acid compounds, but just one form of GAs, GA1, is able to function as a growth hormone in higher plants (Sponsel and Hedden 2004). The GAs positively affect seed germination, stem elongation, leaf expansion, flower and trichome initiation and the development of fruits (Yamaguchi 2008). GAs support the development of plant adaptation and resistance to various abiotic stresses and have protective effects on the toxicity of HMs (Maggio et al. 2010). GAs may act occasionally in the paracrine signaling pathway, but there is still a mystery regarding the exact mechanism of GAs movement/transport in plants (Gupta and Chakrabarty 2013). He et al. (2012) reported the role of GAs in increasing stress tolerance in wheat by enhancing the expression of the TaMYB73 gene. DELLA proteins, which are well-known repressors of GA responses, have recently been shown to be involved in stress avoidance (Wild and Achard 2013). A low concentration of HM (Zn) is able to increase the content of GA3, whereas higher concentrations decrease the GA3 level (Atici et al. 2005).
Other growth plant hormones, CKs, play a regulatory role in modulating plant development and their endogenous concentration under stress conditions can be altered, which indicates the involvement of CKs in stress tolerance (Brien and Benkova 2013). HM stress decreases CK production and transport from roots. CKs during HM stress participate intensively in interactions with other hormones (Ha et al. 2012). CKs are often antagonists of ABA (Pospíšilová 2003a) and changes in the levels of both plant hormones under HM stress can be dependent on each other as a result of their crosstalk.
A naturally occurring phenolic compound, SA, is linked to the defence response of plants under HM stress. SA participates in the coordination of plant growth and development, ripening, and responses to abiotic stresses (Rivas-San and Plasencia 2011). SA together with ABA is implicated in the regulation of drought response (Miura and Tada 2014). Plant resistance to salinity, heat, and cell death under various hypersensitive stresses can also be regulated by the presence of SA (Fahad and Bano 2012; Khanna et al. 2016). Cd exposure increases free SA content of barley (Hordeum vulgare) roots and it has been observed that SA can alleviate Cd toxicity by influencing mechanisms of Cd detoxification, other than the activation of the anti-oxidant defence system (Metwally et al. 2003).
SA often acts in combination with other phytohormones such as JA and ET (Jia et al. 2013). The biosynthesis of hormones and their transport and accumulation generate a cascade of signaling pathways, which are part of the plant stress response (Matilla-Vazquez and Matilla 2014). The activity of ET biosynthetic enzymes increases and, under HM exposure, MAPKs phosphorylate ACS2 and ACS6 (Skottke et al. 2011; Bucker-Neto et al. 2017).
It has been observed that ET, ethane, ethanol, and acetaldehyde production has been increased under HM stress (Gora and Clijsters 1989). Photosynthetic processes regulated by ET depend on the plant’s sensitivity to ET (Iqbal et al. 2012). It was reported that increasing 1-aminocyclopropane carboxylic acid (ACC) synthase (ACS) activity stimulates ET synthesis under the effects of HMs such as Cd and Cu (Pell et al. 1997). At comparably low concentrations of HMs, ET production was enhanced, but under the application of higher concentrations of HMs, this effect progressively disappeared (Gora and Clijsters 1989).
S-Adenosylmethionine synthetase participates in the ET synthesis stage from methionine to S-adenosylmethionine. S-Adenosylmethionine is a capable substrate for synthesis of 1-aminocyclopropane-1-carboxylic acid by 1-aminocyclopropane-1-carboxylic acid synthase. Induction of expression ACS genes in potatoes has been observed under a Cu effect. ACS transcript accumulation under Cu exposure has been found for different varieties of tobacco (Schlagnhaufer et al. 1997). Under chromium (Cr) signaling, the expression of four ET biosynthesis-related genes (ACO4, ACO5, ACS1 and ACS2) confirms the ET participation in rice seedlings (Trinh et al. 2014).
