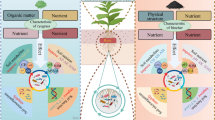
Abstract
Biochar and organic fertilizer are widely supported to maintain crop production and sustainable development of agroecosystems. However, it is unclear how biochar and organic fertilizer alone or in combination regulate soil functional microbiomes and their relationships to ecosystem multifunctionality (EMF). Herein, a long-term (started in 2013) field experiment, containing five fertilization treatments, was employed to explore the effects of biochar and organic fertilizer applications on the EMF (based on 18 functional indicators of crop productivity, soil nutrient supply, element cycling, and microbial biomass) and the functional microbiomes of bulk soil and rhizosphere soil [normalizing the abundances of 64 genes related to carbon (C), nitrogen (N), phosphorus (P), and sulphur (S) cycles]. Compared with single-chemical fertilization, biochar and organic fertilizer inputs significantly enhanced most ecosystem-single functions and, in particular, the EMF significantly increased by 18.7–30.1%; biochar and organic fertilizer applications significantly increased the abundances of soil microbial functional taxa related to C-N-P-S cycles to varying degree. The combined application of biochar and organic fertilizer showed a better improvement in these indicators compared to using them individually. Most functional microbial populations in the soil, especially the taxa involved in C degradation, nitrification, nitrate-reduction, organic P mineralization, and S cycling showed significantly positive associations with the EMF at different threshold levels, which ultimately was regulated by soil pH and nutrient availability. These results highlight the strong links between soil microbiomes and agroecosystem functions, as well as providing scientific support for inclusion of biochar in agricultural production and services with organic amendments.
Graphical Abstract

Highlights
-
1.
8-year field evidence revealed impacts of biochar and pig manure on soil functional microbiome and ecosystem functions.
-
2.
Biochar and pig manure inputs notably enhanced most ecosystem-single functions and the EMF increased by 18.7–30.1%.
-
3.
Biochar and pig manure inputs notably enriched soil functional microbes related to C-N-P-S cycles to varying degree.
-
4.
Increase in EMF was related to microbe-driven soil processes such as C degradation, nitrification, and Po mineralization.
-
5.
Inclusion of biochar in crop production with organic amendments could enhance agro-ecosystem functions and services.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In agricultural production, fertilization, as a major farmland management measure, can alter the nutrient requirements of soil microorganisms and crops as well as affect microbe-mediated soil biochemical reactions and ecosystem functions (Jia et al. 2022). However, long-term over-application of mineral fertilizers can lead to environmental pollution and soil degradation, which further influences the health and processes of entire agroecosystems (Hossain et al. 2022). Organic fertilizers can provide rich organic matter and available nutrients to promote soil microbial activities and metabolisms, and then maintain or promote ecosystem services (Han et al. 2022; Wu et al. 2021). In addition to organic fertilizers, biochar has been widely used as a soil conditioner or organic material in crop production and soil improvement in recent years. Rich pore structure, nutrients, and carbon (C) of biochar can improve soil structure and nutrient supply and increase soil C storage (Liu et al. 2023; Bai et al. 2022). Thus, soil fertility and crop productivity gains may be greater with the mixing of biochar with organic fertilizer than with single applications (Brtnicky et al. 2019; Bai et al. 2022; Liu et al. 2022a; Yan et al. 2023). However, current studies on the effects of their coupling application on the multiple functions (termed “multifunctionality”) of agroecosystems are insufficient.
The services or functions provided by agroecosystems are closely related to the diversity and function of microbial communities in soil (Zabala et al. 2021). Soil microorganisms participate in various functional processes such as nutrient cycling, disease suppression, primary production, and thus special microbial taxa in the soil play a vital role in EMF (Singh et al. 2021; Han et al. 2022). For example, the microbial taxa related to C cycle can drive organic matter degradation and affect soil C storage, thereby regulating ecosystem C balance (Basile-Doelsch et al. 2020). Nitrogen-fixing bacteria can fix nitrogen (N) from atmosphere to ammonia for plant absorption (Aasfar et al. 2021). Some functional taxa also affect ecosystem N cycle by triggering the processes of soil nitrification and denitrification (Dai et al. 2020). Phosphorus (P) soluble microbes can promote insoluble P dissolution and then increase P availability in soil (Wang et al. 2023). Organic sulphur (S) mineralization can provide more S sources for soil microorganisms and plants; and meanwhile, S cycle affects C and N cycles in the soil (Saha et al. 2018). In addition, soil microorganisms can also use enzymes to degrade various derivatives in organic conditioners to meet their own metabolic activities, thereby improving soil material circulation (Saifullah et al. 2018). Although soil microbes can affect multiple functions of agroecosystems, there are few studies on the responses of key functional microbial taxa associated with C-N-P-S cycling processes on the co-applications of biochar and organic fertilizer. This limits our ability to further understand the roles of biochar couping with organic fertilizer in regulating the EMF from the perspective of functional microbiome control.
Changes in management practices such as land use and fertilization may alter the ability of soil microorganisms to maintain the multiple-functions of above- and below-ground ecosystems (Delgado-Baquerizo et al. 2016). Any loss of soil biodiversity may have a negative impact on ecosystem functioning (Delgado-Baquerizo et al. 2020). Therefore, the status of special functional microbial populations in soil can influence EMF and its stability (Luo et al. 2018, 2019). Soil enzyme activity, organic matter and nutrient content, plant nutrient uptake and productivity, and microbial biomass are broadly applied as the indicators for evaluating ecosystem functions and services (Delgado-Baquerizo et al. 2016; Luo et al. 2018; Zak et al. 2019). Functional microorganisms can influence various soil biochemical processes, and various microbial processes occurring in soil can directly or indirectly regulate above-ground biomes (Singh et al. 2021). Thus, assessing how different ecosystem functions show synergies or trade-offs is important for understanding EMF implementation (Yuan et al. 2020). The index of EMF mainly be quantified through mean value method and multi-threshold method, both of which are used together to better assess the effects of soil biodiversity on the EMF (Li et al. 2020; Liu et al. 2019b). Thus, multi-faceted assessment can effectively clarify how the amendments of biochar and organic fertilizer regulate ecosystem functions and their relationships with special functional microbes.
The main objective of this research was to explore the impacts of biochar and organic fertilizer applications on soil functional microbiomes and their relationships with ecosystem functions by setting up a database of a long-term field-based experiment. We investigated the regulations of biochar and organic fertilizer applications on the abundances of C-N-P-S acquiring functional genes in soil via high-throughput q-PCR (Additional file 1: Table S1). We selected 18 ecosystem functions (including soil enzyme activities, soil nutrients, soil microbial biomass, and crop productivity) to explore the effects of biochar and organic fertilizer applications on ecosystem-single functions and EMF (Additional file 1: Table S2). Multiple ecological methods, such as ecological networks and models were applied to reveal the connections between functional microbial abundances and ecosystem-single functions and EMF. We propose the following hypothesis: (1) biochar and organic fertilizer co-applications could better improve the abundances of soil functional microbes and the EMF than their single application; (2) changes in the abundances of special functional microbes in the soil could be the strong predictors of the EMF, and the relationship could be regulated by fertilized-effects on certain soil attributes. This study provides new insights into the effects of biochar and organic fertilizers, especially their co-application, on soil functional microbiomes and agroecosystems multifunctionality, and demonstrates the importance of soil microbe-driven function processes to stabilize or increase ecosystem functions and services.
