Abstract
Introduction
Central serous chorioretinopathy (CSCR) as a clinical entity is potentially damaging and may significantly affect retinal morphology and function, especially in the chronic form. Our study aimed to determine the amount of deficit of best corrected visual acuity (BCVA) and individual retinal layers, including ganglion cells, in different types of CSCR and with reference to its duration.
Methods
The retrospective analysis included 69 eyes of patients with resolved CSCR managed in Dobry Wzrok Ophthalmological Clinic between 1 January 2019 and 30 June 2022. The diagnosis of CSCR was based on the criteria outlined by the Central Serous Chorioretinopathy International Group. The analysis included data obtained from medical history, BCVA testing, and spectral domain optical coherence tomography (SD-OCT) measurements, with specific thickness values for individual retinal layers. The results were compared among affected eyes, unaffected fellow eyes, and healthy controls.
Results
BCVA values were significantly lower in acute (0.08 ± 0.12 logMAR) and chronic (0.26 ± 0.19 logMAR) cases versus controls (0.0 logMAR). The thickness of all retinal layers (central subfoveal thickness, CST; inner retina with ganglion cell complex, GC; outer retina, ORT; and photoreceptor outer segments, POS) and macular volume (MV) were significantly decreased in chronic eyes versus controls (p < 0.01). Acute eyes had significant thinning of the outer retina and POS only compared to control eyes (p < 0.01). The amount of deficit in CST, ORT, GC, and MV was strongly correlated with poorer BCVA (p < 0.001), and the deficit of CST, ORT, and GC was correlated with disease duration (p < 0.05). The subfoveal choroidal thickness was significantly greater in affected and fellow eyes versus controls (p < 0.001).
Conclusion
Damage to the outer retina and photoreceptors occurs early in the course of CSCR, with a deficit in ganglion cells noted adjunctively in chronic forms of the disease. Further studies are required to precisely determine correlation between visual loss in CSCR and deficits in individual retinal layers.
Plain Language Summary
Central serous chorioretinopathy (CSCR) is a common condition typically affecting young and middle-aged individuals. In its chronic form, it can lead to changes in retinal morphology and significant visual impairment. This disorder occurs basically in two forms: acute and chronic, depending on its duration. The study aimed to determine the amount of morphological and functional deficit occurring in the course of acute and chronic CSCR and refer it to healthy controls. The analysis of 69 resolved cases of CSCR was performed, including results of visual acuity testing and spectral domain coherence tomography (SD-OCT) measurements. Visual acuity was significantly lower in both acute and chronic groups compared to healthy control eyes, with greater deficit in the chronic group. Analysis revealed also a significant thinning of the retina in the chronic group versus control group. Chronic group demonstrated substantial loss of ganglion cells, which was not noted in acute form of the disease. Acute eyes demonstrated only a partial deficit in the outer retina, with the ganglion cell layer remaining intact. The amount of deficit of all retinal layers was strongly correlated with poorer visual acuity and disease duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Central serous chorioretinopathy is potentially damaging and may significantly affect retinal morphology and function, especially in the chronic form. |
The study aimed to determine the amount of deficit of best corrected visual acuity and individual retinal layers, including ganglion cells, in different types of central serous chorioretinopathy and with reference to its duration. |
What was learned from the study? |
Significant loss of visual acuity and reduction of the thickness of all retinal layers is noted in chronic forms of central serous chorioretinopathy, whereas inner retinal layers (ganglion cell complex) remain intact in acute forms of the disease. |
Damage to the outer retina and photoreceptors occurs early in the course of central serous chorioretinopathy, with ganglion cell deficit following in chronic forms of the disease. |
Introduction
Central serous chorioretinopathy (CSCR) is a common disorder traditionally divided into acute and chronic types depending on its duration with time point of division typically between 4 and 6 months [1, 2]. Most of the cases resolve spontaneously within a few months of disease onset without significant functional and morphological impairment; however, as many as 16% of patients develop a chronic form of the disease that might result in retinal thinning [3, 4]. Morphological deficit observed in chronic forms of CSCR can lead to significant visual impairment [5,6,7]. The origin and timing of vision loss in CSCR are still discussed and not fully understood [8]. Spectral domain optical coherence tomography (SD-OCT) testing has made it possible to evaluate individual retinal layers in the course of different retinal disorders, including CSCR [9, 10]. Because this chorioretinopathy typically presents with subretinal fluid (SRF) and detachment of photoreceptors from the retinal pigment epithelium (RPE), most of the available CSCR studies concentrate on changes to the photoreceptor outer segments layer (POS) and outer nuclear layer (ONL) comprised of photoreceptor nuclei [11,12,13,14,15,16,17,18,19,20]. Literature evaluating the impact of CSCR on more inner retinal layers, including the ganglion cell complex layer (GC), is scarce [21,22,23]. In just a few studies on that subject, SD-OCT is employed as the primary diagnostics method to evaluate inner retinal damage. Presented conclusions are not always unequivocal, and results are sometimes contradictory. Another question related to vision loss in CSCR is its correlation to the damage to specific retinal layers projected at the timeline of the disease course. Again, most of the studies analyze ONL in that respect, with analysis of inner retina rarely found in the medical literature [24,25,26,27,28]. Our study aimed to determine the amount of deficit of best corrected visual acuity (BCVA) and individual retinal layers, GC in particular, in different types of CSCR and with reference to its duration.