Exogenous Application of Hormones in the Adaptation of Plants Under HMs Stress
The study in the area of exogenous application of phytohormones in plant responses under HMs stress effects is in a naïve form, which is due to its high potential in plant tolerance mechanisms, being eco-friendly and cost effective. Recent studies have shown how plant hormones can regulate and integrate growth responses to various environmental cues to sustain plant development and growth (Bücker-Neto et al. 2017). The exogenous application of phytohormones has been observed to alleviate the toxic impact of HMs (Table 1). The plant responses to CKs have been evaluated mostly by exogenous application, and external stress also enhances their endogenous levels via uptake and enhanced biosynthesis of proteins and some secondary metabolites (Pospíšilová 2003b).
Under HMs, exogenous phytohormones stimulated the increase of soluble phenolics and free proline contents in wheat seedlings (Ergün and Öncel 2012). The ABA and GA3 hormones in interaction with Pb and Zn may cause an increase in total phenolic content, but hormones in interaction with Cd prevent an increase in phenolic compounds (Fuhrer 1982; Ahmad et al. 2015). The effect of HMs alone or in combination with phytohormones have completely different influences on plants, depending on the type of plant, its development stage, HM concentration and the duration of treatment. Therefore, prolonged Pb treatment together with GA3 influenced the soluble protein content in a positive manner (Nuray and Işil 2012).
IAA at low and high concentrations was observed to influence Cd toxicity differently, which was dependent on the regulation of the ascorbate–glutathione cycle and other anti-oxidant activities (Bashri and Prasad 2015). IAA increased Cd, Pb and Zn phytoextraction and stimulated root and shoot growth (Fässler et al. 2010; Hadi et al. 2010). Exogenous application of IAA, GA3, and citric acid increased plant biomass (Aderholt et al. 2017). Application of GAs regulated enzymatic activities in nitrogen assimilation with further reduction of nitric oxide accumulation (Gangwar et al. 2011; Zhu et al. 2012). GAs inhibited iron (Fe) translocation by suppressing OsYSL2 gene expression in addition to regulation of Fe transport and translocation (Wang et al. 2017).
An increase in the biomass production via stimulation of cell division, anti-oxidant capacity and shoot initiation has been observed after exogenous application of CKs (Tassi et al. 2008). CK application was able to increase the transpiration rate which can affect the photosynthesis process and stimulate an increase in plant biomass (Cassina et al. 2011; Piotrowska-Niczyporuk et al. 2012).
Arabidopsis as a plant model has been investigated for interactions between BRs and stress signaling to understand the correlation between plant growth and signaling pathways under stress. It was established that exogenous application of BRs represses microbe-associated molecular patterns with further activation of the general stress response, but enhances the wound-triggered general stress response (Bjornson et al. 2016). Under exogenous BRs, there is enhancement in the anti-oxidant machinery (catalase, peroxidase, superoxide dismutase and glutathione reductase enzymes) together with a decrease in ROS, malonaldehyde (MDA) and carbonyl levels (Rady 2011; Ramakrishna and Rao 2012). BRs also stimulate the synthesis of IAA which helped in the stimulation of growth processes (Choudhary et al. 2011).
Salicylic acid was also observed to decrease the level of ROS, and lipid peroxidation, whereas it increased the electrolyte leakage, chlorophyll content, total lipids, and linolenic acid contents (Shi and Zhu 2008; Belkhadi et al. 2010; Kazemi et al. 2010). This helps in the increase in improved photosynthesis, and mineral nutrition (Xu et al. 2015a, b). Capability of plants to HM stress on the physiological responses level is significant in cases of plant productivity (Bücker-Neto et al. 2017). Literature analysis had shown that IAAs, CKs, and GAs positively affect the level of metal accumulation and improve plant growth and tolerance to the stress (Bulak et al. 2014). Exogenously applied MeJA to Cd-stressed O. sativa showed modulation in the activity of CAT, SOD and GR along with glutathione pools (Per et al. 2018; Singh and Shah 2014). In Kandelia obovate, exogenous application of MeJA also maintained endogenous levels of JA and controlled stomatal aperture, reduced the transpiration rate and inhibited Cd uptake and reduced photosynthetic damage (Chen et al. 2014; Per et al. 2018). Additionally, the expression was enhanced in Cd-treated plants, whereas exogenously applied MeJA significantly restored the expression of the type-2 metallothionein gene (KoMT2) in leaves (Chen et al. 2014).