2 Materials and methods
2.1 Experimental design and field management
Starting from 2013, a field localization experiment was conducted in Liuyang City, Hunan Province, China (28°19′ N, 113°49′ E). Average local precipitation of this location is about 1429 mm and the average temperature is 24 ℃. The experimental soil is light-loamy fluvo-aquic soil formed by alluvial sediments. The basic soil properties (0–20 cm) at the beginning are shown in Additional file 1: Table S3.
The experiment included five treatments: (1) no fertilizer, CK; (2) inorganic fertilizer, CF; (3) mixed input of inorganic fertilizer with biochar, BF; (4) mixed input of inorganic fertilizer and pig manure (replacing 20% of inorganic N with manure), OF; and (5) BOF, OF mixed with biochar. Every treatment consisted of three plots, each of which was 4 m × 5 m in size. The experiment was conducted in a rotations of maize and cabbage. Fertilizer application rates, fertilizer types, and their homologous nature are provided in Additional file 1: Table S4. The fertilization regime was maintained from the beginning of the trial. Crop residues were cleaned up after the harvest.
2.2 Soil and plant sample collection
Maize cobs and straw were harvested separately by hand at maturity in 2021. Maize kernels were sun-dried for one month, adjusted to a kernel moisture of less than 14%, and then weighted to determine yield. Maize cobs and straw samples were dried in an oven at 70 °C to constant dry weight. The N (P) accumulation (uptake) of the maize plants was calculated by measuring maize plant biomass and N (P) content.
Soil was sampled using the five-point method and shake-down method. Five portions of soil mass with intact root systems were dug from each plot and brought back to laboratory immediately. Large patches of soil that did not contain roots were gently shaken off first, and then the soil adhering to the root perimeter removed with a knife was used as bulk soil. The soil adhering to the root perimeter was removed using a brush as rhizosphere soil. Five soil samples from each plot were mixed into one portion and then stored for subsequent analysis.
2.3 Soil physiochemical property determination
Soil pH was measured by a soil–water (1:2.5, w/v) slurry with a compound electrode (PE-10; Sartorius, Germany). Soil NH4+–N and NO3−–N contents were measured with a Discrete Auto Analyzer Smart Chem200 (Westco Scientific Instruments, Inc., Italy). Soil alkali-hydrolyzed N content was determined by alkaline diffusion method (Bao 2000). Soil dissolved organic C (DOC) content was measured by TOC analyser (ELEMENTER, Germany). Soil total dissolved N (TDN) content was measured via Potassium persulfate oxidation-UV spectrophotometry and the DON content was determined by calculating the difference between TDN and inorganic N (NO3−–N + NH4+–N) as described by Doyle et al. (2004). Soil available P (AP) content was determined by spectrophotometer (Bao 2000). Soil total dissolved P (TDP) content was measured via ammonium molybdate spectrophotometric method (Galhardo and Masini 2000). PO43− content was measured by phospho-molybdenum blue method (Jarvie et al. 2002). Soil dissolved organic P (DOP) content was calculated as TDP-PO43− (Liu et al. 2019a). Total and available potassium in soil were determined using flame photometer (Bao 2000).
2.4 Ecosystem multifunctionality assessment
In this study, 18 variables were used as the indicators of ecosystem functions (Additional file 1: Table S3). Eight of them were the activities of soil enzymes correlated with C, N, and P biogeochemical cycles, including α-1,4-glucosidase (AG), β-1,4-glucosidase (BG), β-d-cellobiosidase (CBH), β-1,4-xylosidase (BX), β-1,4-n-acetylglucosaminidase (NAG), leucine amino peptidase (LAP), alkaline phosphatase (ALP), acid phosphatase (ACP). These enzymes can promote C and nutrient cycles by catalyzing organic matter degradation and nutrient transformations (Hu et al. 2021). The contents of soil organic C (SOC), total N (TN), and total P (TP) were devoted to represent soil C, N, and P storage, respectively (Hu et al. 2021). Microbial biomass C (MBC), N (MBN), and P (MBP) contents are used to indicate C, N, and P bio-availability (Ren et al. 2023). Specific measurement details for the above indicators are given in the supplementary material. Plant nutrient uptake, biomass, and grain yield were used to represent plant nutrient acquirement and productivity (Zak et al. 2019).
Single functional indicators were Z-score transformed and then were averaged to gain EMF index on the basis of averaging method (Delgado-Baquerizo et al. 2016; Luo et al. 2018). In addition, a multiple-threshold method was adapted to calculate the EMF index (Liu et al. 2019b). This method can reveal the impacts of special functional microbial taxa in soil on the EMF over a range of thresholds from 0 to 100%. To adopt the multiple-threshold method, we first normalized each function, and then each function was respectively convert to a percentage of its maximum value (Liu et al. 2019b).
2.5 Functional gene abundance quantification
High-throughput quantitative PCR was executed by a real-time PCR system (Wafergen, Fremont, CA, USA). A total of 64 primer sets were adapted to target the genes correlated with C-N-P-S cycles (Additional file 1: Table S1), including C degradation, C fixation, methane metabolism, N fixation, nitrification, denitrification, nitratereduction, anammox, N mineralization, inorganic-P (Pi) solubilization, organic-P (Po) mineralization, and S cycling. Melting process was automatically generated and analyzed by SmartChip qPCR Software. Specific procedures of this analysis are offered in former literature (Zheng et al. 2018; Wang et al. 2022).
2.6 Data analysis
Principal component analysis (PCA) and heat-map in R software tool (version 4.0.5) were adapted to contrast the diversity between treatments in the abundances of soil functional genes or microbial populations. Co-occurring relationships between ecosystem-single functions and functional gene abundances in bulk and rhizosphere soil were revealed by structuring ecological networks. Relationships between functional microbial abundances and the EMF index were further explored by regression analysis and multi-threshold approach.
The main predictors of the EMF index were deciphered by random forest analysis proposed by Breiman (2001). Random forest algorithms added an additional layer of randomness to bagging, and each tree used a different bootstrap sample of the data. The “randomForest” and “rfPermute” packages were used to carry out and estimate the significance of importance metrics for a random forest model by permuting the response variable. Details on the analysis process have been given in former publications (Luo et al. 2019, 2020). Structural equation model was developed by analyzing soil properties and functional microbial abundances to identify the main drivers and pathways of the EMF index. A priori model of the known potential correlations between the EMF and the above mentioned drivers was developed on the basis of data mining with random forest analysis. AMOS software (SPSS AMOS 20.0.0) was used for the construction and evaluation of the model. The following metrics were used to evaluate the goodness of fit of our model: the root mean squared error of approximation (RMSEA), chi-square value, Fisher’s P statistic, and the Akaike information criterion (AIC). Ultimately, the priori model was optimized to obtain the model that satisfied the goodness of fit condition, and the significance of the path was further verified.
3 Results
3.1 The impacts of fertilization regimes on ecosystem functions
Compared with the CK, fertilization treatments showed higher EMF index and 11 ecosystem-single functions (P ≤ 0.05; Fig. 1). Compared to the CF, the BF, OF, and BOF treatments notably increased soil enzyme (BG, CBH, BX, AG, ACP, and ALP) activity, soil C and N (TN and SOC) content, MBP content, N and P uptake by plants, yield, and plant biomass, and the BOF showed the highest increment of these functional indicators. The BF, OF, and BOF significantly increased the EMF index by 18.7%, 19.5%, and 30.1%, respectively, compared to the CF.
