Abstract
Background
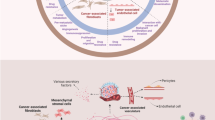
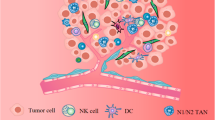
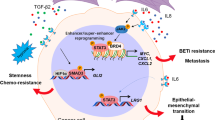
Various cancers have been found to be associated with heterogeneous and adaptive tumor microenvironments (TMEs) and to be driven by the local TMEs in which they thrive. Cancer heterogeneity plays an important role in tumor cell survival, progression and drug resistance. The diverse cellular components of the TME may include cancer-associated fibroblasts, adipocytes, pericytes, mesenchymal stem cells, endothelial cells, lymphocytes and other immune cells. These components may support tumor development through the secretion of growth factors, evasion from immune checkpoints, metabolic adaptations, modulations of the extracellular matrix, activation of oncogenes and the acquisition of drug resistance. Here, we will address recent advances in our understanding of the molecular mechanisms underlying stromal-tumor cell interactions, with special emphasis on basic and pre-clinical information that may facilitate the design of novel personalized cancer therapies.
Conclusions
This review presents a holistic view on the translational potential of the interplay between stromal cells and cancer cells. This interplay is currently being employed for the development of promising preclinical and clinical biomarkers, and the design of small molecule inhibitors, antibodies and small RNAs for (combinatorial) cancer treatment options. In addition, nano-carriers, tissue scaffolds and 3-D based matrices are being developed to precisely and safely deliver these compounds.





Similar content being viewed by others
References
M. Plummer, C. de Martel, J. Vignat, J. Ferlay, F. Bray, S. Franceschi, Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 4, e609–e616 (2016)
J. Bishop, The molecular genetics of cancer. Science 235, 305–311 (1987)
M.M. Gottesman, Mechanisms of cancer drug resistance. Annu. Rev. Med. 53, 615–627 (2002)
A.A. Alizadeh, V. Aranda, A. Bardelli, C. Blanpain, C. Bock, C. Borowski, C. Caldas, A. Califano, M. Doherty, M. Elsner, M. Esteller, R. Fitzgerald, J.O. Korbel, P. Lichter, C.E. Mason, N. Navin, D. Peer, K. Polyak, C.W.M. Roberts, L. Siu, A. Snyder, H. Stower, C. Swanton, R.G.W. Verhaak, J.C. Zenklusen, J. Zuber, J. Zucman-Rossi, Toward understanding and exploiting tumor heterogeneity. Nat. Med. 21, 846–853 (2015)
J.W. Clark, B.A. Chabner, Limits to precision cancer medicine. N. Engl. J. Med. 376, 95–97 (2017)
L.A. Liotta, E.C. Kohn, The microenvironment of the tumour host interface. Nature 411, 375–379 (2001)
B.S. Wiseman, Stromal effects on mammary gland development and breast cancer. Science 296, 1046–1049 (2002)
J.W. Pollard, Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 4, 71–78 (2004)
V. Kumar, L. Donthireddy, D. Marvel, T. Condamine, F. Wang, S. Lavilla-Alonso, A. Hashimoto, P. Vonteddu, R. Behera, M.A. Goins, C. Mulligan, B. Nam, N. Hockstein, F. Denstman, S. Shakamuri, D.W. Speicher, A.T. Weeraratna, T. Chao, R.H. Vonderheide, L.R. Languino, P. Ordentlich, Q. Liu, X. Xu, A. Lo, E. Puré, C. Zhang, A. Loboda, M.A. Sepulveda, L.A. Snyder, D.I. Gabrilovich, Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32 e655, 654–668 (2017)