The concentrations of exogenous phytohormones turn out to be significant in plant growth regulation and reactions to environmental condition changes (Song et al. 2014; Piotrowska-Niczyporuk and Bajguz 2014). A significant difference in the plant growth effects of natural and synthetic auxins was not observed, but dose-dependent changes have been observed (Piotrowska-Niczyporuk and Bajguz 2014).
Plant growth and development is dependent upon photosynthetic activity (Xu et al. 2015a, b), and the correlation between phytohormones and photosynthetic processes under HM stress conditions has been studied (Gururani et al. 2015). Under HM stress, the capacity of energy trapping by PSII reaction centers is increased by IAAs (Ouzounidou and Ilias 2005). BRs supported increased chlorophyll accumulation together with higher PN and stomatal conductivity is improved (Hayat et al. 2007; Ali et al. 2008a, b). Under exogenous BR application on plants growing under HMs conditions, the PSII reaction centers have elevated electron transport, energy absorption with further trapping and effective oxygen-evolution (Janeczko et al. 2005).
Exogenous ET has an important role during senescence and promotes ripening in horticultural crops (Agarwal et al. 2012). The potential of combined application of exogenous ET and sulfur could synergistically upgrade photosynthetic performance under Cd effects (Khan et al. 2016). The authors claimed that the impact was observed due to an increase in the concentration of sulfur-containing amino acids and thiol production.
The most recently discovered phytohormone class called as strigolactones (SLs), are carotenoid-derived phytohormones. SLs are found in many different plants including higher plants like rice, Arabidopsis, and so on (Xie et al. 2010). Plant development and their interactions with the environment are related to SLs (Gomez-Roldan et al. 2008; Leyser 2009). A synthetic SL analog, GR24, was discovered recently and widely used for the regulation of plant growth and development (Marzec 2016). However, a few reports related to the role of SL in HM stress and phytoextraction upon exogenous use exist including the effects on plant growth under Cd stress. There are many ameliorative ways for Cd toxicity but mechanisms of Cd toxicity removal in plants, by the exogenous application of phytohormone, are important. Upon the exogenous use of GR24 (a synthetic SL phytohormone), Cd phytotoxicity and uptake in switchgrass, a warm-season C4 perennial grass, a reduction of Cd concentration was exhibited. However, Fe and Zn levels increased due to additional GR24 which was related to the competition between Cd and other elements (Tai et al. 2017). GR24 acted as a positive regulator of chlorophyll synthesis and exogenous GR24 alleviated this decrease in chlorophyll content. By contrast, Woo et al. (2004) reported that leaf senescence symptoms were delayed in (max2/ore9) mutants with SL deficiency. An SL-deficient mutant, dad1, was observed in petunias showing the role of a SL negative effect on chlorophyll synthesis (Snowden et al. 2005). The upcoming data related to SLs suggest crosstalk with other hormones and particularly during HM stress there also exists crosstalk between phytohormones.
Conclusion and Future Perspective
Exogenous application of BRs, CKs, IAAs, GAs, SA and SLs can increase the level of anti-oxidants, decrease the level of ROS together with lipid peroxidation, and stimulate plant growth (Fig. 2). Thus, plants may survive better with phytohormone priming in HM-contaminated areas (Fig. 3). This review provides insight on the role of exogenous phytohormone application for enhancing and developing defence strategies of plants. The data presented in this mini review can be used for developing agro-ecological technology based on the exogenous application of phytohormones to improve tolerance under HM contamination which may contribute to the agricultural or ecological sectors, and explore ways for further improvisation. However, suitable bio-formulations may be laboratory-tested after further research to be used for reclamation of contaminated soils. This low input technique is sustainable and will help in reclamation of HM contaminated soils, thus increasing the quality and crop yield in such areas.