Methods
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by a local bioethics committee (Komisja Bioetyczna OIL in Gdańsk, approval no. KB-45/22, dated 06.09.2022).
Study Design
The study aimed to evaluate the damage to individual retinal layers after resolved CSCR. For that purpose, the results of spectral domain optical coherence tomography (SD-OCT) and visual acuity measurements were retrospectively analyzed in a group of consecutive patients with resolved CSCR and compared to healthy control eyes and the unaffected fellow eyes. Additionally, the resolved CSCR cases were divided into acute and chronic according to the duration of the symptoms, with cases lasting > 4 months considered chronic. The acute and chronic groups were compared according to age, duration of symptoms, and retinal morphology to indicate whether the amount of functional and morphological damage was related to CSCR type. Finally, the study sought to find the correlation between the disease duration and the deficit of BCVA, between the duration of the disease and intensity of morphological alterations in retinal layers and between BCVA loss and morphological damage measured by the deficits in specific retinal layers. Subgroup analysis according to applied treatment was not performed because of significant disproportions in sample sizes.
Patient Population
The retrospective analysis included 69 eyes of 69 patients who were diagnosed, observed, and/or treated for CSCR in Dobry Wzrok Ophthalmological Clinic between 1 January 2019 and 30 June 2022 and presented with or achieved complete resolution of subretinal fluid during that period. The treatment modalities used in the management of these patients were observation in 6 eyes, subthreshold micropulse laser in 55, photodynamic therapy (PDT) in 6, and classic laser photocoagulation in 3 (some of the eyes had more than one modality applied). Only the resolved cases were included in the study, that is, the cases of CSCR without any subretinal fluid on SD-OCT examination. Cases complicated by choroidal neovascularization were excluded from the study (2 cases). Diagnosis of CNV was based on results of fluorescein angiography (leakage of dye within neovascular membrane) and angio-OCT (vascular network within the CNV body visible on the scans). All the patients were interviewed for the duration and character of symptoms associated with their disorder. The duration of symptoms reported by the patients was noted in months. In cases with recurrences or subacute courses, when the exact length of the disease was difficult to assess, the duration of CSCR was considered the time from the onset of the first episode of chorioretinopathy.
The study group was compared to healthy fellow eyes and control group.
The fellow eyes were included in the analysis only if without resolved form of CSCR or any other retinal disorder that could affect retinal morphology. Active bilateral form of disease was not observed in our material; however, resolved form of CSCR was noted in 25 eyes. Exclusion of previously affected eyes was based on medical history and ophthalmological imaging. Finally, 44 eyes were eligible for the healthy fellow eyes group.
The control group consisted of 70 healthy eyes of consecutive patients examined at the clinic for the occupational program. The group was matched for age with the study group. Control eyes with any ocular pathology or amblyopia were excluded from the study.
Methods
All patients underwent the basic ophthalmological examination, which included BCVA testing, biomicroscopic assessment of the anterior and posterior segment of the eye, and intraocular pressure measurements. The diagnosis of CSCR was confirmed based on analysis of the symptoms observed at the fundus of the eye, fundus autofluorescence (FAF), fluorescein angiography findings, and SD-OCT assessment as well as indocyanine green (ICGA) images in cases suspected of polypoidal choroidal vasculopathy (PCV). The diagnostic criteria outlined by the Central Serous Chorioretinopathy International Group were used to confirm the diagnosis of CSCR [29]. The case was considered resolved if no subretinal fluid or PED was present in the posterior pole covered by the SD-OCT scan of 6 × 6 mm and previous medical history confirmed the presence of CSCR according to the described criteria.
