Abstract
Adding organic amendments to mining tailings to ameliorate extreme conditions that limit plant growth is a common practice in reclamation projects; still, the impact on microbial activity is not commonly considered. This work aimed to explore the use of the metabolic quotient and specific enzymatic activity as indicators of microbial carbon use efficiency in response to adding organic amendments to mining tailings. An experiment in vitro on adding organic amendments: compost, biochar, a mixture of them, and no addition on mining tailing from Taxco, Guerrero, Mexico, was established. Carbon mineralization, microbial biomass, and the enzymatic activity of β-glucosidase, phosphatase, polyphenol oxidase, and dehydrogenase were measured, while specific enzymatic activity and metabolic quotient were calculated. The results showed that microbial activity increased by adding all organic amendments in the following order: compost > mixture > biochar. In the treatment with the addition of compost, we observed a higher carbon mineralization and a greater enzymatic activity. The treatment with adding biochar showed similarities with the control treatment in parameters related to carbon dynamics, such as β-glucosidase, dehydrogenase, and carbon mineralization. This reflects microorganisms’ trade-off between investing energy in searching for resources or using them to improve their biomass clearly to view the specific enzymatic activity and metabolic quotient indicators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mining is an essential economic activity, particularly in México, which has a presence in 26 of the 32 states (Cárdenas 2013), and their wastes represent 65% of the total industrial waste (Mejía et al. 1999). The primary residues this activity generates are sediments deposited in open sites, sometimes confined, called tailings. These residues are characterized by having high concentrations of potentially toxic elements (PTE), such as Zn, Hg, Pb, Cd, Fe, Cu, and Mn, among others; pH in a wide range (2–9); low concentration of organic carbon (C) and essential nutrients (e.g., N, P, K, Mg) (Ye et al. 2002; Mendez et al. 2007; Mendez and Maier 2008). Silt-sized sand particles are the dominant, lack structure and aggregation, and regularly are uncovered by vegetation. In tailing mining, the microbial activity is dominated by autotrophic bacteria that oxidize iron and sulfur, promoting the production of acid leachates, while the heterotrophic microorganisms are restricted by organic matter scarcity (Moynahan et al. 2002; Li and Huang 2015; Lottermoser 2010).
To ameliorate the extreme conditions that limit plant growth, adding organic residues is a common practice in reclamation projects; biochar and compost are two organic amendments of significant interest for application in mining tailings. Biochar is a charcoal produced by pyrolysis of organic materials, which generates condensation of organic molecules to give rise to aromatic compounds, increasing as the temperature increases during its formation (Kleber 2010; Lehmann and Joseph 2015). Biochar has been proposed to improve mining tailings because it buffers acid pH, immobilizes toxic elements, and improves moisture retention, promoting plant establishment (Fellet et al. 2011; Kelly et al. 2014; Paz-Ferreiro et al. 2014; Hmid et al. 2015). The maximum temperature raised during their production defines the chemical composition, as aromaticity, of biochar and the influence on microbial activity; for example, the biochar produced over 700 °C inhibits microbial communication compared with biochar produced at 300 °C with possible negative consequences on nitrogen fixation, enzymatic activity, and immobilization of C in microbial biomass (Masiello et al. 2013); these could result in limited decomposition of organic matter and recycling of nutrients (Lehmann et al. 2011; Paz-Ferreiro et al. 2012; Gul et al. 2015; Foster et al. 2016). The compost is produced through aerobic biodegradation of different organic residues; it has been observed that its addition in mining tailings favors the regulation of temperature, moisture retention, structure, and increased availability of nutrients (Cambardella et al. 2003; de Araújo et al. 2010), which in turn promote microbial growth and activity (Saison et al. 2006; Fini et al. 2016). In particular, the compost has positive effects on heterotrophic microbial activity, as it has been observed that its addition increases microbial biomass and the activity of enzymes such as dehydrogenase, β-glucosidase, arylsulfatase, phosphatase, cellulase, protease, and urease (de Mora et al. 2005; Alvarenga et al. 2008; de Varennes et al. 2010; de Varennes and Cunha-Queda 2014), which will promote the depolymerization and mineralization of organic matter and with it the availability of nutrients (Lehmann and Joseph 2015).
Particularly, in mining tailings it has been concluded that enzymes activity such as dehydrogenase, phosphatase, urease, protease, and arylsulfatase has been reduced in the presence of high concentrations of PTE (Moynahan et al. 2002; Dai et al. 2004; Aponte et al. 2022) but that adding any organic amendment applied to mining tailings will show a positive effect on microbial activity (Kandeler et al. 1996; Chen et al. 2005; Malley et al. 2006). Some authors have observed that C mineralization and enzymatic activity are sensible indicators that will record immediate changes in the properties of amended soils and sediments (Acosta-Martínez and Tabatai 2000; Ekenler and Tabatabai 2003). Still, sometimes the interpretation of C mineralization and enzymatic activity will be unclear because it strongly depends on factors such as the size, commonly measured as microbial C concentration, and metabolic specialization of the microbial community (Chávez-Vergara et al. 2014). These functional parameters of microbial activity are sensitive to the chemical composition of resource availability, but they can show convergency in a different way related to the size of microbial communities and nutrient use efficiency.