References
Aderholt M, Vogelien DL, Koether M, Greipsson S (2017) Phytoextraction of contaminated urban soils by Panicum virgatum L. enhanced with application of a plant growth regulator (BAP) and citric acid. Chemosphere 175:85–96
Agami RA, Mohamed GF (2013) Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol Environ Saf 94:164–171
Agarwal G, Choudhary D, Singh VP, Arora A (2012) Role of ethylene receptors during senescence and ripening in horticultural crops. Plant Signal Behav 7(7):827–846
Ahmad MS, Ashraf M (2011) Essential roles and hazardous effects of nickel in plants. Rev Environ Contam Toxicol 214:125–167
Ahmad A, Hadi F, Ali N (2015) Effective phytoextraction of Cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int J Phytorem 17:56–65
Alam MM, Hayat S, Ali B, Ahmad A (2007) Effect of 28-homobrassinolide treatment on nickel toxicity in Brassica juncea. Photosynthetica 45:139–142
Ali B, Hasan SA, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A (2008a) A role for brassinosteroids in the amelioration of aluminium stress through anti-oxidant system in mung bean (Vigna radiata L. Wilc-zek). Environ Exp Bot 62:153–159
Ali B, Hayat S, Fariduddin Q, Ahmad A (2008b) 24-Epi-brassinolide protects against the stress generated by salinity and nickel in Brassica juncea. Chemosphere 72:1387–1392
Anuradha S, Rao SSR (2009) Effect of 24-Epibrassinolide on the photosynthetic activity of radish plants under cadmium stress. Photosynthetica 47:317–320
Atici Ö, Ağar G, Battal P (2005) Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biol Plantarum 49(2):215–222
Bajguz A (2000) Blockade of HMs accumulation in Chlorella vulgaris cells by 24-epibrassinolide. Plant Physiol Biochem 38:797–801
Bajguz A, Hayat S (2009) Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol Biochem 47:1–8
Bashri G, Prasad SM (2015) Indole acetic acid modulates changes in growth, chlorophyll a fluorescence and anti-oxidant potential of Trigonella foenum-graecum L. grown under cadmium stress. Acta Physiol Plant 37:1745
Belkhadi A, Hediji H, Abbes Z, Nouairi I, Barhoumi Z, Zarrouk M, Chaïbi W, Djebali W (2010) Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol Environ Saf 73:1004–1011
Bjornson M, Dandekar AM, Chory J, Dehesh K (2016) Brassinosteroid’s multi-modular interaction with the general stress network customizes stimulus-specific responses in Arabidopsis. Plant Sci 250:165–177
Bradl H, Xenidis A (2005) Remediation techniques. Interface Sci Technol 6:165–261
Brien JAO, Benkova E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4:451
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and HMs responses. Genet Mol Biol 40:373–386
Bulak P, Walkiewicz A, Brzezińska M (2014) Plant growth regulators-assisted phytoextraction. Biol Plant 58:1–8
Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J, González-Fontes A (2015) Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. J Exp Bot 66:3831–3840
Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B (2005) Loss-of-function mutations in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant 123:57–66
Cassina L, Tassi E, Morelli E, Giorgetti L, Remorini D, Chaney RL, Barbafieri M (2011) Exogenous cytokinin treatments of an NI Hyper-Accumulator, Alyssum Murale, grown in a serpentine soil: implications for phytoextraction. Int J Phytoremediation 13:90–101
Chen TM, Gokhale J, Shofer S, Kuschner WG (2007) Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci 333:249–256
Chen J, Yan Z, Li X (2014) Effect of methyl jasmonate on cadmium uptake and anti-oxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Saf 104:349–356
Choudhary SP, Bhardwaj R, Gupta BD, Dutt P, Gupta RK, Biondi S, Kanwar M (2010) Epibrassinolide in-duces changes in indole-3-acetic acid, abscisic acid and polyamine concentrations and enhances anti-oxidant potential of radish seedlings under copper stress. Plant Physiol 140:280–296
Choudhary SP, Kanwar M, Bhardwaj R, Gupta BD, Gupta RK (2011) Epibrassinolide ameliorates Cr (VI) stress via influencing the levels of indole-3-acetic acid, abscisic acid, polyamines and anti-oxidant system of radish seedlings. Chemosphere 84:5592–5600