Y. Qiao, C. Zhang, A. Li, D. Wang, Z. Luo, Y. Ping, B. Zhou, S. Liu, H. Li, D. Yue, Z. Zhang, X. Chen, Z. Shen, J. Lian, Y. Li, S. Wang, F. Li, L. Huang, L. Wang, B. Zhang, J. Yu, Z. Qin, Y. Zhang, IL6 derived from cancer-associated fibroblasts promotes chemoresistance via CXCR7 in esophageal squamous cell carcinoma. Oncogene 37, 873–883 (2017). https://doi.org/10.1038/onc.2017.387
H.G. Augustin, G.Y. Koh, Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 357, eaal2379 (2017)
M. Spaw, S. Anant, S.M. Thomas, Stromal contributions to the carcinogenic process. Mol. Carcinog. 56, 1199–1213 (2017)
T. Shibue, R.A. Weinberg, EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017)
M. Cheng, S. Ho, J.H. Yoo, D.H. Tran, K. Bakirtzi, B. Su, D.H. Tran, Y. Kubota, R. Ichikawa, H.W. Koon, Cathelicidin suppresses colon cancer development by inhibition of cancer associated fibroblasts. Clin. Exp. Gastroenterol. 8, 13–29 (2015)
V. Gkretsi, A. Stylianou, P. Papageorgis, C. Polydorou, T. Stylianopoulos, Remodeling components of the tumor microenvironment to enhance cancer therapy. Front. Oncol. 5, 214 (2015)
D. Jahagirdar, S. Purohit, A. Jain, N.K. Sharma, Export of microRNAs: A bridge between breast carcinoma and their neighboring cells. Front. Oncol. 6, 147 (2016)
M. Najar, H. Fayyad-Kazan, W.H. Faour, B. Badran, F. Journe, L. Lagneaux, Breast cancer cells and bone marrow mesenchymal stromal cells: A regulated modulation of the breast tumor in the context of immune response. Inflamm. Res. 66, 129–139 (2017)
P. Nilendu, A. Kumar, A. Kumar, J.K. Pal, N.K. Sharma, Breast cancer stem cells as last soldiers eluding therapeutic burn: A hard nut to crack. Int. J. Cancer 142, 7–17 (2017)
R. Kalluri, The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016)
P.J. Morin, in Cancer Drug Resistance, ed. by B.A. Teicher (Humana Press, Totowa, NJ, 2006), pp. 201–210
A. Morandi, E. Giannoni, P. Chiarugi, Nutrient exploitation within the tumor–stroma metabolic crosstalk. Trends Cancer 2, 736–746 (2016)
K. Cyll, E. Ersvær, L. Vlatkovic, M. Pradhan, W. Kildal, M.A. Kjær, A. Kleppe, T.S. Hveem, B. Carlsen, S. Gill, S. Löffeler, E.S. Haug, H. Wæhre, P. Sooriakumaran, H.E. Danielsen, Tumour heterogeneity poses a significant challenge to cancer biomarker research. Br. J. Cancer 117, 367–375 (2017)
M. Majidinia, B. Yousefi, Breast tumor stroma: A driving force in the development of resistance to therapies. Chem. Biol. Drug Des. 89, 309–318 (2017)
N. McGranahan, C. Swanton, Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 168, 613–628 (2017)
N. Picco, E. Sahai, P.K. Maini, A.R.A. Anderson, Integrating models to quantify environment-mediated drug resistance. Cancer Res. 77, 5409–5418 (2017)
R.F. Schwabe, C. Jobin, The microbiome and cancer. Nat. Rev. Cancer 13, 800–812 (2013)
A.L. Ribeiro, O.K. Okamoto, Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 868475, 2015 (2015)
C.C. Maley, A. Aktipis, T.A. Graham, A. Sottoriva, A.M. Boddy, Classifying the evolutionary and ecological features of neoplasms. Nat. Rev. Cancer 17, 605–619 (2017)
H. Omar Al-Hassi, O. Ng, M. Brookes, Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 67, 395 (2018)
E. Perez-Chanona, G. Trinchieri, The role of microbiota in cancer therapy. Curr. Opin. Immunol. 39, 75–81 (2016)