For example, in the face of the high availability of high labile compounds that contain high N or P, low molecular weight, are rapidly hydrolyzable, and have non-hydrophobic characteristics, the microbial biomass grows rapidly and releases relatively low enzymes to the environment, are reflected as high enzyme activity. On the contrary, when the labile resources are limited, the inhibited microbial biomass releases more enzymes producing high enzyme activity. This will be improved using the metabolic coefficient (qCO2) and specific enzymatic activity (SEA). The first is related to microbial C mineralization. The second to enzymatic activity, these indicators of microbial activity clarify the interpretation of changes in microbial metabolic efficiency in the context of “decisions” related to the use of C for growth (incorporate in biomass) or investing C to obtain energy (mineralization) and nutrient acquisition (enzymatic activity). Hence weighting the microbial activity by microbial biomass as qCO2 and SEA will be suitable indicators of microbial C use efficiency changes by adding quality organic amendments in mining tailings. The qCO2 and SEA have been shown to help detect metabolic adjustments influenced by management, as by addition of organic amendments, in agroecosystems (Raiesi and Beheshti 2014; Arcand et al. 2017; Chavarria et al. 2018; Rodríguez-Bustos et al. 2022) and by species composition in forest ecosystems (Chávez-Vergara et al. 2018; Choreño-Parra et al. 2022) showing that when the microbial community are less stressed and had a C use more efficient these two indicators show lower values than in opposed conditions. Of these two indicators, only the qCO2 has been used in mining tailing studies as a microbial activity parameter (Gul et al. 2015) but not the SEA, which will be useful for evaluating microbial C use efficiency related to the organic matter decomposition process. We hypothesize that the adjustments in microbial C use efficiency produced by adding contrasting organic amendments be easy to identify using qCO2 and SEA as microbial activity functional indicators. This work carried out in 2021 aimed to explore the use of qCO2 and SEA as indicators of the efficiency of microbial C in response to adding organic amendments to mine tailings located in Taxco de Alarcon, Guerrero, Mexico. Mining tailings were amended with compost, biochar, and a mixture for this. Subsequently, they were incubated in vitro for 108 days and contrasted against mining tailing non-amended to evaluate the effect of organic amendments on microbial metabolic adjustments related to C use efficiency.
Materials and methods
Study area
The sampling site was the tailings of “El Fraile” inactive mine, located approximately 12 km southwest of Taxco de Alarcón, in the state of Guerrero, Mexico (Fig. 1), which contains mining tailings that originated between 1940 and 1970 as a result of the exploitation of Ag, Zn and, Pb (Talavera et al. 2006; Romero et al. 2008). To establish the experiment, a representative sample of 30 kg of mining tailings from the “El Fraile” mine was collected in January 2016 by the staff of the Environmental Geochemistry Laboratory, coordinated by Dr. Francisco Martín Romero.
Microcosm experiment
Substrates
Four substrates were made for the experiment with the sample of mining tailings. These were: residues without amendment addition (NA), residues amended with biochar (BC), residues amended with compost (CM), and residues amended with equal proportions of biochar and compost (MX). In treatments with amendments, we add the equivalent to reach 4% in mass; this simulates the mean of soil organic matter in adjacent soils to mine tailings (data not shown). The biochar (total organic carbon 79%, total nitrogen 0.08%) used was produced by coconut fiber after pyrolysis to obtain synthesis gas at > 800 °C for electricity generation; the Green to Energy (G2E) company donated the BC. The compost (total organic carbon 21%, total nitrogen 1.4%) was made from municipal organic waste donated by the Secretariat of the Environment of Mexico City (SEDEMA). Before starting the experiments, each of the substrates was homogenized. Subsequently, the total organic C (TOC) concentration was determined by coulometric detection using a CM150 carbon analyzer (TC/TIC/TOC) UIC Inc, the pH in water 10:1 ratio using a Versa Star pH meter Thermo Scientific. The concentration of heavy metals was analyzed using a Portable X-ray Fluorescence analyzer NITON XL3t, according to the EPA 6200 method (US-EPA 2006) using certified calibration standards (Romero et al. 2008; Martinez-Jardines et al. 2012; Chávez-Vergara et al. 2015;) (Table 1).
Experimental design
Seventy-five grams of each substrate with ten replicates was placed in polyvinyl chloride (PVC) tubes of 15 × 5 cm (height × diameter) adapted with a nylon mesh with 100 mesh (0.149 mm) opening at the lower end. Subsequently, treatments were brought to field capacity by capillarity with deionized water and incubated in the dark at 25 °C for 72 h. After that time, five samples of each treatment were randomly collected and processed for the initial analysis (T0). In comparison, the other five samples of each treatment were placed in hermetic glass flasks with a capacity of 1L and incubated at 25 °C in the dark for 108 days (T108), with periodic quantification of CO2, as will be described later (Fig. 2). The experiment was conducted to reach stable microbial conditions reflecting the start of the stable phase, defined as a change of less than 5% in CO2 emitted by microbial respiration, that was reached to 108 days (Fig. S1).
Laboratory analyses
Microbial carbon
The C immobilized in microbial biomass or microbial C (Cmic) was determined at the beginning (T0) and the end of the experiment (T108) and was extracted using the fumigation method with chloroform (CHCl3) (Vance et al. 1987; Chávez-Vergara et al. 2016). Two subsamples of each treatment (20 g) were taken; one was fumigated in an atmosphere saturated with chloroform for 30 min. Both subsamples were incubated for 24 h at 25 °C. The extraction with K2SO4 0.5 N was carried out in a 1:4 ratio (mass/volume) under agitation for 30 min at 120 rpm and filtered through Whatman #42 paper filter. The determination of total C concentration was carried out on a Shimadzu C analyzer, and the final concentration (expressed in µgC g−1) was determined from the difference of the concentration of Cmic in fumigated samples minus the non-fumigated samples and divided by the extraction coefficient KEC 0.45 (Joergensen and Mueller 1996). Since the pH of the samples was not higher than 8.5, the presence of carbonates was neglected, so total C is assumed to be equivalent to organic C. (Chávez-Vergara et al. 2015).
Enzymatic activity
In T0 and T108 samples, the activity of four exoenzymes was determined: β-glucosidase (BG), polyphenol oxidase (PPO), and phosphomonoesterase (PHO) and dehydrogenase (DHG) as a measure of microbial metabolic activity (Kandeler 2015), according to Chávez-Vergara et al. (2014, 2018).