Fertilization-driven changes in ecosystem single functions and multifunctionality. Mean ± SD of all variables were expressed as the ratio of the fertilization treatments in the presence and no fertilization. A ratio < 1.0 represents a decrease in the ecosystem function, while a ratio > 1.0 indicates a increase (P ≤ 0.05) in the certain function. CF inorganic fertilizers, BF inorganic fertilizers and biochar, OF organic fertilizer N replaces 20% of fertilizer N, BOF OF and biochar. MB microbial biomass
3.2 The impacts of organic amendments on soil functional microbial abundances
There was a significant difference in the abundances of functional microbiomes (ADONIS: R2 = 0.89, P ≤ 0.001; ANOSIM: R2 = 0.81, P ≤ 0.001; A) and C-N-P-S functional microbial populations (ANOSIM: R2 = 0.54–0.75, P ≤ 0.001; Fig. 2B–E) among different treatments. There was also a significant difference in the C-N-P-S cycling microbial abundances between bulk and rhizosphere soil, and the soil place showed a bigger effect on the microbial abundances than the treatments (P ≤ 0.001; Fig. 2A). The abundance of each functional gene showed a distinct response to fertilization treatments (Fig. 2F and G). The single or combined inputs of biochar and organic fertilizer increased the abundances of functional microbial groups to varying degree, with the rhizosphere soil having bigger impacts on the abundances than the bulk soil (Fig. 3). Compared with the CF, the microbial abundances related to C mineralization of the BF, OF, and BOF in bulk and rhizosphere soil increased by 65.7–162.2% and 39.2–129.5%; The microbial abundances related to C fixation of the OF and BOF in both soils increased by 73.4–113.1% and 28.8–76.4%; And the microbial abundances related to methane metabolism of the BF, OF, and BOF in both soils increased by 73.4–113.1% and 28.8–76.4%, respectively (Fig. 3A–C).
The dissimilarity of functional microbial abundance profiles between five treatments based on ADONIS and ANOSIM test (A). Two-way PERMANOVA comparing the main and interactive effects of the treatments and soil places on the functional gene abundance profiles (999 permutations). Based on the results, the dissimilarity of four specific functional microbial populations was further explored as the bulk soil and rhizosphere soil among five treatments (B–E). Asterisks (*, **, and ***) indicate significant differences at P ≤ 0.05, ≤ 0.01, and ≤ 0.001 probability levels, respectively. Changes in abundance of 64 functional genes in the bulk soil (F) and rhizosphere soil (G) between five treatments. The greener the color, the greater the value of the variable. Conversely, the redder the color, the smaller the value. CK no fertilizers, CF inorganic fertilizers, BF inorganic fertilizers and biochar, OF organic fertilizer N replaces 20% of fertilizer N, BOF OF and biochar
Differences in the abundances of functional microbial populations associated to C degradation, C fixation, methane metabolism, N fixation, nitrification, nitrate-reduction, anammox, N mineralization, denitrification, Pi-solubilization, Po-mineralization, and S-cycling between five treatments in bulk soil and rhizosphere soil. CK no fertilizers, CF inorganic fertilizers, BF inorganic fertilizers and biochar, OF organic fertilizer N replaces 20% of fertilizer N, BOF OF and biochar
The microbial abundances related to (a) N fixation of the OF and BOF in the bulk and rhizosphere soil increased by 18.5–26.5% and 57.3–121.3%, (b) nitrification increased by 85.6–125.5% and 50.3–50.5%, (c) nitrate-reduction increased by 49.7–171.5% and 36.9–233.0%, (d) anammox increased by 25.5–120.0% and 14.6–97.4%, (e) N mineralization increased by 99.9–131.3% and 68.5–144.3%, (f) denitrification increased by 87.5–90.9% and 47.6–91.5%, respectively, compared to the CF (Fig. 3D–I). Compared to the CF, the microbial abundances related to Pi-solubilization of the BF, OF, and BOF in bulk and rhizosphere soil increased by 38.3–69.6% and 29.6–87.5%, and those related to Po-mineralization of the BF, OF, and BOF in the two soils increased by 37.0–247.3% and 54.5–237.5%, respectively (P ≤ 0.05; Fig. 3J and K). The microbial abundances related to S-cycling of the OF and BOF in bulk and rhizosphere soil increased by 16.5–84.4% and 65.0–66.1%, respectively (P ≤ 0.05; Fig. 3J–L).
3.3 Relationship between ecosystem functions and functional microbial abundances
The profiles of ecosystem functions and soil functional microbial populations were clustered in different treatments based on procrustes analysis, and meanwhile, the profile of ecosystem functions was found to be correlated to those of soil functional microbial abundances (R2 = 0.57–0.87%; P ≤ 0.001; Fig. 4A). Mantel test affirmed that ecosystem functions were significantly correlated with soil functional microbial abundances (r = 0.41–0.62; P ≤ 0.01). The dissimilarity of ecosystem function profiles showed a significant relationship with that of soil functional microbial profiles (R = 0.36–0.54; P ≤ 0.001; Fig. 4B).
Principal component analysis depicting the dissimilarity of ecosystem functions and functional microbial abundances (A). The relationships between the dissimilarities of functional microbial abundance profiles and those of ecosystem multifunctionality (B). The solid lines represent significant linear regressions, and the 95% confidence bands are shaded in gray. Co-occurrence networks between ecosystem functions and functional gene abundances in bulk soil and rhizosphere soil (C). The blue nodes indicate the parameters of ecosystem functions, and the pink nodes indicate the functional gene abundances, the size of nodes is proportional to the degree of connectivity. The larger the circle, the stronger the relationships. The relationships between ecosystem functions and functional microbial abundances in bulk soil and rhizosphere soil (D). CK no fertilizers, CF inorganic fertilizers, BF inorganic fertilizers and biochar, OF organic fertilizer N replaces 20% of fertilizer N, BOF OF and biochar. ALP alkaline phosphatase, ACP acid phosphatase, MBC, microbial biomass C, MBN microbial biomass N, MBP microbial biomass P
The co-occurring networks of functional gene abundances and ecosystem functional indicators showed multiple associations, dominated by positive edges (Fig. 4C). The functional indicators of BG, ACP, ALP activities, plant N uptake, and plant P uptake showed high connections with other notes. Meanwhile, the abundances of pqq-mdh, mcrA, korA, nirK2 and amoa2 in bulk soil showed high connections with other notes, and those of ppx, napA, nasA, glx and nirK2 genes in rhizosphere soil showed high connections with other notes (Fig. 4C). Heat-map analysis showed that the EMF and some ecosystem-single functions, especially yield, biomass and nutrient uptake, showed significant correlations with the abundances of most functional microbial populations we studied in the bulk and rhizosphere soil (Fig. 4D).