Z. Benyahia, N. Dussault, M. Cayol, R. Sigaud, C. Berenguer-Daize, C. Delfino, A. Tounsi, S. Garcia, P.M. Martin, K. Mabrouk, L. Ouafik, Stromal fibroblasts present in breast carcinomas promote tumor growth and angiogenesis through adrenomedullin secretion. Oncotarget 8, 15744–15762 (2017)
L. Bolm, S. Cigolla, U.A. Wittel, U.T. Hopt, T. Keck, D. Rades, P. Bronsert, U.F. Wellner, The role of fibroblasts in pancreatic cancer: Extracellular matrix versus paracrine factors. Transl. Oncol. 10, 578–588 (2017)
N. Cohen, O. Shani, Y. Raz, Y. Sharon, D. Hoffman, L. Abramovitz, N. Erez, Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene 36, 4457–4468 (2017)
N.A. Bhowmick, E.G. Neilson, H.L. Moses, Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (2004)
A. Aboussekhra, Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int. J. Dev. Biol. 55, 841–849 (2011)
U.M. Polanska, A. Orimo, Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting mesenchymal cells. J. Cell. Physiol. 228, 1651–1657 (2013)
H. Luo, G. Tu, Z. Liu, M. Liu, Cancer-associated fibroblasts: A multifaceted driver of breast cancer progression. Cancer Lett. 361, 155–163 (2015)
A. Orimo, R.A. Weinberg, Stromal fibroblasts in cancer: A novel tumor-promoting cell type. Cell Cycle 5, 1597–1601 (2006)
G.S. Markopoulos, E. Roupakia, M. Tokamani, E. Chavdoula, M. Hatziapostolou, C. Polytarchou, K.B. Marcu, A.G. Papavassiliou, R. Sandaltzopoulos, E. Kolettas, A step-by-step microRNA guide to cancer development and metastasis. Cell. Oncol. 40, 303–339 (2017)
P. Mishra, D. Banerjee, A. Ben-Baruch, Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. J. Leukoc. Biol. 89, 31–39 (2011)
K.O. Osuala, M. Sameni, S. Shah, N. Aggarwal, M.L. Simonait, O.E. Franco, Y. Hong, S.W. Hayward, F. Behbod, R.R. Mattingly, B.F. Sloane, Il-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer 15, 584 (2015)
Ö. Sağlam, Z.S. Ünal, C. Subaşı, E. Ulukaya, E. Karaöz, IL-6 originated from breast cancer tissue-derived mesenchymal stromal cells may contribute to carcinogenesis. Tumour Biol. 36, 5667–5677 (2015)
D. von Ahrens, T.D. Bhagat, D. Nagrath, A. Maitra, A. Verma, The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 10, 76 (2017)
S.-F. Hendrayani, H.H. Al-Khalaf, A. Aboussekhra, The cytokine IL-6 reactivates breast stromal fibroblasts through transcription factor STAT3-dependent up-regulation of the RNA-binding protein AUF1. J. Biol. Chem. 289, 30962–30976 (2014)
T. Ishimoto, K. Miyake, T. Nandi, M. Yashiro, N. Onishi, K.K. Huang, S.J. Lin, R. Kalpana, S.T. Tay, Y. Suzuki, B.C. Cho, D. Kuroda, K. Arima, D. Izumi, M. Iwatsuki, Y. Baba, E. Oki, M. Watanabe, H. Saya, K. Hirakawa, H. Baba, P. Tan, Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 153, 191-204. e116 (2017), 204.e16
E. Sjöberg, M. Augsten, J. Bergh, K. Jirström, A. Östman, Expression of the chemokine CXCL14 in the tumour stroma is an independent marker of survival in breast cancer. Br. J. Cancer 114, 1117–1124 (2016)
M. Yao, E. Yu, V. Staggs, F. Fan, N. Cheng, Elevated expression of chemokine C-C ligand 2 in stroma is associated with recurrent basal-like breast cancers. Mod. Pathol. 29, 810–823 (2016)
L. Zhao, G. Ji, X. Le, Z. Luo, C. Wang, M. Feng, L. Xu, Y. Zhang, W.B. Lau, B. Lau, Y. Yang, L. Lei, H. Yang, Y. Xuan, Y. Chen, X. Deng, T. Yi, S. Yao, X. Zhao, Y. Wei, S. Zhou, An integrated analysis identifies STAT4 as a key regulator of ovarian cancer metastasis. Oncogene 36, 3384–3396 (2017)