The extraction of enzymes was carried out with 30 mL of modified universal buffer (MUB) to 2 g of the fresh substrate to quantify the exoenzyme activity. Subsequently, an aliquot was taken for each of the activities to be evaluated from the extract and incubated at 25° C for 2 h with the specific substrate for each enzyme, pNP-glucopyranoside for BG, pNP-phosphate for PHO and ABTS (2, 20-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) for PPO. Subsequently, they were centrifuged at 10,000 rpm for 2 min. An aliquot of 0.75 mL was taken to which 0.075 mL of 1N NaOH and 3 mL of deionized water were added, except for samples for determining the PPO activity. Enzyme activities were determined by absorbance in a Thermo Spectronic model 4001/4 spectrophotometer at 410 nm for BG and PHO and 460 nm for PPO. The activity of the enzymes was expressed in mmol pNP g−1 h−1.
For determination of DHG activity, the method is based on the reduction of triphenyl tetrazolium chloride (TTC) to triphenyl formazan (TPF) after incubation. Two grams of each fresh substrate was weighed to which 2 mL 0.1% TTC solution (mass/volume) in Tris–HCl buffer was added (Alef and Nannipieri 1995; Chávez-Vergara et al. 2018). The samples with the substrate were incubated in the dark and shaken at 25° C for 24 h, to add 8 ml of acetone later and shaken for 2 h at 120 rpm. Subsequently, the samples were centrifuged at 3,000 rpm for 2 min, and the supernatant was measured in a Thermo Spectronic model 4001/4 spectrophotometer set at 560 nm. DHG activity was expressed in µg TPF g−1 d−1.
C potential mineralization
In each 1L hermetic flask, together with the PVC tubes, a vial with 10 ml of 0.5 N NaOH was placed, which functioned as a trap to absorb CO2. The vial with NaOH was replaced every seven days to quantify captured CO2 until the end of the experiment. Once the vial with NaOH had been removed, 5 ml of 1N BaCl2 and two drops of 1% phenolphthalein with ethanol were added and titrated with 0.5N HCl. The HCl expenditure is proportional to the amount of CO2 precipitated. With these data, the cumulative mass (T108) of C in the form of CO2 produced in each treatment (µgC-CO2 g−1) was calculated (Coleman et al. 1977; Chavez-Vergara et al. 2014).
Metabolic quotient and specific enzymatic activity
The qCO2 (1) and SEA (2) were calculated by dividing the accumulated production of C-CO2 and the concentration of each of the enzymes, respectively, by the concentration of C microbial biomass (Cmic) (Waldrop et al. 2000; Vinhal-Freitas et al. 2010; Chávez-Vergara et al. 2014).
where A is the C-CO2 production of each treatment expressed in µgC g−1 and B is the concentration of any enzyme (BG, PHO, PPO, and DHG).
Data analysis
A repeated-measures analysis of variance (RMANOVA) was performed on Cmic, enzyme activity, and specific enzyme activity data. This was done to determine differences among treatments at the beginning to the end of the experiment, as a factor “between” the four treatments (NA, CP, BC, MX) and as a factor “within” the initial and final time of the experiment. The data of C mineralization and qCO2 were used in a one-way ANOVA; if p ≤ 0.05, a post hoc Tukey HSD analysis was performed for both analyses. All analyses were performed in Statistica v.13 software by StatSoft.
Results and discussion
Microbial carbon
The concentration of Cmic presented significant differences in the interaction between the analyzed factors (F = 6.85, p < 0.01). At the beginning of the incubation (T0), no significant differences were observed in Cmic between treatments. However, after 108 days of incubation (T108), Cmic showed significant differences between treatments in the following order CM = MX > BC > NA. Regarding the effect of incubation time, there were significant differences only in CM and MX at the end of the incubation (T108) compared to the beginning of the incubation (T0). The Cmic increased by 80 and 70%, respectively (Fig. 3).
Mean (± standard error) of microbial biomass (Cmic) for RMANOVA analysis. NA No amendment, CM compost, BC biochar, MX biochar and compost. Different lowercase letters represent significant differences (p < 0.05) in factor “time” and different capital letters represent significant differences (p < 0.05) between treatments
Enzymatic activity
The interaction between dates and treatment showed to be significant in the activity of BG (F = 7.06, p < 0.01), PPO (F = 7.95, p < 0.01), and PHO (F = 6.09, p = 0.01), while in DHG both factors were significant independently. At the beginning of the incubation (T0), we observed no significant differences between treatments and BG activity. Still, in T108, the treatments were significantly different because NA was higher and different from the other treatments, while CM presented the lowest value, which differed from NA and BC. BG activity showed differences between dates only in NA and BC; in T108, it was about three- and two-times higher concerning T0, respectively (Fig. 4a). The PPO activity did not show differences in T0. However, there tended to be higher in NA and lower in MX. On the other hand, PPO at T108 showed significant differences in treatments in the following order CM = BC > NA = MX. Only in CM and BC did PPO activity increase at T108 concerning T0, as it was approximately 3.5-fold higher in both cases (Fig. 4b).
Mean (± standard error) of enzymatic activity: a β-glucosidase (BG), b polyphenol oxidase (PPO) c phosphatase (PHO), d dehydrogenase (DHG) for RMANOVA analysis. NA No amendment, CM compost, BC biochar, MX biochar and compost. Different lowercase letters represent significant differences (p < 0.05) in factor "time" and different capital letters represent significant differences (p < 0.05) between treatments
The activity of PHO in T0 showed differences because NA had the highest activity compared to all other treatments. At the same time, in T108, the pattern was modified since NA was different from only BC (Fig. 4c). All treatments showed decreased PHO activity from T0 to T108, except for MX (Fig. 4c). DHG activity did not show differences in T0. Still, in T108, NA was lower than in the other treatments. In all cases, DHG activity increased in T108 concerning T0 (Fig. 4d).
Potential C mineralization
The accumulated C potential mineralization at 108 days showed significant differences between the treatments in the following order: CM > MX > NA = BC, where the first two treatments were 1.6- and 1.3-times higher concerning NA and BC, respectively (Fig. 5).
Metabolic coefficient and specific enzymatic activity
The SEA showed different patterns. SEA PPO and SEA PHO showed significant differences between dates and treatments independently, and in SEA BG, the interaction between factors was significant (F = 12.9, p < 0.01).