SD-OCT measurements were performed with the use of spectral optical tomograph with angio-OCT option (REVO NX 2018, software version 11.07, Optopol Technology, Zawiercie, Poland). The device scanning speed is 110,000 A scans per second, axial resolution of − 2.8 μm digital and 5 μm in the tissue, overall scan depth from 2.8 to 6 mm in full range mode and scan range from 5 to 15 mm at the posterior pole. Retina analysis provided in the device include retinal thickness, inner retinal thickness, outer retinal thickness, retinal nerve fiber layer (RNFL) + ganglion cell layer (GCL) + inner plexiform layer (IPL) thickness, GCL + IPL thickness, RNFL thickness, retinal pigment epithelium (RPE) deformation, myoid zone/ellipsoid zone (MZ/EZ) – RPE thickness.
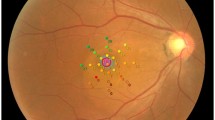
For the purpose of the study, the following SD-OCT measurements were considered: central subfoveal thickness (CST), macular volume (MV), outer retina thickness (ORT), myoid zone/ellipsoid zone—RPE distance practically corresponding to the POS, mean ganglion cell complex thickness (GC mean), minimal ganglion cell complex thickness (GC min), and subfoveal choroidal thickness (SFCT). CST value refers to the mean retinal thickness within the central circle of 1 mm diameter. MV refers to the retinal volume within the central circle of 6 mm in diameter. ORT refers to the retinal thickness measured at the central foveal point between the inner nuclear/outer plexiform (INL/OPL) layer line and the RPE. POS thickness refers to the retinal thickness measured at the central foveal point between the myoid zone/ellipsoid zone line and the RPE/Bruch’s membrane complex. GC refers to the mean thickness of the complex of the ganglion cell layer and inner plexiform layer measured at the central oval ring area (Fig. 1). GC min value refers to minimal thickness value of that complex recorded within the measured area. GC min indicates focal damage to GC layer, which can be significant, despite normal or moderately decreased mean values of GC thickness.
Manual corrections to the segmentation lines were performed in each case if needed. The SFCT was manually measured at the central foveal point with the software provided by the manufacturer of the SD-OCT equipment.
The fellow eye group and control group were subject to the same ophthalmological procedures and measurements as the study group. The baseline characteristics of the groups are presented in Table 1.
Statistical Analysis
Categorical variables are depicted using integers and percentages. Numerical traits are described with their mean, median, standard deviation, and minimum–maximum values. The normality of distribution was assessed using the Anderson-Darling test. The homogeneity of variances was appraised using the Levene test. The statistical significance of between-group differences in the numerical variables was tested using multifactor ANOVA without replication (for normally distributed variables) or the Kruskal-Wallis test (for non-normally distributed ones). Generalized linear models with robust standard errors were fitted when carrying out a multifactor model incorporating non-normally distributed traits. Spearman’s correlation coefficients were computed to expose relationships between the investigated numerical variables. The correction for multiple comparisons was applied when building and performing the multivariate models.
A level of p < 0.05 was deemed statistically significant. The minimal sufficient sample size was calculated for significance at p < 0.05, statistical power of at least 80%, and a minimal amount of parameter difference of 10% (for the GC layer); this equaled 23 eyes. With 70 eyes included in the analyzed group, the study results reached 99.9% of statistical power. All the statistical procedures and the sample size were calculated using Statistica™, release 14 (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Patients in both groups had similar ages, close to 48 years on average. The mean duration of CSCR was almost 2 years (23.6 ± 27.0 months). Patients with CSCR had a significantly lower BCVA compared to healthy controls (0.22 ± 0.19 logMAR). The thickness of individual retinal layers was also significantly lower in the study group for all SD-OCT measurements. The difference, although significant, was less prominent for inner retina, especially GC layer. Results of the descriptive statistics analysis for the study and control groups are presented in Table 2.
Analysis of the same parameters for the acute versus chronic groups revealed that the patients from the acute group were statistically significantly younger compared to the chronic group, with a difference of close to 7 years. The acute group also showed significantly lesser deficits in BCVA, CST, ORT, and minimal GC value.
Table 3 presents the differences in measured parameters between the acute and chronic CSCR groups.
Analysis of the differences between the measured parameters among the affected CSCR group, fellow eyes, and control eyes with the multifactor model applied revealed statistically significant differences in all analyzed variables (Table 4). Pairwise comparisons (Table 5) showed differences in selected pairs of variables.