N. Kramer, J. Schmöllerl, C. Unger, H. Nivarthi, A. Rudisch, D. Unterleuthner, M. Scherzer, A. Riedl, M. Artaker, I. Crncec, D. Lenhardt, T. Schwarz, B. Prieler, X. Han, M. Hengstschläger, J. Schüler, R. Eferl, R. Moriggl, W. Sommergruber, H. Dolznig, Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene 36, 5460–5472 (2017)
A. Avgustinova, M. Iravani, D. Robertson, A. Fearns, Q. Gao, P. Klingbeil, A.M. Hanby, V. Speirs, E. Sahai, F. Calvo, C.M. Isacke, Tumour cell-derived Wnt7a recruits and activates fibroblasts to promote tumour aggressiveness. Nat. Commun. 7, 10305 (2016)
J.S.K. Chan, M.K. Sng, Z.Q. Teo, H.C. Chong, J.S. Twang, N.S. Tan, Targeting nuclear receptors in cancer-associated fibroblasts as concurrent therapy to inhibit development of chemoresistant tumors. Oncogene 37, 160–173 (2017)
M.P. Pinto, W.W. Dye, B.M. Jacobsen, K.B. Horwitz, Malignant stroma increases luminal breast cancer cell proliferation and angiogenesis through platelet-derived growth factor signaling. BMC Cancer 14, 735 (2014)
Y. Kitadai, T. Sasaki, T. Kuwai, T. Nakamura, C.D. Bucana, S.R. Hamilton, I.J. Fidler, Expression of activated platelet-derived growth factor receptor in stromal cells of human colon carcinomas is associated with metastatic potential. Int. J. Cancer 119, 2567–2574 (2006)
S. Neri, T. Miyashita, H. Hashimoto, Y. Suda, M. Ishibashi, H. Kii, H. Watanabe, T. Kuwata, M. Tsuboi, K. Goto, T. Menju, M. Sonobe, H. Date, A. Ochiai, G. Ishii, Fibroblast-led cancer cell invasion is activated by epithelial–mesenchymal transition through platelet-derived growth factor BB secretion of lung adenocarcinoma. Cancer Lett. 395, 20–30 (2017)
P.S.H. Soon, E. Kim, C.K. Pon, A.J. Gill, K. Moore, A.J. Spillane, D.E. Benn, R.C. Baxter, Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells. Endocr. Relat. Cancer 20, 1–12 (2013)
A. Orimo, P.B. Gupta, D.C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V.J. Carey, A.L. Richardson, R.A. Weinberg, Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335–348 (2005)
N.L.E. Harris, C. Vennin, J.R.W. Conway, K.L. Vine, M. Pinese, M.J. Cowley, R.F. Shearer, M.C. Lucas, D. Herrmann, A.H. Allam, M. Pajic, J.P. Morton, A.V. Biankin, M. Ranson, P. Timpson, D.N. Saunders, SerpinB2 regulates stromal remodelling and local invasion in pancreatic cancer. Oncogene 36, 4288–4298 (2017)
N. Perurena, C. Zandueta, S. Martínez-Canarias, H. Moreno, S. Vicent, A.S. Almeida, E. Guruceaga, R.R. Gomis, M. Santisteban, M. Egeblad, EPCR promotes breast cancer progression by altering SPOCK1/testican 1-mediated 3D growth. J. Hematol. Oncol. 10, 23 (2017)
S. Busch, A. Acar, Y. Magnusson, P. Gregersson, L. Ryden, G. Landberg, TGF-beta receptor type-2 expression in cancer-associated fibroblasts regulates breast cancer cell growth and survival and is a prognostic marker in pre-menopausal breast cancer. Oncogene 34, 27–38 (2015)
A.M. Hammer, G.M. Sizemore, V.C. Shukla, A. Avendano, S.T. Sizemore, J.J. Chang, R.D. Kladney, M.C. Cuitiño, K.A. Thies, Q. Verfurth, A. Chakravarti, L.D. Yee, G. Leone, J.W. Song, S.N. Ghadiali, M.C. Ostrowski, Stromal PDGFR-α activation enhances matrix stiffness, impedes mammary ductal development, and accelerates tumor growth. Neoplasia 19, 496–508 (2017)