A decrease in SEA PHO was observed in all treatments from T0 to T108, except NA. Likewise, in this same treatment, the highest value was obtained for this parameter (Fig. 6c). The SEA PPO showed an increase from T0 to T108 in BC treatment, which showed the highest value compared to the other treatments only at the end of the incubation (Fig. 6b). SEA BG increased over time only in NA. At the end of the incubation, it was higher, up to six times higher than the other treatments (Fig. 6a), while SEA DHG did not show differences between the factors analyzed (Fig. 6d). Likewise, the highest value of qCO2 was observed in NA. It was double that in the treatments with organic amendments (Fig. 7).
Mean (± standard error) of specific enzyme activity: a β-glucosidase (SEA BG), b polyphenol oxidase (SEA PPO), c phosphatase (SEA PHO) and d. dehydrogenase (SEA DHG) for RMANOVA analysis. NA No amendment, CM compost, BC biochar, MX biochar and compost. Different lowercase letters represent significant differences (p < 0.05) in the factor “time” and different capital letters represent significant differences (p < 0.05) between treatments
Change in processes related to search and use of C
The limiting conditions for developing biological activity in mining tailings are related to high concentrations of potentially toxic elements, low moisture retention capacity, and low availability of C and other nutrients (Cross et al. 2021; Tardif et al. 2019). This is why it is considered that adding organic matter can promote the development of biological activity by mitigating the adverse conditions of tailings (Bacchetta et al. 2015; Gil-Loaiza et al. 2016; Arvizu-Valenzuela et al. 2020). In mining tailings without vegetation cover, the C input is dominated by photoautotrophs, and their organic residues are fast used by chemotrophs, particularly chemoheterotrophs, exhausting the available C (Newsome and Falagán, 2021) for this, the addition of organic amendments represents a new, maybe excessive, available C condition that now can support the more complex functional microbial community with high activity of microbial heterotroph (Abujabhah et al. 2016; Risueño et al 2021). The fast response of heterotroph bacteria has been observed after organic amendment addition, with compost addition (Risueño et al 2021); still, we observe that time was required to couple the microbial community to the new conditions promoted by adding water and organic amendments once the differences between treatments were observed at the end of the experiment. However, the activity of the BG enzyme increased immediately after conditioning in control and BC treatment, as did the PPO enzyme in CM and BC treatments (Fig. 4a, b), which is interesting as it suggests a greater sensitivity in the response of microorganisms to these activities related to organic C compared to the stimulation of P mineralization by phosphatases (Allison et al. 2007; Shukla and Varma 2010; Vinhal-Freitas et al. 2010) because the main limitation for heterotrophic microbial communities in mining tailings is the scarcity of organic compounds and the C acquisition is the key feature to gain biomass and energy for continues mining others resources (Risueño et al 2021; Solís-Hernández et al. 2022).
After 108 days, it was observed that microbial activity responded differently to the organic C source added. Still, the chemical composition associated with organic amendments type is essential in regulating C immobilization, enzymatic activity, and C mineralization (Hale et al. 2021). This is evident once C immobilization in microbial biomass shows an inverse pattern with the concentration of organic C in treatments (Table 1; Fig. 3). Of all treatments, it is in CM where a higher proportion of labile compounds is present, as it has not gone through the pyrolysis process, which allows C to be assimilated and immobilized in cells of microorganisms more easily by requiring less energy investment (Garcia-Gil et al. 2000; Bhattacharyya et al. 2003; Farrell et al. 2010; Emmerling et al. 2010; de Mora et al. 2014), which is opposite case with the addition of biochar, produced at high temperature (1000 °C), and in which it has been reported that during the pyrolysis process, labile compounds volatilize between 200 and 400 °C, and the remaining components are recalcitrant compounds, which are highly stable and resistant to degradation by the microbial community (Lehmann et al. 2011; Al-Wabel et al. 2013; Ameloot et al. 2014).
In the same sense, C mineralization was promoted in CM and MX treatments (Fig. 5), as it was higher by 59 and 26%, respectively, concerning control, and by 73 and 36%, respectively, concerning BC, suggesting that mineralization process was faster in compost addition treatments and is attributable to the presence of labile compounds (Cardelli et al. 2017; Mingorance et al. 2017; Liu and Takahashi 2019; Lorenz and Lal 2018). In addition, it is observed in these same treatments that there is an increased activity of the DHG enzyme (Fig. 4d), indicating that adding this type of amendment provides compounds that allow the maintenance and activity of microorganisms. There are several studies where the DHG enzyme was evaluated in soils contaminated with mine tailings after improvement with compost addition, and significant increases in this enzyme are reported because of microbial activity stimulation by ameliorating adverse conditions (de Mora et al. 2005; Alvarenga et al. 2008; de Varennes et al. 2010; Kohler et al. 2015).
Adding biochar to tailings did not significantly affect the C microbial biomass and C mineralization process (Figs. 3, 5, respectively). Although it slightly promoted C immobilization in microbial biomass after conditioning, mineralization was lower than in treatments with compost addition and was like the control. This is consistent with the activity of BG and PPO enzymes (Fig. 4a, b), which in both cases was promoted in treatments with biochar addition and where there is labile C limitation and a higher proportion of recalcitrant compounds. In this case, the higher activity in enzymes with highly contrasting substrates converges as a strategy in the face of labile C limitation (Alvarenga et al. 2008; Fialho et al. 2010; Lehmann et al. 2011; Moreno-Barriga et al. 2017). However, by itself, the conditioning of the mining tailings with the addition of water promotes the activity of the BG enzyme. This suggests that, when faced with a pulse of moisture, the microorganisms initiate the search for labile forms of C (Solís-Hernández et al. 2022) (Fig. 6).
The results obtained show that enzyme activity responds to two factors proved in this main work factors: (1) the characteristics of the organic amendments (i.e., whether they are labile or recalcitrant) and (2) the effects of changing temperature and humidity conditions during incubation that alleviate the natural conditions in that these microorganisms are exposed as high temperatures and low water availability which means a mitigation of the stress conditions. Still, the lack of nutrients such as P or the formation of organo-mineral compounds can be crucial for explaining the availability of microbial resources and need to be expanded in future research.