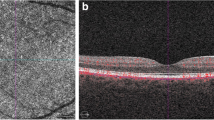
CSCR eyes had lower retinal parameters and BCVA and higher choroidal thickness compared to the eyes from the control group. Comparison of the CSCR eyes and fellow eyes proved the same, except for the GC mean value, which was not significantly thinner in the CSCR group. Nevertheless, focal GC thinning (minimal GC value) was clear in the CSCR eyes vs. fellow eyes (p = 0.019). Fellow eyes had similar retinal parameters compared to healthy controls, with the exceptions of BCVA, which was significantly better in the controls, and SFCT, which was significantly thicker in CSCR fellow eyes. Examples of GC measurements in acute and chronic cases of CSCR are presented in Figs. 2 and 3.
Comparison of the measured parameters among CSCR subtypes and controls revealed significant differences (Tables 6 and 7) in each variable. Pairwise comparisons (Table 7) revealed significantly poorer BCVA, thinner all retinal layers, and thicker choroid in chronic CSCR eyes versus controls. This comparison of acute cases proved significant thinning of the outer retinal layers and photoreceptor layer but not the CST, MV, or inner retina with GC layer. The SFCT in acute cases was also significantly thicker compared to controls. The comparison of acute versus chronic cases revealed significant thinning of the outer retina, with CST, MV, and GC preserved relatively intact.
Correlations
The correlation of disease duration and visual loss was significant for all cases of CSCR (r = 0.32, p < 0.001) but not for the acute and chronic subtypes of CSCR (acute: r = 0.30, p = 0.321; chronic: r = 0.27, p = 0.071).
The results of the analysis of the correlations between the BCVA and disease duration and the loss of retinal layers are presented in Table 8. Loss of visual acuity was strongly associated with the CST, ORT, and MV thinning and loss of ganglion cells but not necessarily with the photoreceptor outer segment deficit. The visual deficit was not correlated with choroidal thickness either. Similarly, a longer disease duration had a strong relationship to the loss of CST, ORT, and GC but not the outer photoreceptor segment loss or increased choroidal thickness.
Discussion
Chronic form of CSCR causes alterations in retinal morphology that result in visual impairment. In most of the studies this deficit was attributed to the damage to outer retinal layers; nevertheless, direct proportional correlation between retinal thinning and functional deficit was not found [4, 5]. Exploration of that subject raises the question of whether the significant decline of BCVA in long-lasting cases of CSCR could be attributed to deficits in GC layer related to outer retinal thinning.
In our study, analysis of the whole CSCR cohort and its comparison to the control group confirmed damage to all retinal layers in the course of this chorioretinopathy. Changes in retinal morphology were strongly correlated to the decrease in BCVA. This refers to thinning of the CST, MV, ORT, and inner retina with GC that is observed in the course of CSCR. Loss of CST, ORT, and GC is also related to the duration of the disease. These data are consistent with other studies, but to our knowledge, this is the first such large analysis of GC changes in CSCR.
Most of the studies on retinal morphology alterations in the course of CSCR have concentrated on compromised ONL and POS layers [11,12,13,14,15,16,17,18,19,20, 24,25,26,27,28]. ONL and POS thinning is consequently reported in chronic cases of CSCR and usually correlated with BCVA deficit [11,12,13,14,15,16,17,18, 20]. Interestingly, Ooto et al. [19] reported ONL thinning but not POS thinning in 36 eyes with CSC versus healthy controls. Acute cases are analyzed less often, but usually, some deficit in the outer retinal layers is reported. In a large study of 60 eyes, Hata et al. observed ONL thinning beginning a month after the onset of the disease and correlated to BCVA loss and duration of CSCR [28]. Similar findings and correlations were presented by Ozdemir et al., who reported ONL deficit as early as 3 months after the onset [25]. On the other hand, Yu et al. did not observe significant ONL thinning versus controls in a comparative study of 29 acute cases, but distinct POS alterations were noted [18].
The GC layer is rarely observed in ophthalmic research on CSCR. The results of Han et al. [23] are consistent with our findings. The authors analyzed 34 spontaneously resolved cases of CSCR of short duration (< 6 months). They reported significant ONL and CST thinning but an unaffected GC layer. Nam et al. observed transient GC thinning in the acute phase of CSCR, but deeper analysis of that phenomenon showed that this is due to segmentation errors in automated analysis, not real damage [21]. In an analysis of 35 eyes, Demirok et al. [22] showed deficits in the GC layer in both acute and chronic forms of CSCR, with prominent changes attributed to long-lasting cases and only sectoral changes in short-lasting cases. Notably, analysis of retinal layer deficits based solely on resolved eyes with CSCR has been performed in only a few studies [17, 23, 25, 27, 28]. Generally, outer retinal thinning is reported in this research, correlated with BCVA deficit. The morphological deficit reported in our study was reflected in visual acuity loss. With the final BCVA at 0.26 ± 0.19 logMAR, chronic patients lost around four Snellen lines during the course of the disease (30 months on average), whereas in acute cases (2 months on average), the loss was less than two Snellen lines, and the final BCVA equaled 0.08 ± 0.12 logMAR. These findings are similar to those presented in previous work by the same authors [5, 30].