Y. Kinugasa, T. Matsui, N. Takakura, CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells 32, 145–156 (2014)
B.Y. Owusu, S. Thomas, P. Venukadasula, Z. Han, J.W. Janetka, R.A. Galemmo Jr., L. Klampfer, Targeting the tumor-promoting microenvironment in MET-amplified NSCLC cells with a novel inhibitor of pro-HGF activation. Oncotarget 8, 63014–63025 (2017)
J. Paulsson, L. Rydén, C. Strell, O. Frings, N.P. Tobin, T. Fornander, J. Bergh, G. Landberg, O. Stål, A. Östman, High expression of stromal PDGFRβ is associated with reduced benefit of tamoxifen in breast cancer. J. Pathol. Clin. Res. 3, 38–43 (2016)
C.A.S. Corsa, A. Brenot, W.R. Grither, S. Van Hove, A.J. Loza, K. Zhang, S.M. Ponik, Y. Liu, D.G. DeNardo, K.W. Eliceiri, P.J. Keely, G.D. Longmore, The action of discoidin domain receptor 2 in basal tumor cells and stromal cancer-associated fibroblasts is critical for breast cancer metastasis. Cell Rep. 15, 2510–2523 (2016)
X. Li, W. Zhu, Z. Chen, L. Luo, J. Huang, F. Zhang, M. Li, Y. Guo, L. Guo, Fibroblast growth factor-inducible 14 regulates cell growth and multidrug resistance of small-cell lung cancer through the nuclear factor-κB pathway. Anti-Cancer Drugs 25, 1152–1164 (2014)
E.M. De Francesco, A.H. Sims, M. Maggiolini, F. Sotgia, M.P. Lisanti, R.B. Clarke, GPER mediates the angiocrine actions induced by IGF1 through the HIF-1alpha/VEGF pathway in the breast tumor microenvironment. Breast Cancer Res. 19, 129 (2017)
S.Y. Yeo, S.Y. Ha, K.W. Lee, Y. Cui, Z.T. Yang, Y.H. Xuan, S.H. Kim, Twist1 is highly expressed in cancer-associated fibroblasts of esophageal squamous cell carcinoma with a prognostic significance. Oncotarget 8, 65265–65280 (2017)
D.E. Kim, M.-G. Procopio, S. Ghosh, S.-H. Jo, S. Goruppi, F. Magliozzi, P. Bordignon, V. Neel, P. Angelino, G.P. Dotto, Convergent roles of ATF3 and CSL in chromatin control of cancer-associated fibroblast activation. J. Exp. Med. 214, 2349–2368 (2017)
W.N. Brennen, D.M. Rosen, H. Wang, J.T. Isaacs, S.R. Denmeade, Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J. Natl. Cancer Inst. 104, 1320–1334 (2012)
U.E. Martinez-Outschoorn, M.P. Lisanti, F. Sotgia, Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin. Cancer Biol. 25, 47–60 (2014)
B.B. Patel, E. Ackerstaff, I.S. Serganova, J.E. Kerrigan, R.G. Blasberg, J.A. Koutcher, D. Banerjee, Tumor stroma interaction is mediated by monocarboxylate metabolism. Exp. Cell Res. 352, 20–33 (2017)
B. Chiavarina, D. Whitaker-Menezes, U.E. Martinez-Outschoorn, A.K. Witkiewicz, R. Birbe, A. Howell, R.G. Pestell, J. Smith, R. Daniel, F. Sotgia, M.P. Lisanti, Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated fibroblasts drives stromal nutrient production and tumor growth. Cancer Biol. Ther. 12, 1101–1113 (2011)
F. Lopes-Coelho, S. Andre, A. Felix, J. Serpa, Breast cancer metabolic cross-talk: Fibroblasts are hubs and breast cancer cells are gatherers of lipids. Mol. Cell. Endocrinol. 462, 93–106 (2018)
X. Tang, Y. Hou, G. Yang, X. Wang, S. Tang, Y.E. Du, L. Yang, T. Yu, H. Zhang, M. Zhou, S. Wen, L. Xu, M. Liu, Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 23, 132–145 (2016)