3.4 Relationship between the EMF and soil functional microbial abundances
The EMF showed significant relationships with the abundances of functional microbe related to C (C degradation, C fixation, and methane metabolism), N (nitrification, denitrification, nitrate-reduction, and anammox), P (Pi-solubilization and Po-mineralization), and S cycle in the bulk and rhizosphere soil (P ≤ 0.05; Fig. 5). The abundances of microbial taxa related to C degradation, nitrification, and S cycling in the bulk soil were the important predictors of the EMF (P ≤ 0.05; Additional file 1: Fig. S1A). Multi-threshold method showed that the abundances of the microbial taxa relating to C degradation, nitrification, and S cycling in the bulk soil were positively correlated with the EMF (Fig. 6A–C). As shown by the regressions between microbial abundance related to C degradation, nitrification, and S-cycling and the EMF at each threshold, the minimum threshold (Tmin) was 19%, 65%, and 30%, and the threshold of maximum effect (Tmde) was 74%, 88%, and 82%, respectively (Fig. 6A–C). When mapping the slopes of multiple regressions with 95% confidence interval (Fig. 6D–F), the maximum threshold (Tmax) was infinite. The realized maximum effect (Rmde) of functional microbial abundance associated with C degradation, nitrification, and S-cycling was 24.12, 17.73, and 15.84, respectively. The results showed that, in the bulk soil, the abundances of microbial taxa related to C degradation, nitrification, and S cycling needed to increase by at least 0.04, 0.06, 0.07 Lg copies g−1 soil, respectively, to improve one function of the ecosystem.
Relationships between functional microbial abundances and ecosystem multifunctionality index in bulk soil (A–C) and rhizosphere soil (D–F) based on the results of the Fig. 4D. The fitted lines are from the regression relationship. Only significant fitted lines are displayed on the graphs. Shaded areas show 95% confidence intervals of the fit. C degrad. C degradation, meth. met. methane metabolism, nit.-red. nitrate-reduction, N minera. N mineralization, Pi solubi. Pi solubilization, Po minera. Po mineralization
Associations between functional microbial abundance and the number of ecosystem functions at or above a threshold of the maximum observed function in bulk soil (A–C) and rhizosphere soil (F–I) across the full range from 0 to 100%. Colors indicate different thresholds, with cooler colors indicating lower thresholds and warmer colors indicating higher thresholds. The slope of the relationship between functional microbial abundance and the number of ecosystem functions at or above a threshold of the maximum observed ecosystem functions in bulk soil (D–F) and rhizosphere soil (J–L). The dashed line and shadowed area indicate the slope and the 95% confidence interval of the regressions
The abundances of the microbial taxa related to C degradation, nitrate-reduction, and Po-mineralization in the rhizosphere soil were the important predictors of the EMF (P < 0.05; Additional file 1: Fig. S1B). Multi-threshold method showed that the abundances of the taxa related to C degradation, nitrate-reduction, and Po-mineralization in rhizosphere soil were positively correlated with the EMF (Fig. 6G–I). As shown by the regression relationships between microbial abundance related to C degradation, nitrate-reduction, and Po-mineralization and the EMF at each threshold, the Tmin was 20%, 27%, and 26%, and the Tmde was 81%, 82%, and 82%, respectively (Fig. 6G–I). The realized maximum effect of microbial abundance associated with C degradation, nitrate-reduction, and Po-mineralization was 26.73, 17.22, and 16.03, respectively (Fig. 6J–L). The results showed that, in the rhizosphere soil, the abundances of microbial taxa related to C degradation, nitrate-reduction, and Po-mineralization needed to increase by at least 0.04, 0.06, 0.06 Lg copies g−1 soil, respectively, to improve one function of the ecosystem.
3.5 Regulation of soil functional microbial abundances on the EMF
In the bulk soil, pH, available N:P ratio, and DON content were the main predictors of the abundances of microbial groups relating to C degradation, nitrification, and S-cycling, respectively, followed by AP and inorganic N contents (P ≤ 0.05; Additional file 1: Fig. S2A–C). In the rhizosphere soil, pH, available N:P ratio, pH were the main predictors of the abundances of the microbial taxa related to C degradation, nitrate-reduction, and Po-mineralization, respectively, followed by AP and inorganic N contents (P ≤ 0.05; Additional file 1: Fig. S2D–F).
The abundances of soil microbial taxa related to C degradation showed the largest direct effect on the EMF, followed by soil available N:P ratio and nitrification and nitrate-reduction microbial abundance (P ≤ 0.05; Fig. 7). The abundances of S cycling microbial population in the bulk soil and those related to Po-mineralization also showed direct effects on the EMF. Soil pH, available N:P ratio, AP, DON, and inorganic N contents indirectly influenced the EMF by mediating the abundances of the microbial taxa related to C degradation, nitrification, S-cycling, nitrate-reduction, and Po-mineralization. Soil pH and C degradation microbial abundance showed the greatest positive total impacts on the EMF, followed by soil AP and nitrate-reduction microbial abundance. Soil N:P ratio and nitrification microbial abundance showed a negative total effect on the EMF (Fig. 7).
Structural equation model showing the direct and indirect regulating effects of the physicochemical properties and functional microbial abundances in bulk soil (A and B) and rhizosphere soil (C and D) on the ecosystem multifunctionality (EMF). Blue and red arrows indicate the positive and negative effects, respectively. Width of the arrows shows the strength of the causal relationship. Numbers on arrows are standardized path coefficients. All of the drivers of the EMF were chosen based on previous associations and random forest analysis. Numbers on arrows are standardized path coefficients. R2 indicates the proportion of variance explained. χ2 chi-square, RMSEA root mean square error of approximation, AIC akaike information criterion, AP available P, DON soluble organic N, N:P ratio of available N and available P, C degrad. C degradation, nitrifi. Nitrification, nit.-red. nitrate-reduction, Po minera. Po mineralization, micro. abun. microbial abundance
4 Discussion
4.1 Biochar and organic fertilizer applications improve ecosystem functions
Similar to previous studies (Biederman and Harpole. 2013; Luo et al. 2018), the amendments of biochar and organic fertilizer significantly increase most ecosystem-single functions such as crop productivity, soil nutrient storage, and soil enzyme activities (Fig. 1). Biochar inputs can improve soil nutrient availability by regulating soil microbial activities and metabolisms, and then stimulate plant growth, change root structure, and increase photosynthesis (Backer et al. 2018; Liu et al. 2022b), thereby improving plant nutrient uptake, biomass, and yield (Fig. 1). Organic fertilizers have a positive impact on plant growth by improving soil nutrient status and physical conditions (Zhang et al. 2021). Additionally, soil C, N, and P reserves also have significant positive impacts on plant nutrient uptake and crop yield (Waqas et al. 2020). Organic C from organic fertilizers and C, N and P resources from biochar are key factors in the increase of C and nutrient stocks in the soil (Fig. 1; Waqas et al. 2020; Ibrahim et al. 2021). Biochar and organic fertilizer inputs also can reduce nutrient losses by adsorbing nutrients from the soil and improving soil water holding capacity, further improving nutrient uptake by plants (Fig. 1; Zhang et al. 2021; Yan et al. 2023). Meanwhile, biochar can slow down the decomposition of organic fertilizers, and the combination of the two materials has a greater promotion effect on maintaining the balance of soil nutrient supply and plant nutrient requirement (Kätterer et al. 2019). However, organic matter decomposition and nutrient transformations in soil cannot be separated from the regulation of soil extracellular enzymes (Dotaniya et al. 2019).