The SFCT in CSCR patients is also significantly thicker than in control eyes, supporting the pachychoroid etiopathogenesis of this disease [31]. Interestingly, choroidal thickness is also increased in the fellow unaffected eyes. This finding is consistent with the results of other studies [32,33,34]. This morphological feature possibly puts the fellow eyes of CSCR patients at risk of developing CSCR. That assumption is plausible given the data, which show that CSCR affects both eyes of the same patient in as many as 30–40% of cases [35, 36].
The evaluation of the results of our study in the subgroups according to disease duration (acute and chronic) provides information on the possible scenario of retinal cellular damage in CSCR. It seems that early deficit occurs in the outer retinal layers because it is visible in the thinning of the whole outer retina and specifically the layer of photoreceptor segments. On the other hand, loss of ganglion cells is distinct in chronic cases. Comparison of the GC of chronic versus acute cases shows a significant difference in minimal GC values, which can be explained by the focal loss of ganglion cells in long-lasting cases of CSCR.
Relationship between morphological deficits and BCVA needs further investigation. We proved a progressive loss of individual retinal layers and BCVA with disease duration; however, the deficit of which particular layers underlies significant visual impairment in CSCR is yet to be determined. It is plausible that in long-lasting cases the progression of morphological deficit toward the inner retina is responsible for visual deterioration; nevertheless, that theory should be confirmed in functional tests, such as electroretinography.
Among electrophysiological testing relevant to GC assessment, pattern electroretinography (PERG) could provide valuable information as its N95 wave refers solely to GC function and P50 wave to combined photoreceptor (30%) and GC (70%) function. Unfortunately electrophysiological testing in CSCR is not performed routinely, and available research is scarce. Nevertheless, it provides results proving GC involvement in CSCR. Goyal et al. analyzed results of PERG examination in acute CSCR cases and reported prominent reduction of P50 persistent after resolution of SRF and lesser N95 reduction [37]. That finding is consistent with our results and proves mainly photoreceptor damage in acute CSCR cases and minor GC involvement. On the other hand, analysis of acute CSCR cases by Doguizi et al. showed decreases in both P50 and N95 waves that improved but still persisted after resolution of CSCR [38]. That result indicates GC deficit even in acute CSCR. Unfortunately, our literature search did not reveal any PERG employing studies of chronic CSCR.
Results of our study enable drawing a possible scenario for CSCR-related retinal damage (Table 9). Patients with the short-lasting form of the disorder present with just mild BCVA loss, outer retinal layer deficit, and intact inner retina. Chronic patients lose outer retinal layers and additionally ganglion cell layers and show significant visual impairment.
Detecting GC loss in CSCR might encourage further research on the subject, especially on the relationship between the leak type and location visible on fluorescein angiography and location of GC deficit. While GC measurements are performed in sectors, as presented in Fig. 1, a possible relation to ganglion cell damage and leak is to be determined. Previous research related the POS thinning to the location of the leakage point on fluorescein angiography [39, 40]; thus, a relationship between inner retina damage and leakage cannot be excluded.
Strengths and Limitations of the Study
The present study is among the largest, considering the number of cases, enabling reliable statistical analysis. Moreover, this is the largest study so far analyzing alterations of the GC layer in the course of CSCR. We believe that another strength of our study is the uniformity of cases: only the resolved ones were included in the study. Measurements of retinal layers in the active phase of CSCR are difficult because of retinal edema and might bear segmentation errors despite manual corrections. Additionally, some studies show an improvement in ONL thickness after treatment (usually with PDT) [15, 41]. This phenomenon is not necessarily related to cellular response but rather to the elimination of the pressure on retinal layers elicited by the SRF. Thus, ONL thinning, reported in many studies in the active phase of the disease, might not actually reflect a permanent deficit. In our opinion, only the analysis of resolved cases can provide consistent data on the changes in retinal morphology and visual impairment in the course of CSCR. Another strength of the study is that the control group was matched according to age with the affected cohort. Comparative study allows quantifying the actual damage to retinal layers in the course of CSCR.
The main limitation of the study is its retrospective design. In some cases, it was impossible to precisely indicate the time point of the resolution of SRF and the timespan between this moment and the acquisition of the SD-OCT scans.