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217:67–75
Ergün N, Öncel I (2012) Effects of some HMs and HM hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afr J Agric Res 7:1518–1523
Fahad S, Bano A (2012) Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pak J Bot 44:1433–1438
Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, Khan FA, Khan F, Chen YT, Wu C, Tabassum MA, Chun MX, Afzal M, Jan A, Jan MT, Huang JL (2015) Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: consequences for changing environment. Environ Sci Pollut Res 22:4907–4921
Fässler E, Evangelou MW, Robinson BH, Schulin R (2010) Effects of indole-3-acetic acid (IAA) on sunflower growth and HM uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 80:901–907
Fuhrer J (1982) Early effects of excess cadmium uptake in Phaseolus vulgaris. Plant Cell Environ 5:263–270. https://doi.org/10.1111/1365-3040.ep11572648
Gangwar S, Singh VP, Srivastava PK, Maurya JN (2011) Modification of chromium (VI) phytotoxicity by exogenous gibberellic acid application in Pisum sativum (L.) seedlings. Acta Physiol Plant 33:1385
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194. https://doi.org/10.1038/nature07271
Gora L, Clijsters H (1989) Effect of copper and zinc on the ethylene metabolism in Phaseolus Vulgaris L. In: Clijsters H, De Proft M, Marcelle R, Van Poucke M (eds) Biochemical and physiological aspects of ethylene production in lower and higher plants. Advances in Agricultural Biotechnology, vol 26. Springer, Dordrecht, pp 219–228
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:e311
Gupta R, Chakrabarty SK (2013) Gibberellic acid in plant: Still a mystery unresolved. Plant Signal Behav 8(9):e25504
Gururani MA, Mohanta TK, Bae H (2015) Current understanding of the interplay between phytohormones and photosynthesis under environmental stress. Int J Mol Sci 16(8):19055–19085
Ha S, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Phan Tran LS (2012) Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends Plant Sci 17:172–179
Hadi F, Bano A, Fuller MP (2010) The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): the role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 80(4):457–462
Hadi F, Ali N, Ahmad A (2014) Enhanced phytoremediation of cadmium-contaminated soil by Parthenium hysterophorus plant: effect of gibberellic acid (Ga3) and synthetic chelator, alone and in combinations. Bioremediat J 18:46–55
Hasan SA, Hayat S, Ali B, Ahmad A (2008) 28-Homo- brassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating anti-oxidants. Environ Pollut 151:60–66
Hayat S, Ali B, Aiman Hasan S, Ahmad A (2007) Brassinosteroid enhanced the level of anti-oxidants under cadmium stress in Brassica juncea. Environ Exp Bot 60:33–41
He Y, Li W, Lv J, Jia Y, Wang M, Xia G (2012) Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J Exp Bot 63(3):1511–1522
Hollenbach B, Schreiber L, Hartung W, Dietz KJ (1997) Cadmium leads to stimulated expression of the lipid transfer protein genes in barley: implications for the involvement of lipid transfer proteins in wax assembly. Planta 203(1):9–19
Iqbal N, Nazar R, Khan MIR, Khan NA (2012) Variation in photosynthesis and growth of mustard cultivars: role of ethylene sensitivity. Sci Hortic 135:1–6
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some HMs. Interdiscip Toxicol 7(2):60–72
Jan S, Alyemeni MN, Wijaya L, Alam P, Siddique KH, Ahmad P (2018) Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of anti-oxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol 18(1):146
Janeczko A, Kościelniak J, Pilipowicz M, Lukaszewska S (2005) Protection of winter rape photosystem 2 by 24-Epibrassinolide under cadmium stress. Photosynthetica 43(2):293–298
Jia C, Zhang L, Liu L, Wang J, Li C, Wang Q (2013) Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternate f. sp. lycopersici. J Exp Bot 64(2):637–650
Kaur R, Yadav P, Sharma A, Kumar Thukral A, Kumar V, Kaur Kohli S, Bhardwaj R (2017) Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol Environ Saf 145:466–475