As indicators of ecosystem functions, soil enzyme activities participate in multiple soil functional processes (Wang et al. 2021). Biochar and organic fertilizer amendments could enhance the activities of soil enzymes correlated with C and nutrients cycles (Fig. 1). Biochar and organic fertilizer can enhance soil C storage and bio-availability, thereby stimulating microbial growth and synthesis of enzymes associated with C degradation (Dominchin et al. 2021; Zhou et al. 2020). This is also confirmed by our results (Fig. 1). Biochar and organic matter can increase soil pH and improve the adaptability of some soil enzymes such as organic P hydrolases (Neina et al. 2019). In addition, organic amendments also increase phosphatase activity by regulating soil aggregates (Hu et al. 2023). The increase of phosphatase activity improves the acquisition of nutrients by microbes, thus increasing soil MBP content (Fig. 1; Azeem et al. 2019). These findings suggest that special functional microbial taxa in soil have the role in improving soil C and nutrient mining by controlling biomass and enzyme synthesis, which in turn positively affects ecosystem functions (Li et al. 2023). Ultimately, these enhanced ecosystem-single functions by biochar and organic fertilizer co-applications had a high positive impact on the EMF (Fig. 1), which is closely related to special functional microbes in the soil (Luo et al. 2018; Han et al. 2022; Ren et al. 2023).
4.2 Biochar and organic fertilizer amendments regulate soil functional microbiomes
Our data revealed the significant increases in the abundances of soil microbes related to C degradation by biochar and organic fertilizer amendments (Fig. 3A). This result is consistent with previous researches (Guo et al. 2019; Li et al. 2020). The microbial taxa related to C degradation such as Chloromyces and Acidobacterium in croplands are abundant and contain a wide range of cellulose, hemicellulose, and polysaccharide degrading genes (Ibrahim et al. 2021). Organic fertilizers and biochar can provide abundant C sources to stimulate soil microbial degradation of soil organic matter (Zhou et al. 2020; Ibrahim et al. 2021). Fixation of CO2 by soil autotrophic microorganisms can slow down the increase of CO2 in the atmosphere and reduce greenhouse effect (Gao et al. 2023). Improvement of soil pH by biochar and organic fertilizers is a key factor in increased abundance of CO2-fixed autotrophs (Huang et al. 2018). In addition, the rich nutrients provided by biochar and organic fertilizers can create suitable micro-habitats for autotrophic microbes, resulting in a significant enrichment of C-fixation microbes (Fig. 3B; Wang et al. 2021). Organic amendments such as biochar and organic fertilizers could significantly increase microbial abundance related to methane metabolism (Fig. 3C). The high porosity and aeration of biochar are conducive to the growth of methanotrophs (Zhao et al. 2022a). Organic amendments can also enhance soil agglomeration, increase soil porosity, and offer more active substances for methanotrophs (Gong et al. 2022). Thus, biochar and organic fertilizer co-application is a promising management practice enhancing soil C sequestration and reducing the risk of methane emissions in agroecosystems.
Nitrogen mineralization is a key process that regulates soil available N and affects N uptake by plants (Liu et al. 2017). Our data showed increases of N mineralization microbes in the soils with biochar and organic fertilizer inputs (Fig. 3H), which is consistent with previous studies (Zhang et al. 2015). Organic N such as amino acids and amino sugars provided by organic fertilizers are the important sources of microbial N mineralization in soil, and the increase of its rate is chiefly correlated with the increase of soil TN and SOC (Zhang et al. 2015). In this study, the applications of biochar and organic fertilizers also could significantly enhance the abundances of N fixation and nitrification microbial groups (Fig. 3D and E). Incorporating organic materials with a high C/N ratio into soil can increase N fixation in the soil (Zhang et al. 2015). Increase in biological N fixation is also closely related to increased phosphatase production by organic amendments (Sun et al. 2015; Hu et al. 2023). Soil nitrification may reduce ammonia volatilization loss while leading to nitrate leaching and N2O emission (Li et al. 2018). Zhao et al. (2022b) found that biochar can enhance nitrification in acidic soils, which is related to amoA gene abundance. Nitrate reduction, denitrification, and anammox are important pathways for N losses and transformations in soil (Giles et al. 2012). Biochar and organic fertilizer amentments could significantly enrich soil microbial groups related to nitrate-reduction, denitrification, and anammox (Fig. 3F, G and I). Biochar and organic fertilizers can provide abundant active C compounds to denitrifying and nitrate-reduction microbes. Increased activity and metabolism of these functional microbial taxa further triggering N transformations and N2O emissions (Lazcano et al. 2021; Cao et al. 2019). Our results also confirmed the regulation of soil NO3−–N content on the abundances of denitrifying and nitrate-reduction microbial taxa (Additional file 1: Fig. S2). Thus, biochar and organic fertilizer amendments can regulate soil N transformations via microbial action.
Biochar and organic fertilizer inputs could significantly enrich soil microbial taxa related to Po mineralization and Pi solubilization (Fig. 3J and K). The two functional processes play a vital role in soil available P mining and P uptake by plants (Alori et al. 2017). Abundant organic C sources in biochar and manure can stimulate the proliferation of soil P mining microbes and the secretion of organic acid and phosphatase (Zhang et al. 2022; Hu et al. 2023). The microbial taxa dissolving inorganic P are closely related to soil pH, and the increase of soil pH by biochar and organic fertilizer applications may be one of the reasons for enrichment of Po-mineralization and Pi-solubilization microbes (Additional file 1: Fig. S2; Zheng et al. 2019). Most S in soil exists in the form of organic S such as sulfate, which needs to be mineralized by microorganisms to be absorbed and utilized by plants (Saha et al. 2018). Sulfate reduction and oxidation in microorganisms are key steps in S biogeochemical cycle (Anantharaman et al. 2018). Biochar and organic fertilizer amendments significantly enriched S-cycling microbes (Fig. 3L). Soil S mineralization is related to soil oxygen content (Zhou et al. 2005). The improvement of soil aeration by organic amendments may be one of the reasons affecting S-cycling microbes (Xiao et al. 2018). These findings highlight the positive regulation of biochar and organic fertilizer applications on ecosystem functions and services by influencing functional microbes related to C-N-S-P cycles.
4.3 Regulating pathways of soil functional microbiomes on ecosystem functions
A variety of enzymes encoded by functional genes are associated with C and nutrient cycles in soil. Thus, changes in functional microbial abundance have a large impact on EMF (Wang et al. 2022). Consistent with former studies (Luo et al. 2019; Wang et al. 2022), our results demonstrated the linear relationship between the EMF and the abundances of most soil microbial populations related to C-N-P-S cycles (Fig. 4). This result may be related to a high redundancy of microbial communities in the soil for general functions (Li et al. 2021). More abundant microbes ensure more interactions among them to drive the EMF, thus improving ecosystem stability (Chen et al. 2022). In addition, the mechanism of multifunctional niche complementarity and interspecific functional differences can also explain the positive relationship between the EMF and soil functional microbial abundances (Luo et al. 2018). Both averaging and multi-threshold method proved the significant associations between the EMF and the abundances of soil microbes (Figs. 5, 6). Most microbes in soil are heterotrophic organisms, and organic matter degradation is one of the most redundant functions of soil microbial communities (Maron et al. 2018). Thus, microbe-driven C degradation in soil is an important driver regulating ecosystem functions.