Metabolic adjustments of microbial community
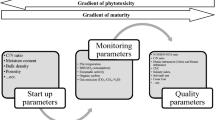
The main interest in mining tailing amelioration strategies is focused on vegetal growth and rarely considers the microbial response, but now is clearly recognized that microorganisms are key for plant establishment (Kolaříková et al. 2017; Sun et al. 2018). The general interest in the microbial community is centered on the composition, and functional traits are poorly explored (Risueño et al. 2021). For it, the present work explores with affordable indicators the metabolic adjustments in C use efficiency promoted by initial conditioning and during the most intense response as priming effect (Kuzyakov 2010). The qCO2 value (Fig. 7) in all treatments with organic amendments was significantly lower than the control, as reported by Vinhal-Freitas et al. (2010) and Paz-Ferreiro et al. (2012). These values indicate that adding organic amendments decreased the microorganisms' stress by using the added C source to increase their microbial biomass and maintain their metabolic activity, promoting the microbial C accumulation that derives in later C microbial incorporation as a critical way for organic carbon accumulation and stabilization through the “microbial carbon pump” (Zhu et al. 2020). Microbial communities invest C not only in biomass and energy production but also in metabolites needed for extracellular resources acquisition, a good example of this is the exoenzymes (Lehmann and Joseph 2015). The chemical composition of organic residues influences the energy investment for their degradation; for example, molecules with a high proportion of aromatic compounds need more energy investment and high specialized enzymes depolymerization (Gunina and Kuzyakov 2022), making a high resources investment in PPO also the scarcity in easy hydrolyzable C compounds promotes high investment in BG production (Chávez-Vergara et al. 2018). These are noticeable in the treatments lacking labile C (NA and BC) were the ones that presented increases in the values of SEA BG and SEA PPO at the end of incubation (Fig. 6a, b), respectively, and concerning other treatments. This indicates that these treatments present greater stress conditions since microorganisms invest their energy in enzyme synthesis, not increasing their biomass. This reflects microorganisms’ trade-off between investing energy in searching for resources or using these resources to improve their biomass (Fig. 8). This is evident in the increased SEA values in treatments with stress conditions (NA and BC) due to labile C compared to treatments with favorable conditions in terms of nutrient availability (CM and MX) which are not observable only with enzyme activity.
Conclusion
Mining tailings with the addition of organic amendments and better temperature and humidity conditions promoted enzymatic activity and, at the same time, decreased stress conditions by functioning as a source of C, which caused a metabolic change in the microbial community by allowing microorganisms to invest their energy in biomass increase and not in search for resources. Also, the processes of C mineralization and microbial C depend partially on the composition of organic amendments. It is concluded that the addition of pyrolyzed biochar at high temperatures is not a good option to promote microbial activity in these mining tailings due to its recalcitrant composition since less microbial C was observed and similarities with control treatment in indicators related to C dynamics, such as mineralization and BG enzyme activity. On the contrary, adding compost is the most convenient scenario to promote microbial activity in mining tailings, which could favor their stabilization. These findings are visible using microbial C use efficiency indicators, principally the specific enzymatic activity (SEA); therefore, they are considered better indicators than only microbial C, CO2 production, or enzymatic activity indicators, because SEA allows a functional approach to understanding the response to amendments in addition to tailing mining.
References
Abujabhah IS, Bound SA, Doyle R, Bowman JP (2016) Effects of biochar and compost amendments on soil physico-chemical properties and the total community within a temperate agricultural soil. Appl Soil Ecol 98:243–253. https://doi.org/10.1016/j.apsoil.2015.10.021
Acosta-Martínez V, Tabatabai MA (2000) Enzyme activities in a limed agricultural soil. Biol Fertil Soils 31(1):85–91. https://doi.org/10.1007/s003740050628
Alef K, Nannipieri P (eds) (1995) Methods in applied soil microbiology and biochemistry. Academic Press, San Diego. https://doi.org/10.1016/B978-0-12-513840-6.X5014-9
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39(7):1770–1781. https://doi.org/10.1016/j.soilbio.2007.02.006
Alvarenga P, Gonçalves AP, Fernandes RM, De Varennes A, Vallini G, Duarte E, Cunha-Queda AC (2008) Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ 406(1–2):43–56. https://doi.org/10.1016/j.scitotenv.2008.07.061
Al-Wabel MI, Al-Omran A, El-Naggar AH, Nadeem M, Usman ARA (2013) Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Biores Technol 131:374–379. https://doi.org/10.1016/j.biortech.2012.12.165
Ameloot N, Sleutel S, Case SDC, Alberti G, McNamara NP, Zavalloni C, Vervisch B, Vedove G, Delle, De Neve, S. (2014) C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol Biochem 78:195–203. https://doi.org/10.1016/j.soilbio.2014.08.004
Arcand MM, Levy-Booth DJ, Helgason BL (2017) Resource legacies of organic and conventional management differentiate soil microbial carbon use. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02293