The chronic and acute groups are uneven in number with the acute group significantly less numerous, what might produce a certain bias in statistical comparisons. In real life acute cases do not occur less frequently than the chronic ones; however, the chronic ones are usually diagnosed and treated at ophthalmic clinics, while acute cases are often not recorded at all. That results in sample size imbalance between acute and chronic groups if records of consecutive patients are analyzed. Hence, such disproportion is difficult to avoid in research studies.
Another limitation could be related to treatment that was applied in 91% of studied cases and theoretically could influence retinal morphology. Nevertheless, the vast majority of patients were treated with subthreshold micropulse laser, which, if carefully performed, according to current knowledge, does not leave any significant trace on the retina [42, 43]. PDT, which was performed in six eyes, potentially affects choroid and outer retinal layers, but not inner retina, as was proved in one study [44]. Finally, classic laser photocoagulation, applied in a small number of eyes (3) with extrafoveal leakage, typically caused only focal damage outside the foveal center, usually limited to the RPE and outer retinal layers [45]. Thus, we believe that treatment-related bias of the results in the study group, especially for GC layer thickness, can be considered insignificant.
The study lacks electrophysiological examinations that could provide information on the function of specific retinal cells. Potential correlation of results of such tests with retinal morphology could provide solid knowledge on functional impact of our findings.
Conclusions
Our study proves that CSCR is damaging in all its forms, particularly chronic. Persistent chorioretinopathy results in significant deficits in all retinal layers, including ganglion cells. Damage to the GC layer typically occurs later in the course of the disease and is strongly correlated with poor visual acuity.
The relationship between visual loss and damage to individual retinal layers needs further research. The present study, performed on a relatively large number of resolved cases, provides grounds for studies that would precisely evaluate the role of ganglion cell loss in visual impairment attributed to some cases of CSCR. It is possible that lesions to GC adjunctive to an earlier occurring outer retinal layer deficit are responsible for progressive visual loss. Nevertheless, this theory needs more research employing electrophysiological testing of the retina.
Approaching patients with CSCR often requires the evaluation of prognosis for the preservation or improvement of visual acuity. If results of our study are confirmed in subsequent research including electrophysiological testing, it is possible that the evaluation of GC may provide information on the possible BCVA loss in the course of CSCR. Finding focal deficits in GC at the early stage of disease might indicate poor visual prognosis after resolution of SRF. Because GC is measured in the area outside the central 2 mm (Fig. 1), it is usually not significantly affected by the pressure from SRF, which is greatest at the SRF cavity apex, typically located close to the foveal center. Thus, the obtained values are more likely to reflect the actual state of this layer, unlike automated measurements of ONL. Nevertheless, GC measurement in the active form of the disease, with SRF present, might require manual corrections to segmentation lines because segmentation errors of the SD-OCT are more common in edematous retina. We believe that it is worth making efforts to evaluate the GC in every problematic CSCR patient because this can provide information on the prognosis and speed the initiation of more radical treatment (e.g., PDT). The procedure is likely to become easier and less time-consuming with machine learning employed in modern SD-OCT devices.
References
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, Jaisser F, Behar-Cohen F. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82–118.
Arora S, Chhablani J, Hariprasad SM. Acute versus chronic central serous chorioretinopathy: classification strategies and management. Ophthalmic Surg Lasers Imaging Retina. 2022;53(8):418–20.
Daruich A, Matet A, Marchionno L, De Azevedo JD, Ambresin A, Mantel I, Behar-Cohen F. Acute central serous chorioretinopathy: factors influencing episode duration. Retina. 2017;37(10):1905–15.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Grzybowski A. Functional and morphological outcome in patients with chronic central serous chorioretinopathy treated by subthreshold micropulse laser. Graefes Arch Clin Exp Ophthalmol. 2017;255(12):2299–306.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Kneba M, Grzybowski A. Impairment of visual acuity and retinal morphology following resolved chronic central serous chorioretinopathy. BMC Ophthalmol. 2019;19(1):160.
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, Yannuzzi NA, Patel S, Chen KC, Yu S, Stoffels G, Spaide RF, Freund KB, Yannuzzi LA. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126(4):576–88.
Breukink MB, Dingemans AJ, den Hollander AI, Keunen JE, MacLaren RE, Fauser S, Querques G, Hoyng CB, Downes SM, Boon CJ. Chronic central serous chorioretinopathy: long-term follow-up and vision-related quality of life. Clin Ophthalmol. 2016;20(11):39–46.
Gawęcki M, Jaszczuk A, Grzybowski A. Short term presence of subretinal fluid in central serous chorioretinopathy affects retinal thickness and function. J Clin Med. 2020;9(11):3429.