Kazan K (2013) Auxin and the integration of environmental signals into plant root development. Ann Bot 112:1655–1665
Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T (2010) Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and anti-oxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hortic 126(3):402–407
Khan MIR, Khan NA (2014) Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and anti-oxidant metabolism. Protoplasma 251:1007–1019
Khan NA, Asgher M, Per TS, Masood A, Fatma M, Khan MI (2016) Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front Plant Sci 7:1628
Khanna P, Kaur K, Gupta AK (2016) Salicylic acid induces differential anti-oxidant response in spring maize under high temperature stress. Indian J Exp Biol 54:386–393
Kohli SL, Handa N, Sharma A, Kumar V, Kaur P, Bhardwaj R (2017) Synergistic effect of 24-epibrassinolide and salicylic acid on photosynthetic efficiency and gene expression in Brassica juncea L. under Pb stress. Turk J Biol 41:943–953
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Wijaya L, Alyemeni MN, Ahmad P (2018a) Interaction of 24-epibrassinolide and salicylic acid regulates pigment contents, anti-oxidative defense responses, and gene expression in Brassica juncea L. seedlings under Pb stress. Environ Sci Pollut Res 25:15159
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2018b) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 255(1):11–24
Kohli KS, Handa N, Bali S, Arora S, Sharma A, Kaur R, Bhardwaj R (2018c) Modulation of anti-oxidative defense expression and osmolyte content by co-application of 24-epibrassinolide and salicylic acid in Pb exposed Indian mustard plants. Ecotoxicol Environ Saf 147:382–393
Kumar R, Mishra RK, Mishra V, Qidwai A, Pandey A, Shukla SK, Pandey M, Pathak A, Dikshit A (2016) Detoxification and tolerance of HMs in plants. In: Ahmad P (ed) Plant metal interaction, Elsevier, Amsterdam, pp 335–359
Lajayer AB, Ghorbanpour M, Nikabadi S (2017) HMs in contaminated environment: Destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol Environ Saf 145:377–390
Leyser O (2009) The control of shoot branching:an example of plant information processing. Plant Cell Environ 32:694–703
Li K, Kamiya T, Fujiwara T (2015) Differential roles of PIN1 and PIN2 in root meristem maintenance under low-B conditions in Arabidopsis thaliana. Plant Cell Physiol 56(6):1205–1214
Maggio A, Barbieri G, Raimondi G, Pascale SD (2010) Contrasting effects of GA3 treatments on tomato plants exposed to increasing salinity. J Plant Growth Regul 29:63–72
Mandava NB (1988) Plant growth-promoting brassinosteroids. Annu Rev Plant Physiol Plant Mol Bioi 39:23–52
Marzec M (2016) Strigolactones as part of the plant defence system. Trends Plant Sci 21:900–903
Masood A, Khan MI, Fatma M, Asgher M, Per TS, Khan NA (2016) Involvement of ethylene in gibberellic acid-induced sulfur assimilation, photosynthetic responses, and alleviation of cadmium stress in mustard. Plant Physiol Biochem 104:1–10
Matilla-Vazquez MA, Matilla AJ (2014) Ethylene:Role in plants under environmental stress. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment, vol 2. Springer, New York, pp 189–222
Metwally A, Finkemeier I, Georgi M, Dietz K-J (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4
Munzuroglu O, Geckil H (2002) Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Arch Environ Contam Toxicol 43:203
Nanda R, Agrawal V (2016) Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ Exp Bot 125:31–41
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely non overlapping transcriptional responses. Cell 126:467–475
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoto T, Sakakibara H, Schmülling T, Tran L (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Nuray E, Işil Ö (2012) Effects of some HMs and HM hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afr J Agric Res 7:1518–1523
Ouzounidou G, Ilias I (2005) Hormone-induced protection of sunflower photosynthetic apparatus against copper toxicity. Biol Plant 49:223–228