Soil pH and available nutrients could directly influence the EMF or indirectly by regulating soil special functional microbes (Fig. 7; Luo et al. 2018; Li et al. 2023). Increased unstable C and available nutrients such as AP and inorganic N by biochar and organic fertilizer can promote the growth and metabolism of soil special microbes, thereby stimulating them to degrade organic matter by secreting a series of enzymes (Zhou et al. 2020; Ibrahim et al. 2021). Nutrients in the process of organic matter degradation are released by soil microbes and utilized by above-ground community again, thus improving plant nutrient uptake and biomass (Delgado-Baquerizo et al. 2016). In addition, higher C availability in soil can enable the recruitment of specific microorganisms associated with N and P transformations, which in turn promotes the positive relationships of the EMF with soil nutrient cycles (Ren et al. 2023). Higher soil pH results in the disruption of the binding between organic components and clay, affecting soil microbiomes, and thus positively affecting C and nutrient cycles (Luo et al. 2018; Wan et al. 2020). For example, biochar and organic fertilizer inputs can increase soil pH to promote the growth of phosphorolytic microbes and phosphatase activity, and then strengthen organic P mineralization. Increased P mineralization further improves soil P availability and promotes crop P uptake and yield (Zheng et al. 2019; Hu et al. 2023). Thus, biochar and organic fertilizer applications can improve the living habitats of special functional microbial taxa by influencing soil pH and available nutrients, thereby positively regulating EMF. However, a wide and diverse range of functions and services across multiple ecosystem categories, e.g., greenhouse gases, plant functional traits, etc., also need to be investigated in order to provide a representative measure of overall ecosystem functioning (Garland et al. 2021). Meanwhile, comprehensive assessment of the EMF under organic amendment applications requires additional effort in disclosing rhizosphere effects that is regulate the associations between above- and below-ground communities.
5 Conclusion
This study demonstrated the applications of biochar and organic fertilizer, either alone or in combination, could significantly increase most ecosystem-single functions such as crop productivity, soil nutrient storage, and soil enzyme activities, which contributed to increase of EMF. Biochar and organic fertilizer inputs could significantly enrich the microbial taxa in soil related to C, N, P, and S cycles to varying degree. Co-applications of biochar and organic fertilizer showed a higher positive impact on ecosystem functions and soil functional microbial abundances than their individual applications. The abundances of most these microbial populations were positively associated with the EMF and most ecosystem-single functions. The microbial taxa related to C degradation, nitrification, nitrate-reduction, Po-mineralization, and S-cycling showed large and significant prediction and association with the EMF, which is regulated by soil pH and nutrient availability. These findings suggest that biochar and organic fertilizer applications, especially their co-application, could promote the propagation of soil functional microbiomes by regulating soil attributes and then improve ecosystem functions and services.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Additional files).
References
Aasfar A, Bargaz A, Yaakoubi K, Hilali A, Bennis I, Zeroual Y, Meftah Kadmiri I (2021) Nitrogen fixing Azotobacter species as potential soil biological enhancers for crop nutrition and yield stability. Front Microbiol 12:628379
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971
Anantharaman K, Hausmann B, Jungbluth SP, Kantor RS, Lavy A, Warren LA, Rappé MS, Pester M, Loy A, Thomas BC, Banfield JF (2018) Expanded diversity of microbial groups that shape the dissimilatory sulfur cycle. ISME J 12(7):1715–1728
Azeem M, Hayat R, Hussain Q, Tahir MI, Imran M, Abbas Z, Sajid M, Latif A, Irfan M (2019) Effects of biochar and NPK on soil microbial biomass and enzyme activity during 2 years of application in the arid region. Arab J Geosci 12:1–13
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473
Bai SH, Omidvar N, Gallart M, Kämper W, Tahmasbian I, Farrar MB, Singh K, Zhou G, Muqadass B, Xu CY, Koech R, Li Y, Nguyen TTN, van Zwieten L (2022) Combined effects of biochar and fertilizer applications on yield: a review and meta-analysis. Sci Total Environ 808:152073
Bao SD (2000) Soil and Agro-Chemistry Analysis, 3rd ed. China Agric. Press, Beijing
Basile-Doelsch I, Balesdent J, Pellerin S (2020) Reviews and syntheses: the mechanisms underlying carbon storage in soil. Biogeosciences 17(21):5223–5242
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5(2):202–214
Breiman L (2001) Random forest. Mach Learn 45:5–32
Brtnicky M, Dokulilova T, Holatko J, Pecina V, Kintl A, Latal O, Vyhnanek T, Prichystalova J, Datta R (2019) Long-term effects of biochar-based organic amendments on soil microbial parameters. Agronomy 9(11):747
Cao H, Ning L, Xun M, Feng F, Li P, Yue S, Song J, Zhang W, Yang H (2019) Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl Soil Ecol 135:25–32
Chen W, Wang J, Chen X, Meng Z, Xu R, Duoji D, Zhang J, He J, Wang Z, Chen J, Liu K, Hu T, Zhang Y (2022) Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol Biochem 172:108766
Dai Z, Yu M, Chen H, Zhao H, Huang Y, Su W, Xia F, Chang SX, Brookes PC, Dahlgren RA, Xu J (2020) Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Global Change Biol 26(9):5267–5276
Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, Gaitan JJ, Encinar D, Berdugo M, Campbell CD, Singh BK (2016) Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat Commun 7(1):10541
Delgado-Baquerizo M, Reich PB, Trivedi C, Eldridge DJ, Abades S, Alfaro FD, Bastida F, Berhe AA, Cutler NA, Gallardo A, García-Velázquez L, Hart CC, Hayes PE, He JZ, Hseu ZY, Hu HW, Kirchmair M, Neuhauser S, Pérez CA, Reed SC, Santos F, Sullivan BW, Trivedi P, Wang JT, Weber-Grullon L, Williams MA, Singh BK (2020) Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat Ecol Evol 4:210–220
Dominchin MF, Verdenelli RA, Berger MG, Aoki A, Meriles JM (2021) Impact of N-fertilization and peanut shell biochar on soil microbial community structure and enzyme activities in a Typic Haplustoll under different management practices. Eur J Soil Biol 104:103298
Dotaniya ML, Aparna K, Dotaniya CK, Singh M, Regar KL (2019) Role of soil enzymes in sustainable crop production. In: Kuddus M (ed) Enzymes in food biotechnology. Academic Press, Cambridge, pp 569–589
Doyle A, Weintraub MN, Schimel JP (2004) Persulfate digestion and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil Sci Soc Am J 68:669–676