Y. Liu, J. Zhang, X. Sun, Q. Su, C. You, Down-regulation of miR-29b in carcinoma associated fibroblasts promotes cell growth and metastasis of breast cancer. Oncotarget 8, 39559–39570 (2017)
C.J. Clarke, T.J. Berg, J. Birch, D. Ennis, L. Mitchell, C. Cloix, A. Campbell, D. Sumpton, C. Nixon, K. Campbell, V.L. Bridgeman, P.B. Vermeulen, S. Foo, E. Kostaras, J.L. Jones, L. Haywood, E. Pulleine, H. Yin, D. Strathdee, O. Sansom, K. Blyth, I. McNeish, S. Zanivan, A.R. Reynolds, J.C. Norman, The initiator methionine tRNA drives secretion of type II collagen from stromal fibroblasts to promote tumor growth and angiogenesis. Curr. Biol. 26, 755–765 (2016)
K.H. Vousden, Lane D.P. p53 in health and disease. Nat. Rev. 8, 275–283 (2007)
A.J. Levine, p53, the cellular gatekeeper for growth and division. Cell 88, 323–331 (1997)
M.S. Song, L. Salmena, P.P. Pandolfi, The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell Biol. 13, 283–296 (2012)
M.G. Procopio, C. Laszlo, D. Al Labban, D.E. Kim, P. Bordignon, S.H. Jo, S. Goruppi, E. Menietti, Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat. Cell Biol. 17, 1193–1204 (2015)
P. Dauer, X. Zhao, V.K. Gupta, N. Sharma, K. Kesh, P. Gnamlin, Inactivation of Cancer-associated-fibroblasts disrupts oncogenic signaling in pancreatic Cancer cells and promotes its regression. Cancer Res. 78, 1321–1333 (2018)
Y. Hayashi, M. Tsujii, T. Kodama, T. Akasaka, J. Kondo, H. Hikita, T. Inoue, p53 functional deficiency in human colon cancer cells promotes fibroblast-mediated angiogenesis and tumor growth. Carcinogenesis 37, 972–984 (2016)
J.O. Schmid, M. Dong, S. Haubeiss, G. Friedel, S. Bode, A. Grabner, G. Ott, T.E. Mürdter, Cancer cells cue the p53 response of cancer-associated fibroblasts to cisplatin. Cancer Res. 72, 5824–5832 (2012)
C.P. Liao, H. Adisetiyo, M. Liang, P. Roy-Burman, Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 70, 7294–7303 (2010)
A.J. Trimboli, C.Z. Cantemir-Stone, F. Li, J.A. Wallace, A. Merchant, N. Creasap, Pten in stromal fibroblasts suppresses mammary epithelial tumors. Nature 461, 1084–1091 (2009)
G.M. Sizemore, H.A.M. Balakrishnan, K.A. Thies, A.J. Trimboli, J.A. Wallace, Stromal PTEN inhibits the expansion of mammary epithelial stem cells through Jagged-1. Oncogene 36, 2297–2308 (2017)
J. Park, P.E. Scherer, Adipocyte-derived endotrophin promotes malignant tumor progression. J. Clin. Invest. 122, 4243–4256 (2012)
L. Bochet, C. Lehuede, S. Dauvillier, Y.Y. Wang, B. Dirat, V. Laurent, C. Dray, R. Guiet, I. Maridonneau-Parini, S. Le Gonidec, B. Couderc, G. Escourrou, P. Valet, C. Muller, Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013)
K.M. Nieman, I.L. Romero, B. Van Houten, E. Lengyel, Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 1831, 1533–1541 (2013)
J. Zhang, L. Zhang, C. Li, C. Yang, L. Li, S. Song, H. Wu, F. Liu, L. Wang, J. Gu, LOX-1 is a poor prognostic indicator and induces epithelial-mesenchymal transition and metastasis in pancreatic cancer patients. Cell. Oncol. 41, 73–84 (2018)
Y. Lee, W.H. Jung, J.S. Koo, Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res. Treat. 153, 323–335 (2015)
C.-K. Huang, P.-H. Chang, W.-H. Kuo, C.-L. Chen, Y.-M. Jeng, K.-J. Chang, J.-Y. Shew, C.-M. Hu, W.-H. Lee, Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via β-hydroxybutyrate. Nat. Commun. 8, 14706 (2017)