Pell EJ, Schlagnhaufer CD, Arteca RN (1997) Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plant 100:264–273
Pena LB, Azpilicueta CE, Gallego SM (2011) Sunflower cotyledon scope with copper stress by inducing catalase subunits less sensitive to oxidation. J Trace Elem Med Biol 25:125–129
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Piotrowska-Niczyporuk A, Bajguz A (2014) The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul 73(1):57–66
Piotrowska-Niczyporuk A, Bajguz A, Zambrzycka E, Godlewska-Żyłkiewicz B (2012) Phytohormones as regulators of HM biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol Biochem 52:52–65
Pospíšilová J (2003a) Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biol Plant 46:491–506
Pospíšilová J (2003b) Interaction of cytokinins and abscisic acid during regulation of stomatal opening in bean leaves. Photosynthetica 41:49–56
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, anti-oxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237
Ramakrishna B, Rao SSR (2012) 24-Epibrassinolide alleviated zinc-induced oxidative stress in radish (Rap-hanus sativus L.) seedlings by enhancing anti-oxidative system. Plant Growth Regul 68:249–259
Rivas-San VM, Plasencia J (2011) Salicylic acid beyond defence:its role in plant growth and development. J Exp Bot 62:3321–3338
Rui H, Chen C, Zhang X, Shen Z, Zhang F (2016) Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J Hazard Mater 301:304–313
Schlagnhaufer CD, Arteca RN, Pell EJ (1997) Sequential expression of two 1-aminocyclopropane-1-carboxylate synthase genes in response to biotic and abiotic stresses in potato (Solanum tuberosum L.) leaves. Plant Mol Biol 35:683–688
Schnoor J (1997) Phytoremediation. Technology Evaluation Report TE-98-01. Groundwater Remediation Technologies Analysis Center, Pittsburgh
Sethy SK, Ghosh S (2013) Effect of HMs on germination of seeds. J Nat Sc Biol Med 4(2):272–275
Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Zhaorong D (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotox Env Saf 147:935–944
Sharma P, Bhardwaj R (2007) Effects of 24-epibrassino- lide on growth and metal uptake in Brassica juncea L. under copper metal stress. Acta Physiol Plant 29:259–263
Sharma SS, Kumar V (2002) Responses of wild type and abscisic acid mutants of Arabidopsis thaliana to cadmium. J Plant Physiol 159(12):1323–1327
Shi Q, Zhu Z (2008) Effects of exogenous salicylic acid on manganese toxicity, element contents and anti-oxidative system in cucumber. Environ Exp Bot 63:317–326
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66
Singh HP, Kaur G, Batish DR, Kohli RK (2011) Lead (Pb)-inhibited radical emergence in Brassica campestris involves alterations in starch-metabolizing enzymes. Biol Trace Elem Res 144:295–301
Skottke KR, Yoon GM, Kieber JJ, DeLong A (2011) Protein phosphatase 2A controls ethylene biosynthesis by differentially regulating the turnover of ACC synthase isoforms. PLoS Genet 7(4):e1001370
Smiri M, Chaoui A, Rouhier N, Gelhaye E, Jacquot JP, El Ferjani E (2011) Cadmium affects the glutathione/glutaredoxin system in germinating pea seeds. Biol Trace Elem Res 142:93–105
Sneideris LC, Gavassi MA, Campos ML, D’Amico-Damião V, Carvalho RF (2015) Effects of hormonal priming on seed germination of pigeon pea under cadmium stress. An Acad Bras Cienc 87(3):1847–1852
Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ (2005) The Decreased apical dominance1/Petunia hybrid a CAROTENOID CLEAVAGE DIOXYGENASE 8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17:746–759
Song W-Y, Yang H-Ch, Shao H-B, Brestic ZAi-Zh M (2014) The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by HMs. Clean: Soil Air Water 42(1):88–97
Sponsel VM, Hedden P (2004) Gibberellin, biosynthesis and inactivation. In: Davies PJ (ed) Plant hormones biosynthesis, signal transduction, action! Springer, Dordrecht, pp 63–94
Srivastava S, Srivastava AK, Suprasanna P, D’Souza SF (2013) Identification and profiling of arsenic stress-induced microRNAs in Brassica juncea. J Exp Bot 64(1):303–315