Arvizu-Valenzuela LV, Cruz-Ortega R, Meza-Figueroa D, Loredo-Portales R, Chávez-Vergara B, Mora L, Molina-Freaner F (2020) Barriers for plant establishment in the abandoned tailings of Nacozari, Sonora, Mexico: the influence of compost addition on seedling performance and tailing properties. Environ Sci Pollut Res Int 27(31):39635–39650. https://doi.org/10.1007/s11356-020-09841-7
Bacchetta G, Cappai G, Carucci A, Tamburini E (2015) Use of native plants for the remediation of abandoned mine sites in mediterranean semiarid environments. Bull Environ Contam Toxicol 94(3):326–333. https://doi.org/10.1007/s00128-015-1467-y
Bhattacharyya P, Chakrabarti K, Chakraborty A (2003) Effect of MSW compost on microbiological and biochemical soil quality indicators. Compost Sci Util 11(3):220–227. https://doi.org/10.1080/1065657x.2003.10702130
Cambardella CA, Richard TL, Russell A (2003) Compost mineralization in soil as a function of composting process conditions. Eur J Soil Biol 39(3):117–127. https://doi.org/10.1016/s1164-5563(03)00027-x
Cardelli R, Becagli M, Marchini F, Saviozzi A (2017) Effect of biochar, green compost, and vermicompost on the quality of a calcareous soil: a 1-year laboratory experiment. Soil Sci 182(7):248–255. https://doi.org/10.1097/ss.0000000000000216
Cárdenas J (2013) La minería en México: despojo a la Nación. Cuest Const Rev Mexic Derecho Const 28:35–74. https://doi.org/10.1016/s1405-9193(13)71275-7
Chavarria DN, Pérez-Brandan C, Serri DL, Meriles JM, Restovich SB, Andriulo AE, Jacquelin L, Vargas-Gil S (2018) Response of soil microbial communities to agroecological versus conventional systems of extensive agriculture. Agr Ecosyst Environ 264:1–8. https://doi.org/10.1016/j.agee.2018.05.008
Chavez-Vergara B, Merino A, Vázquez-Marrufo G, García-Oliva F (2014) Organic matter dynamics and microbial activity during decomposition of forest floor under two native neotropical oak species in a temperate deciduous forest in Mexico. Geoderma 235–236:133–145. https://doi.org/10.1016/j.geoderma.2014.07.005
Chávez-Vergara BM, González-Rodríguez A, Etchevers JD, Oyama K, García-Oliva F (2015) Foliar nutrient resorption constrains soil nutrient transformations under two native oak species in a temperate deciduous forest in Mexico. Eur J for Res 134(5):803–817. https://doi.org/10.1007/s10342-015-0891-1
Chávez-Vergara B, Rosales-Castillo A, Merino A, Vázquez-Marrufo G, Oyama K, García-Oliva F (2016) Quercus species control nutrients dynamics by determining the composition and activity of the forest floor fungal community. Soil Biol Biochem 98:186–195. https://doi.org/10.1016/j.soilbio.2016.04.015
Chávez-Vergara B, Merino A, González-Rodríguez A, Oyama K, García-Oliva F (2018) Direct and legacy effects of plant-traits control litter decomposition in a deciduous oak forest in Mexico. PeerJ 6(e5095):e5095. https://doi.org/10.7717/peerj.5095
Chen C-L, Liao M, Huang CY (2005) Effect of combined pollution by heavy metals on soil enzymatic activities in areas polluted by tailings from Pb-Zn-Ag mine. J Environ Sci (China) 17(4):637–640. http://www.jesc.ac.cn/jesc_en/ch/reader/view_abstract.aspx?file_no=20050423&flag=
Choreño-Parra EM, Ángeles-Pérez G, Villegas-Ríos M, Beltrán-Paz O, Pérez-Pazos E, Quintero-Gradilla S, Chávez-Vergara B (2022) Tree stratum alteration decreases C use efficiency and the stability of litter decomposition in a sacred fir (Abies religiosa) forest. Botan Sci 100(4):857–876. https://doi.org/10.17129/botsci.3029
Coleman DC, Anderson RV, Cole CV, Elliott ET, Woods L, Campion MK (1977) Trophic interactions in soils as they affect energy and nutrient dynamics. IV. Flows of metabolic and biomass carbon. Microb Ecol 4(4):373–380. https://doi.org/10.1007/BF02013280
Cross AT, Ivanov D, Stevens J, Sadler R, Zhong H, Lambers H, Dixon KW (2021) Nitrogen limitation and calcifuge plant strategies constrain the establishment of native vegetation on magnetite mine tailings. Plant Soil 461:181–201. https://doi.org/10.1007/s11104-019-04021-0
Dai J, Becquer T, Rouiller JH, Reversat G, Bernhard-Reversat F, Lavelle P (2004) Influence of heavy metals on C and N mineralisation and microbial biomass in Zn-, Pb-, Cu-, and Cd-contaminated soils. Appl Soil Ecol 25(2):99–109. https://doi.org/10.1016/j.apsoil.2003.09.003
de Mora AP, de, Ortega-Calvo JJ, Cabrera F, Madejón E (2005) Changes in enzyme activities and microbial biomass after ‘in situ’ remediation of a heavy metal-contaminated soil. Appl Soil Ecol 28(2):125–137. https://doi.org/10.1016/j.apsoil.2004.07.006
de Araújo ASF, de Melo WJ, Singh RP (2010) Municipal solid waste compost amendment in agricultural soil: changes in soil microbial biomass. Rev Environ Sci Biotechnol 9(1):41–49. https://doi.org/10.1007/s11157-009-9179-6
de Varennes A, Cunha-Queda C, Qu G (2010) Amendment of an acid mine soil with compost and polyacrylate polymers enhances enzymatic activities but may change the distribution of plant species. Water Air Soil Pollut 208(1–4):91–100. https://doi.org/10.1007/s11270-009-0151-4
Ekenler M, Tabatabai MA (2003) Responses of phosphatases and arylsulfatase in soils to liming and tillage systems. J Plant Nutr Soil Sci 166(3):281–290. https://doi.org/10.1002/jpln.200390045
Emmerling C, Udelhoven T, Schneider R (2010) Long-lasting impact of biowaste-compost application in agriculture on soil-quality parameters in three different crop-rotation systems. J Plant Nutr Soil Sci 173(3):391–398. https://doi.org/10.1002/jpln.200900348
Farrell M, Griffith GW, Hobbs PJ, Perkins WT, Jones DL (2010) Microbial diversity and activity are increased by compost amendment of metal-contaminated soil: Compost increases microbial diversity and activity. FEMS Microbiol Ecol 71(1):94–105. https://doi.org/10.1111/j.1574-6941.2009.00793.x