Kałuzny JJ, Szkulmowska A, Bajraszewski T, Szkulmowski M, Kałuzny BJ, Gorczyńska I, Targowski P, Wojtkowski M. Retinal imaging by spectral optical coherence tomography. Eur J Ophthalmol. 2007;17(2):238–45.
Iida T, Hagimura N, Sato T, Kishi S. Evaluation of central serous chorioretinopathy with optical coherence tomography. Am J Ophthalmol. 2000;129(1):16–20.
Ho M, Kwok SHW, Mak ACY, Lai FHP, Ng DSC, Chen LJ, Iu LP, Young AL, Brelen M. Fundus autofluorescence and optical coherence tomography characteristics in different stages of central serous chorioretinopathy. J Ophthalmol. 2021;31(2021):6649064.
Esen E, Sizmaz S, Demircan N. Microstructural changes after half-dose photodynamic therapy in patients with chronic central serous chorioretinopathy. Photodiagnosis Photodyn Ther. 2021;35: 102347.
Uzlu D, Erdöl H, Kola M, Özbay AD. The efficacy of subthreshold micropulse yellow laser (577 nm) in chronic central serous chorioretinopathy. Lasers Med Sci. 2021;36(5):981–8.
Torres-Costa S, Penas S, Cerqueira AR, Brandão E, Carneiro Â, Rocha-Sousa A, Falcão-Reis F. Long term outer retinal changes in central serous chorioretinopathy submitted to half-dose photodynamic therapy. Photodiagnosis Photodyn Ther. 2021;34: 102235.
Yu J, Jiang C, Xu G. Correlations between changes in photoreceptor layer and other clinical characteristics in central serous chorioretinopathy. Retina. 2019;39(6):1110–6.
Matušková V, Vysloužilová D, Uher M. Half-fluence photodynamic therapy for chronic central serous chorioretinopathy: predisposing factors for visual acuity outcomes. Semin Ophthalmol. 2018;33(5):690–9.
Hasegawa T, Okamoto M, Masuda N, Ueda T, Ogata N. Relationship between foveal microstructures and visual outcomes in eyes with resolved central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):343–50.
Yu H, Xia GY, Gao MH, Yu J, Zhang YY. Morphologic changes in acute central serous chorioretinopathy evaluated by spectral domain optical coherence tomography. Zhonghua Yan Ke Za Zhi. 2011;47(6):508–15.
Ooto S, Tsujikawa A, Mori S, Tamura H, Yamashiro K, Yoshimura N. Thickness of photoreceptor layers in polypoidal choroidal vasculopathy and central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248(8):1077–86.
Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008;145(1):162–8.
Nam KY, Kim JY. Serous retinal detachment causes a transient reduction on spectral domain OCT estimates of ganglion cell layer thickness. Optom Vis Sci. 2019;96(3):156–63.
Demirok G, Kocamaz F, Topalak Y, Altay Y, Sengun A. Macular ganglion cell complex thickness in acute and chronic central serous chorioretinopathy. Int Ophthalmol. 2017;37(2):409–16.
Han KJ, Kim HJ, Woo JM, Min JK. Comparison of retinal layer thickness and capillary vessel density in the patients with spontaneously resolved acute central serous chorioretinopathy. J Clin Med. 2020;10(1):45.
Nakamura T, Ueda-Consolvo T, Oiwake T, Hayashi A. Correlation between outer retinal layer thickness and cone density in patients with resolved central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2347–54.
Ozdemir I, Eren A, Ersöz G. Outer nuclear layer thickness at the central fovea relation with symptom duration in central serous chorioretinopathy. Int Ophthalmol. 2019;39(6):1323–8.
Ozdemir O, Erol MK. Morphologic changes and visual outcomes in resolved central serous chorioretinopathy treated with ranibizumab. Cutan Ocul Toxicol. 2014;33(2):122–6.
Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009;148(1):105-10.e1.
Hata M, Oishi A, Shimozono M, Mandai M, Nishida A, Kurimoto Y. Early changes in foveal thickness in eyes with central serous chorioretinopathy. Retina. 2013;33(2):296–301.
Chhablani J, Cohen FB, Central Serous Chorioretinopathy International Group. Multimodal imaging-based central serous chorioretinopathy classification. Ophthalmol Retina. 2020;4(11):1043–6.
Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, Kneba M, Grzybowski A. Transfoveal micropulse laser treatment of central serous chorioretinopathy within six months of disease onset. J Clin Med. 2019;8(9):1398.
Hanumunthadu D, van Dijk EHC, Dumpala S, Rajesh B, Jabeen A, Jabeen A, Ansari M, Mehta P, Shah S, Sarvaiya C, Meyerle C, Wu L, Banker A, Boon CJ, Chhablani J. Evaluation of choroidal layer thickness in central serous chorioretinopathy. J Ophthalmic Vis Res. 2019;14(2):164–70.