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) HM-induced oxidative damage, defence reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35:985–999
Tai Z, Yin X, Fang Z, Shi G, Lou L, Cai Q (2017) Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switchgrass (Panicum virgatum) seedlings. Int J Environ Res Public Health 14(8):852
Tassi E, Pouget J, Petruzzelli G, Barbafieri M (2008) The effects of exogenous plant growth regulators in the phytoextraction of HMs. Chemosphere 71(1):66–73
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) HM toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6. Review
Trinh N, Huang T, Chi W, Fu S, Chen C (2014) Chromium stress response effect on signal transduction and expression of signaling genes in rice. Physiol Plant 150:205–224
Vardhini BV (2014) Brassinosteroids role for amino acids, peptides and amines modulation in stressed plants—a review. In: Anjum NA, Gill SS, Gill R (eds) Plant adaptation to environmental change: significance of amino acids and their derivatives. CAB International, Wallingford, pp 300–316
Wang H, Zhong G, Shi G, Pan F (2011) Toxicity of Cu, Pb, and Zn on seed germination and young seedlings of wheat (Triticum aestivum L.). In: Li D, Liu Y, Chen Y (eds) Computer and computing technologies in agriculture IV. CCTA 2010. IFIP Advances in Information and Communication Technology, vol 346. Springer, Berlin
Wang B, Wei H, Xue Z, Zhang WH (2017) Gibberellins regulate iron deficiency-response by influencing iron transport and translocation in rice seedlings (Oryza sativa). Ann Bot 119:945–956
Wani SH, Kumar V, Shriram V, Sahd SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wild M, Achard P (2013) The DELLA protein RGL3 positively contributes to jasmonate/ethylene defence responses. Plant Signal Behav 8(4):e23891
Woo HR, Kim JH, Nam HG, Lim PO (2004) The delayed leaf senescence mutants of Arabidopsis, ore1, ore3, and ore9 are tolerant to oxidative stress. Plant Cell Physiol 45:923–932
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117
Xu LL, Fan ZY, Dong YJ, Kong J, Bai XY (2015a) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol Plant 59:171–182
Xu Z, Jiang Y, Zhou G (2015b) Response and adaptation of photosynthesis, respiration, and anti-oxidant systems to elevated CO2 with environmental stress in plants. Front Plant Sci 6:701
Xu YX, Mao J, Chen W, Qian TT, Liu SC, Hao WJ, Li CF, Chen L (2016) Identification and expression profiling of the auxin response factors (ARFs) in the tea plant (Camellia sinensis (L.) O. Kuntze) under various abiotic stresses. Plant Physiol Biochem 98:46–56
Yadav P, Kaur R, Kanwar MK, Sharma A, Verma V, Sirhindi G, Bhardwaj R (2018) Castasterone confers copper stress tolerance by regulating anti-oxidant enzyme responses, anti-oxidants, and amino acid balance in B. juncea seedlings. Ecotoxicol Environ Saf 147:725–734
Yamaguchi S (2008) Gibberellin metabolism and its regulation. Annu Rev Plant Physiol 59:225–251
Zhu XF, Jiang T, Wang ZW, Lei GJ, Shi YZ, Li GX, Zheng SJ (2012) Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J Hazard Mater 239–240:302–307
Zhu XF, Wang ZW, Dong F, Lei GJ, Shi YZ, Li GX, Zheng SJ (2013) Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J Hazard Mater 263:398–403
Acknowledgements
This work was supported by the research project of the Slovak Research and Development Agency under the project APVV-15-0721, VEGA 1/0923/16, and National Science Centre Poland, under the project UMO-2016/21/B/ST10/02271. AR thanks, Slovak Academic Information Agency (SAIA) for providing National Scholarship for research in the year 2017.
Author information
Authors and Affiliations
Contributions
OS and AR discussed the idea. OS, PK, SY and AR, prepared the manuscript. AR, PK prepared the figures. MB corrected the manuscript. All authors read and approved manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sytar, O., Kumari, P., Yadav, S. et al. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J Plant Growth Regul 38, 739–752 (2019). https://doi.org/10.1007/s00344-018-9886-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9886-8