Fellet G, Marchiol L, Delle Vedove G, Peressotti A (2011) Application of biochar on mine tailings: effects and perspectives for land reclamation. Chemosphere 83(9):1262–1267. https://doi.org/10.1016/j.chemosphere.2011.03.053
Fialho LL, Lopes da Silva WT, Milori DMBP, Simões ML, Martin-Neto L (2010) Characterization of organic matter from composting of different residues by physicochemical and spectroscopic methods. Biores Technol 101(6):1927–1934. https://doi.org/10.1016/j.biortech.2009.10.039
Fini A, Degl’Innocenti C, Ferrini, F (2016) Effect of mulching with compost on growth and physiology of Ulmus ‘FL634’ planted in an urban park. Arboric Urban For. https://doi.org/10.48044/jauf.2016.017
Foster EJ, Hansen N, Wallenstein M, Cotrufo MF (2016) Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agr Ecosyst Environ 233:404–414. https://doi.org/10.1016/j.agee.2016.09.029
Garcı́a-Gil JC, Plaza C, Soler-Rovira P, Polo A (2000) Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol Biochem 32(13):1907–1913. https://doi.org/10.1016/s0038-0717(00)00165-6
Gil-Loaiza J, White SA, Root RA, Solís-Dominguez FA, Hammond CM, Chorover J, Maier RM (2016) Phytostabilization of mine tailings using compost-assisted direct planting: translating greenhouse results to the field. Sci Total Environ 565:451–461. https://doi.org/10.1016/j.scitotenv.2016.04.168
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agr Ecosyst Environ 206:46–59. https://doi.org/10.1016/j.agee.2015.03.015
Gunina A, Kuzyakov Y (2022) From energy to (soil organic) matter. Glob Change Biol 28(7):2169–2182. https://doi.org/10.1111/gcb.16071
Hale L, Curtis D, Azeem M, Montgomery J, Crowley DE, McGiffen ME (2021) Influence of compost and biochar on soil biological properties under turfgrass supplied deficit irrigation. Appl Soil Ecol 168:104134. https://doi.org/10.1016/j.apsoil.2021.104134
Hmid A, Al Chami Z, Sillen W, De Vocht A, Vangronsveld J (2015) Olive mill waste biochar: a promising soil amendment for metal immobilization in contaminated soils. Environ Sci Pollut Res Int 22(2):1444–1456. https://doi.org/10.1007/s11356-014-3467-6
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol Biochem 28(1):33–37. https://doi.org/10.1016/0038-0717(95)00101-8
Kandeler E (2015) Physiological and biochemical methods for studying soil Biota and their functions. In: Soil microbiology, ecology and biochemistry. Elsevier, pp 187–222. https://doi.org/10.1016/B978-0-08-047514-1.50007-X
Kandeler F, Kampichler C, Horak O (1996) Influence of heavy metals on the functional diversity of soil microbial communities. Biol Fertil Soils 23(3):299–306. https://doi.org/10.1007/bf00335958
Kelly CN, Peltz CD, Stanton M, Rutherford DW, Rostad CE (2014) Biochar application to hardrock mine tailings: soil quality, microbial activity, and toxic element sorption. Appl Geochem 43:35–48. https://doi.org/10.1016/j.apgeochem.2014.02.003
Kleber M (2010) What is recalcitrant soil organic matter? Environ Chem (collingwood, VIC.) 7(4):320–332. https://doi.org/10.1071/en10006
Kohler J, Caravaca F, Azcón R, Díaz G, Roldán A (2015) The combination of compost addition and arbuscular mycorrhizal inoculation produced positive and synergistic effects on the phytomanagement of a semiarid mine tailing. Sci Total Environ 514:42–48. https://doi.org/10.1016/j.scitotenv.2015.01.085
Kolaříková Z, Kohout P, Krüger C, Janoušková M, Mrnka L, Rydlová J (2017) Root-associated fungal communities along a primary succession on a mine spoil: distinct ecological guilds assemble differently. Soil Biol Biochem 113:143–152. https://doi.org/10.1016/j.soilbio.2017.06.004
Kuzyakov Y (2010) Priming effects: Interactions between living and dead organic matter. Soil Biol Biochem 42(9):1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Lehmann J, Joseph S (eds) (2015) Biochar for environmental management: science, technology and implementation, 2nd edn. Routledge, London. https://doi.org/10.4324/9780203762264
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43(9):1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022
Li X, Huang L (2015) Toward a new paradigm for tailings phytostabilization—nature of the substrates, amendment options, and anthropogenic pedogenesis. Crit Rev Environ Sci Technol 45(8):813–839. https://doi.org/10.1080/10643389.2014.921977
Liu E, Takahashi T (2019) Carbon mineralization characteristics of compost made from pruning material. Landsc Ecol Eng 15(2):199–204. https://doi.org/10.1007/s11355-018-00369-0
Lorenz K, Lal R (2018) Carbon sequestration in agricultural ecosystems, 1st edn. Springer, Cham. https://doi.org/10.1007/978-3-319-92318-5
Lottermoser B (2010) Mine wastes: Characterization, treatment and environmental impacts, 3rd edn. Springer, Berlin. https://doi.org/10.1007/978-3-642-12419-8
Malley C, Nair J, Ho G (2006) Impact of heavy metals on enzymatic activity of substrate and on composting worms Eisenia fetida. Biores Technol 97(13):1498–1502. https://doi.org/10.1016/j.biortech.2005.06.012
Mejía J, Carrizales L, Rodríguez VM, Jiménez ME, Díaz F (1999) Un método para la evaluación de riesgos para la salud en zonas mineras. Salud Pública De México 41(2):S132–S140. https://doi.org/10.1590/s0036-36341999000800010
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments–an emerging remediation technology. Environ Health Perspect 116(3):278–283. https://doi.org/10.1289/ehp.10608
Mendez MO, Glenn EP, Maier RM (2007) Phytostabilization potential of quailbush for mine tailings: growth, metal accumulation, and microbial community changes: growth, metal accumulation, and microbial community changes. J Environ Qual 36(1):245–253. https://doi.org/10.2134/jeq2006.0197