Galhardo CX, Masini JC (2000) Spectrophotometric determination of phosphate and silicate by sequential injection using molybdenum blue chemistry. Anal Chim Acta 417:191–200
Gao Y, Liang A, Zhang Y, Huang D, McLaughlin N, Zhang Y, Wang Y, Chen X, Zhang S (2023) Effect of tillage practices on soil CO2 emissions, microbial C-fixation, and C-degradation functional gene abundance in Northeast China. J Soil Sediment 23(1):446–458
Garland G, Banerjee S, Edlinger A, Miranda Oliveira E, Herzog C, Wittwer R, Philippot L, Maestre FT, van Der Heijden MG (2021) A closer look at the functions behind ecosystem multifunctionality: a review. J Ecol 109(2):600–613
Giles M, Morley N, Baggs EM, Daniell TJ (2012) Soil nitrate reducing processes–drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front Microbiol 3:407
Gong H, Li J, Liu Z, Zhang Y, Hou R, Zhu O (2022) Mitigated greenhouse gas emissions in cropping systems by organic fertilizer and tillage management. Land 11(7):1026
Guo Z, Han J, Li J, Xu Y, Wang X (2019) Effects of long-term fertilization on soil organic carbon mineralization and microbial community structure. PLoS ONE 14(1):e0211163
Han Z, Xu P, Li Z, Lin H, Zhu C, Wang J, Zou J (2022) Microbial diversity and the abundance of keystone species drive the response of soil multifunctionality to organic substitution and biochar amendment in a tea plantation. GCB Bioenergy 14(4):481–495
Hossain ME, Shahrukh S, Hossain SA (2022) Chemical fertilizers and pesticides: impacts on soil degradation, groundwater, and human health in Bangladesh. Environmental degradation: challenges and strategies for mitigation. Springer International Publishing, Cham, pp 63–92
Hu W, Ran J, Dong L, Du Q, Ji M, Yao S, Sun Y, Gong C, Hou Q, Gong H, Chen R, Lu J, Xie S, Wang Z, Huang H, Li X, Xiong J, Xia R, Wei M, Zhao D, Zhang Y, Li J, Yang H, Wang X, Deng Y, Sun Y, Li H, Zhang L, Chu Q, Li X, Aqeel M, Manan A, Akram MA, Liu X, Li R, Li F, Hou C, Liu J, He JS, An L, Bardgett RD, Schmid B, Deng J (2021) Aridity-driven shift in biodiversity–soil multifunctionality relationships. Nat Commun 12(1):5350
Hu W, Zhang Y, Rong X, Fei J, Peng J, Luo G (2023) Coupling amendment of biochar and organic fertilizers increases maize yield and phosphorus uptake by regulating soil phosphatase activity and phosphorus-acquiring microbiota. Agr Ecosyst Environ 355:108582
Huang X, Wang C, Liu Q, Zhu Z, Lynn TM, Shen J, Whiteley AS, Kumaresan D, Tida GE, Wu J (2018) Abundance of microbial CO2-fixing genes during the late rice season in a long-term management paddy field amended with straw and straw-derived biochar. Can J Soil Sci 98(2):306–316
Ibrahim MM, Zhang H, Guo L, Chen Y, Heiling M, Zhou B, Mao Y (2021) Biochar interaction with chemical fertilizer regulates soil organic carbon mineralization and the abundance of key C-cycling-related bacteria in rhizosphere soil. Eur J Soil Biol 106:103350
Jarvie HP, Withers PJA, Neal C (2002) Review of robust measurement of phosphorus in river water: sampling, storage, fractionation and sensitivity. Hydrol Earth Syst Sci 6:113–131
Jia R, Zhou J, Chu J, Shahbaz M, Yang Y, Jones DL, Zang H, Razavi BS, Zeng Z (2022) Insights into the associations between soil quality and ecosystem multifunctionality driven by fertilization management: a case study from the North China Plain. J Cleaner Prod 362:132265
Kätterer T, Roobroeck D, Andrén O, Kimutai G, Karltun E, Kirchmann H, Nybery G, Vanlauwe B, de Nowina KR (2019) Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crop Res 235:18–26
Lazcano C, Zhu-Barker X, Decock C (2021) Effects of organic fertilizers on the soil microorganisms responsible for N2O emissions: a review. Microorganisms 9(5):983
Li Y, Chapman SJ, Nicol GW, Yao H (2018) Nitrification and nitrifiers in acidic soils. Soil Biol Biochem 116:290–301
Li S, Wang S, Fan M, Wu Y, Shangguan Z (2020) Interactions between biochar and nitrogen impact soil carbon mineralization and the microbial community. Soil Tillage Res 196:104437
Li M, Guo J, Ren T, Luo G, Shen Q, Lu J, Guo S, Ling N (2021) Crop rotation history constrains soil biodiversity and multifunctionality relationships. Agr Ecosyst Environ 319:107550
Li QM, Zhang D, Zhang JZ, Zhou ZJ, Pan Y, Yang ZH, Zhu JH, Liu YH, Zhang LF (2023) Crop rotations increased soil ecosystem multifunctionality by improving keystone taxa and soil properties in potatoes. Front Microbiol 14:1034761
Liu Y, Wang C, He N, Wen X, Gao Y, Li S, Niu S, Butterbach-Bahl K, Luo Y, Yu G (2017) A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: latitudinal patterns and mechanisms. Global Change Biol 23(1):455–464
Liu H, Yang X, Liang C, Li Y, Qiao L, Ai Z, Xue S, Liu G (2019a) Interactive effects of microplastics and glyphosate on the dynamics of soil dissolved organic matter in a Chinese loess soil. CATENA 182:104177
Liu T, Chen X, Gong X, Lubbers IM, Jiang Y, Feng W, Li X, Whalen JK, Bonkowski M, Griffiths BS, Hu F, Liu M (2019b) Earthworms coordinate soil biota to improve multiple ecosystem functions. Curr Biol 29(20):3420–3429
Liu H, Du X, Li Y, Han X, Li B, Zhang X, Li Q, Liang W (2022a) Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. J Cleaner Prod 347:131323
Liu M, Linna C, Ma S, Ma Q, Song W, Shen M, Song L, Cui K, Zhou Y, Wang L (2022b) Biochar combined with organic and inorganic fertilizers promoted the rapeseed nutrient uptake and improved the purple soil quality. Front Nutr 9:997151
Liu Q, Meki K, Zheng H, Yuan Y, Shao M, Luo X, Li X, Jiang Z, Li F, Xing B (2023) Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 5(1):45
Luo G, Rensing C, Chen H, Liu M, Wang M, Guo S, Ling N, Shen Q (2018) Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management. Funct Ecol 32(4):1103–1116
Luo G, Wang T, Li K, Li L, Zhang J, Guo S, Ling N, Shen Q (2019) Historical nitrogen deposition and straw addition facilitate the resistance of soil multifunctionality to drying-wetting cycles. Appl Environ Microb 85(8):e02251-e2318
Luo G, Xue C, Jiang Q, Xiao Y, Zhang F, Guo S (2020) Soil carbon, nitrogen, and phosphorus cycling microbial populations and their resistance to global change depend on soil C:N: P stoichiometry. mSystems 5(3):e00162-20
Maron PA, Sarr A, Kaisermann A, Lévêque J, Mathieu O, Guigue J, Karimi B, Bernard L, Dequiedt S, Terrat S, Chabbi A, Ranjard L (2018) High microbial diversity promotes soil ecosystem functioning. Appl Environ Microb 84(9):e02738-e2817
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 2019:1–9
Ren T, Liao J, Jin L, Delgado-Baquerizo M, Ruan H (2023) Application of biogas-slurry and biochar improves soil multifunctionality in a poplar plantation during afforestation processes. Plant Soil. https://doi.org/10.1007/s11104-023-05968-x
Saha B, Saha S, Roy PD, Padhan D, Pati S, Hazra GC (2018) Microbial transformation of sulphur: an approach to combat the sulphur deficiencies in agricultural soils. In: Meena VS (ed) Role of rhizospheric microbes in soil: Volume 2: nutrient management and crop improvement. Springer, Berlin, pp 77–97