Chung YR, Kim JW, Kim SW, Lee K. Choroidal thickness in patients with central serous chorioretinopathy: assessment of Haller and Sattler Layers. Retina. 2016;36(9):1652–7.
Kim RS, Jain RR, Brown DM, Bretana ME, Kegley EN, Singer MA, Aragon AV, Schefler AC. Elevated choroidal thickness and central serous chorioretinopathy in the fellow eyes of patients with circumscribed choroidal hemangioma. Ocul Oncol Pathol. 2018;4(6):375–80.
Arora S, Pyare R, Sridharan P, Arora T, Thakar M, Ghosh B. Choroidal thickness evaluation of healthy eyes, central serous chorioretinopathy, and fellow eyes using spectral domain optical coherence tomography in Indian population. Indian J Ophthalmol. 2016;64(10):747–51.
Gäckle HC, Lang GE, Freissler KA, Lang GK. Chorioretinopathia centralis serosa. Klinische, fluoreszeinangiographische und demographische Aspekte [Central serous chorioretinopathy. Clinical, fluorescein angiography and demographic aspects]. Ophthalmologe. 1998;95(8):529–33.
Bujarborua D, Chatterjee S, Choudhury A, Bori G, Sarma AK. Fluorescein angiographic features of asymptomatic eyes in central serous chorioretinopathy. Retina. 2005;25(4):422–9.
Goyal JL, Ghosh B, Sangit V, Kumar S, Jain P, Veerwal V, Arora R. Pattern ERG in central serous retinopathy. Doc Ophthalmol. 2015;130(2):141–7.
Doguizi S, Sekeroglu MA, Ozkoyuncu D, Yilmazbas P. Pattern electroretinography in patients with unilateral acute central serous chorioretinopathy. Clin Exp Optom. 2020;103(5):656–62.
Maltsev DS, Kulikov AN, Chhablani J. Topography-guided identification of leakage point in central serous chorioretinopathy: a base for fluorescein angiography-free focal laser photocoagulation. Br J Ophthalmol. 2018;102(9):1218–25.
Maltsev DS, Kulikov AN, Chhablani J, Kutik DS, Arsenov NV. Opticheskaia kogerentnaia tomografiia v diagnostike i lechenii tsentral’noĭ seroznoĭ khorioretinopatii [Optical coherence tomography in diagnostics and treatment of central serous chorioretinopathy]. Vestn Oftalmol. 2018;134(6):15–24 (Russian).
Yu J, Lei Y, Chang Q, Xu G, Ye X, Li L, Jiang C. The relationship between foveal outer nuclear layer thickness in the active and resolved phases of central serous chorioretinopathy treated with half-dose photodynamic therapy. BMC Ophthalmol. 2019;19(1):84.
Chang DB, Luttrull JK. Comparison of subthreshold 577 and 810 nm micropulse laser effects on heat-shock protein activation kinetics: implications for treatment efficacy and safety. Transl Vis Sci Technol. 2020;9(5):23.
Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina. 2014;34(10):2010–20.
Agharokh S, Akhlaghi MR, Kianersi F, Dehghani A, Jahanbani-Ardakani H, Abtahi SH. Short-term effects of photodynamic therapy on segmentation of retinal layers in central serous chorioretinopathy. Adv Biomed Res. 2021;26(10):34.
Wood JP, Shibeeb O, Plunkett M, Casson RJ, Chidlow G. Retinal damage profiles and neuronal effects of laser treatment: comparison of a conventional photocoagulator and a novel 3-nanosecond pulse laser. Invest Ophthalmol Vis Sci. 2013;54(3):2305–18.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Conceptualization, methodology, validation, review and editing: Maciej Gawęcki, Andrzej Grzybowski; software, validation, investigation, formal analysis, resources, data curation, writing—original draft preparation: Maciej Gawęcki. All authors have read and agreed to the published version of the manuscript.
Disclosures
Maciej Gawęcki and Andrzej Grzybowski have nothing to disclose.
Compliance with Ethics Guidelines
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by a local bioethics committee (Komisja Bioetyczna OIL in Gdańsk, approval no. KB-45/22, dated 06.09.2022). Informed consent was obtained from all the subjects involved in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gawęcki, M., Grzybowski, A. Ganglion Cell Loss in the Course of Central Serous Chorioretinopathy. Ophthalmol Ther 12, 517–533 (2023). https://doi.org/10.1007/s40123-022-00625-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00625-5