Mingorance MD, Franco I, Rossini-Oliva S (2017) Application of different soil conditioners to restorate mine tailings with native (Cistus ladanifer L.) and non-native species (Medicago sativa L.). J Geochem Explor 174:35–45. https://doi.org/10.1016/j.gexplo.2016.02.010
Moreno-Barriga F, Faz Á, Acosta JA, Soriano-Disla M, Martínez-Martínez S, Zornoza R (2017) Use of Piptatherum miliaceum for the phytomanagement of biochar amended Technosols derived from pyritic tailings to enhance soil aggregation and reduce metal(loid) mobility. Geoderma 307:159–171. https://doi.org/10.1016/j.geoderma.2017.07.040
Moynahan OS, Zabinski CA, Gannon JE (2002) Microbial community structure and carbon-utilization diversity in a mine tailings revegetation study. Restor Ecol 10:77–87. https://doi.org/10.1046/j.1526-100X.2002.10108.x
Newsome L, Falagán C (2021) The microbiology of metal mine waste: bioremediation applications and implications for planetary health. GeoHealth 5(10):e2020GH000380. https://doi.org/10.1029/2020GH000380
Paz-Ferreiro J, Gascó G, Gutiérrez B, Méndez A (2012) Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol Fertil Soils 48(5):511–517. https://doi.org/10.1007/s00374-011-0644-3
Paz-Ferreiro J, Lu H, Fu S, Méndez A, Gascó G (2014) Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth 5(1):65–75. https://doi.org/10.5194/se-5-65-2014
Raiesi F, Beheshti A (2014) Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of northwest Iran. Appl Soil Ecol 75:63–70. https://doi.org/10.1016/j.apsoil.2013.10.012
Risueño Y, Petri C, Conesa HM (2021) A critical assessment on the short-term response of microbial relative composition in a mine tailings soil amended with biochar and manure compost. J Harzadous Mater 471(5):126080. https://doi.org/10.1016/j.jhazmat.2021.126080
Rodríguez-Bustos L, Galicia L, Chavez-Vergara B, Beltrán-Paz O (2022) Microbial metabolic activity as legacy of agricultural management in maize agroecosystems from Mexico Highlands. Trop Subtrop Agroecosyst. https://doi.org/10.56369/tsaes.3911
Romero FM, Armienta MA, Gutiérrez ME, Villaseñor G (2008) Factores geológicos y climáticos que determinan la peligrosidad y el impacto ambiental de jales mineros. Rev Int Contam Ambient 24(2):43–54. https://www.redalyc.org/articulo.oa?id=37024201
Saison C, Degrange V, Oliver R, Millard P, Commeaux C, Montange D, Le Roux X (2006) Alteration and resilience of the soil microbial community following compost amendment: effects of compost level and compost-borne microbial community. Environ Microbiol 8(2):247–257. https://doi.org/10.1111/j.1462-2920.2005.00892.x
Shukla G, Varma A (eds) (2010) Soil enzymology, 11th edn. Springer, Berlin. https://doi.org/10.1007/978-3-642-14225-3
Solís-Hernández AP, Chávez-Vergara B, Rodríguez-Tovar AV, Beltrán-Paz O, Santillán J, Rivera-Becerril F (2022) Effect of the natural establishment of two plant species on microbial activity, on the composition of the fungal community, and on the mitigation of potentially toxic elements in an abandoned mine tailing. Sci Total Environ 802:149788. https://doi.org/10.1016/j.scitotenv.2021.149788
Sun X, Zhou Y, Tan Y, Wu Z, Lu P, Zhang G, Yu F (2018) Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China. Environ Sci Pollut Res 25:22106–22119. https://doi.org/10.1007/s11356-018-2244-3
Talavera O, Armienta MA, Abundis JG, Mundo NF (2006) Geochemistry of leachates from the El Fraile sulfide tailings piles in Taxco, Guerrero, southern Mexico. Environ Geochem Health 28(3):243–255. https://doi.org/10.1007/s10653-005-9037-6
Tardif A, Rodrigue-Morin M, Gagnon V, Shipley B, Roy S, Bellenger J-P (2019) The relative importance of abiotic conditions and subsequent land use on the boreal primary succession of acidogenic mine tailings. Ecol Eng 127:66–74. https://doi.org/10.1016/j.ecoleng.2018.11.003
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19(6):703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Vinhal-Freitas IC, Wangen DRB, de Ferreira A, S, Corrêa GF, Wendling B (2010) Microbial and enzymatic activity in soil after organic composting. Rev Bras Cienc Do Solo 34(3):757–764. https://doi.org/10.1590/s0100-06832010000300017
Waldrop MP, Balser TC, Firestone MK (2000) Linking microbial community composition to function in a tropical soil. Soil Biol Biochem 32(13):1837–1846. https://doi.org/10.1016/s0038-0717(00)00157-7
Ye ZH, Shu WS, Zhang ZQ, Lan CY, Wong MH (2002) Evaluation of major constraints to revegetation of lead/zinc mine tailings using bioassay techniques. Chemosphere 47(10):1103–1111. https://doi.org/10.1016/s0045-6535(02)00054-1
Acknowledgements
Authors acknowledge the Institute of Geology (IGl, UNAM), the National Laboratory of Geochemistry and Mineralogy (LANGEM, CONACYT) for the facilities for the use of equipment and performance of analyses, M. Sc. Iris Suárez Quijada (IGl, UNAM) to loan equipment and facilities to access the temperature-controlled room for the microcosm experiment, Dr. Juan Carlos Durán Álvarez of the University Laboratory of Environmental Nanotechnology (LUNA) (ICAT, UNAM) for the liquid phase carbon analysis, the PAPIME Program UNAM by financial support (Grant Number PE102217), and the company Green to Energy (G2E) for providing the biochar used in the experiment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Editorial responsibility: Palanivel Sathishkumar.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nava-Arsola, N.E., Beltrán-Paz, O., Martínez-Jardines, G. et al. Metabolic quotient and specific enzymatic activity in response to the addition of organic amendments to mining tailings. Int. J. Environ. Sci. Technol. 21, 4239–4250 (2024). https://doi.org/10.1007/s13762-023-05280-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05280-2