Saifullah DS, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ 625:320–335
Singh VK, Rai S, Singh D, Upadhyay RS (2021) Application of soil microorganisms for agricultural and environmental sustainability: a review. In: Dubey SK, Verma SK (eds) Plant, soil and microbes in tropical ecosystems. Springer, Berlin, pp 151–175
Sun R, Guo X, Wang D, Chu H (2015) Effects of long-term application of chemical and organic fertilizers on the abundance of microbial communities involved in the nitrogen cycle. Appl Soil Ecol 95:171–178
Wan W, Tan J, Wang Y, Qin Y, He H, Wu H, Zuo W, He D (2020) Responses of the rhizosphere bacterial community in acidic crop soil to pH: changes in diversity, composition, interaction, and function. Sci Total Environ 700:134418
Wang J, Xie J, Li L, Luo Z, Zhang R, Wang L, Jiang Y (2021) The impact of fertilizer amendments on soil autotrophic bacteria and carbon emissions in maize field on the semiarid Loess Plateau. Front Microb 12:664120
Wang T, Duan Y, Liu G, Shang X, Liu L, Zhang K, Li J, Zou Z, Zhu X, Fang W (2022) Tea plantation intercropping green manure enhances soil functional microbial abundance and multifunctionality resistance to drying-rewetting cycles. Sci Total Environ 810:151282
Wang X, Ge H, Fang Y, Liu C, Eltohamy KM, Wang Z, Liang X (2023) Biochar reduces colloidal phosphorus in leachate by regulating phoD- and phoC-harboring microbial communities during drying/rewetting cycles. Biochar 5(1):1–15
Waqas MA, Li Y, Smith P, Wang X, Ashraf MN, Noor MA, Amou M, Shi S, Zhu Y, Li J, Wan Y, Qin X, Gao Q, Liu S (2020) The influence of nutrient management on soil organic carbon storage, crop production, and yield stability varies under different climates. J Clean Prod 268:121922
Wu J, Sha C, Wang M, Ye C, Li P, Huang S (2021) Effect of organic fertilizer on soil bacteria in maize fields. Land 10(3):328
Xiao Y, Peng Y, Peng F, Zhang Y, Yu W, Sun M, Gao X (2018) Effects of concentrated application of soil conditioners on soil–air permeability and absorption of nitrogen by young peach trees. Soil Sci Plant Nutr 64(3):423–432
Yan B, Zhang Y, Wang Y, Rong X, Peng J, Fei J, Luo G (2023) Biochar amendments combined with organic fertilizer improve maize productivity and mitigate nutrient loss by regulating the C–N–P stoichiometry of soil, microbiome, and enzymes. Chemosphere 324:138293
Yuan Z, Ali A, Ruiz-Benito P, Jucker T, Mori AS, Wang S, Zhang X, Li H, Hao Z, Wang X, Loreau M (2020) Above-and below-ground biodiversity jointly regulate temperate forest multifunctionality along a local-scale environmental gradient. J Ecol 108(5):2012–2024
Zabala JA, Martínez-Paz JM, Alcon F (2021) A comprehensive approach for agroecosystem services and disservices valuation. Sci Total Environ 768:144859
Zak D, Stutter M, Jensen HS, Egemose S, Carstensen MV, Audet J, Strand JA, Feuerbach P, Hoffmann CC, Christen B, Knudsen M, Stockan J, Watson H, Heckrath G, Kronvang B (2019) An assessment of the multifunctionality of integrated buffer zones in Northwestern Europe. J Environ Qual 48(2):362–375
Zhang Y, Wang F, Zhang J, Zhu T, Lin C, Müller C, Cai Z (2015) Cattle manure and straw have contrasting effects on organic nitrogen mineralization pathways in a subtropical paddy soil. Acta Agr Scand B-S P 65(7):619–628
Zhang Y, Yan J, Rong X, Han Y, Yang Z, Hou K, Zhao H, Hu W (2021) Responses of maize yield, nitrogen and phosphorus runoff losses and soil properties to biochar and organic fertilizer application in a light-loamy fluvo-aquic soil. Agr Ecosyst Environ 314:107433
Zhang YJ, Gao W, Luan HA, Tang JW, Li RN, Li MY, Zhang HZ, Huang SW (2022) Effects of a decade of organic fertilizer substitution on vegetable yield and soil phosphorus pools, phosphatase activities, and the microbial community in a greenhouse vegetable production system. J Integr Agr 21(7):2119–2133
Zhao Q, Wang Y, Xu Z, Yun J, Yu Z (2022a) Unravelling how biochar and dung amendments determine the functional structure and community assembly related to methane metabolisms in grassland soils. Biochar 4(1):49
Zhao Y, Wang X, Yao G, Lin Z, Xu L, Jiang Y, Jin Z, Shan S, Ping L (2022b) Advances in the effects of biochar on microbial ecological function in soil and crop quality. Sustainability 14:10411
Zheng B, Zhu Y, Sardans J, Peñuelas J, Su J (2018) QMEC: a tool for high-throughput quantitative assessment of microbial functional potential in C, N, P, and S biogeochemical cycling. Sci China Life Sci 61(12):1451–1462
Zheng B, Zhang D, Wang Y, Hao X, Wadaan MAM, Hozzein WN, Peñuelas J, Zhu Y, Yang X (2019) Responses to soil pH gradients of inorganic phosphate solubilizing bacteria community. Sci Rep 9(1):25
Zhou W, He P, Li S, Lin B (2005) Mineralization of organic sulfur in paddy soils under flooded conditions and its availability to plants. Geoderma 125(1–2):85–93
Zhou G, Gao S, Lu Y, Liao Y, Nie J, Cao W (2020) Co-incorporation of green manure and rice straw improves rice production, soil chemical, biochemical and microbiological properties in a typical paddy field in southern China. Soil Tillage Res 197:104499
Acknowledgements
Our work was supported by National Natural Science Foundation of China (42107262) and Key Field Research and Development Program of Hunan Province (2023NK2028).
Funding
National Natural Science Foundation of China (42107262) and Key Field Research and Development Program of Hunan Province (2023NK2028).
Author information
Authors and Affiliations
Contributions
GL, XZ, and YZ conceived, designed and financially supported the study; WH analyzed the data and wrote the paper, and conducted the analytical work with GL and JF; XR and JP revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Bin Gao.
Supplementary Information
Additional file 1. Supplementary methods, figures and tables: Table
S1. The primers and function of 64 biomaker genes. Table S2. Biogeochemical cycles, group of functions, full names, abbreviations, and services supported by the 18 ecosystem functions. Table S3. Basic soil properties (0–20 cm) at beginning of field experiment. Table S4. Fertilizer application rates and fertilizer types of each treatment. Fig. S1. Random forest mean predictor importance (% increase in MSE) of the soil functional microbial population abundance with respect to ecosystem multifunctionality. Fig. S2. Random forest mean predictor importance (% increase in MSE) of the soil chemical property with respect to Functional microbial population abundance. AP available P, DON dissolved organic N, DOP dissolved organic P, DOC dissolved organic carbon.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, W., Zhang, Y., Rong, X. et al. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality. Biochar 6, 3 (2024). https://doi.org/10.1007/s42773-023-00296-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00296-w