Z. Jia, Y. Liu, S. Cui, Adiponectin induces breast cancer cell migration and growth factor expression. Cell Biochem. Biophys. 70, 1239–1245 (2014)
A. Yao-Borengasser, B. Monzavi-Karbassi, R.A. Hedges, L.J. Rogers, S.A. Kadlubar, T. Kieber-Emmons, Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol. Rep. 33, 2689–2694 (2015)
C. Wang, C. Gao, K. Meng, H. Qiao, Y. Wang, Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS One 10, e0119348 (2015)
X. Catteau, P. Simon, J.-C. NoëL, Stromal expression of matrix metalloproteinase 2 in cancer-associated fibroblasts is strongly related to human epidermal growth factor receptor 2 status in invasive breast carcinoma. Mol. Clin. Oncol. 4, 375–378 (2016)
V. D’Esposito, F. Passaretti, A. Hammarstedt, D. Liguoro, D. Terracciano, G. Molea, L. Canta, C. Miele, U. Smith, F. Beguinot, P. Formisano, Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 55, 2811–2822 (2012)
L. Bochet, A. Meulle, S. Imbert, B. Salles, P. Valet, C. Muller, Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem. Biophys. Res. Commun. 411, 102–106 (2011)
K. Fujisaki, H. Fujimoto, T. Sangai, T. Nagashima, M. Sakakibara, N. Shiina, M. Kuroda, Y. Aoyagi, M. Miyazaki, Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res. Treat. 150, 255–263 (2015)
V. D'Esposito, D. Liguoro, M.R. Ambrosio, F. Collina, M. Cantile, R. Spinelli, G.A. Raciti, C. Miele, R. Valentino, P. Campiglia, M. De Laurentiis, M. Di Bonito, G. Botti, R. Franco, F. Beguinot, P. Formisano, Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget 7, 24495–24509 (2016)
M.N. Duong, A. Cleret, E.L. Matera, K. Chettab, D. Mathe, S. Valsesia-Wittmann, B. Clemenceau, C. Dumontet, Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 17, 57 (2015)
D. Katoh, M. Nishizuka, S. Osada, M. Imagawa, Fad104, a positive regulator of adipocyte differentiation, suppresses invasion and metastasis of melanoma cells by inhibition of STAT3 activity. PLoS One 10, e0117197 (2015)
A.L. Ribeiro, C. Kaid, P.B.G. Silva, B.A. Cortez, O.K. Okamoto, Inhibition of lysyl oxidases impairs migration and angiogenic properties of tumor-associated pericytes. Stem Cells Int. 4972078, 2017 (2017)
K. Hosaka, Y. Yang, T. Seki, C. Fischer, O. Dubey, E. Fredlund, J. Hartman, P. Religa, H. Morikawa, Y. Ishii, M. Sasahara, O. Larsson, G. Cossu, R. Cao, S. Lim, Y. Cao, Pericyte–fibroblast transition promotes tumor growth and metastasis. Proc. Natl. Acad. Sci. U. S. A. 113, E5618–E5627 (2016)
J. Hong, N.P. Tobin, H. Rundqvist, T. Li, M. Lavergne, Y. García-Ibáñez, H. Qin, J. Paulsson, M. Zeitelhofer, M.Z. Adzemovic, I. Nilsson, P. Roswall, J. Hartman, R.S. Johnson, A. Östman, J. Bergh, M. Poljakovic, G. Genové, Role of tumor pericytes in the recruitment of myeloid-derived suppressor cells. J. Natl. Cancer Inst. 107, djv209 (2015)
J.H. Trevino-Villarreal, R. Sepulveda, D.A. Cotanche, R.A. Rogers, Effect of pericytes on epithelial-to-mesenchymal transition in melanoma. J. Clin. Oncol. 30, 84–84 (2012)
M. Teichert, L. Milde, A. Holm, L. Stanicek, N. Gengenbacher, S. Savant, T. Ruckdeschel, Z. Hasanov, K. Srivastava, J. Hu, S. Hertel, A. Bartol, K. Schlereth, H.G. Augustin, Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 8, 16106 (2017)
L.E. Reynolds, G. D'Amico, T. Lechertier, A. Papachristodoulou, J.M. Muñoz-Félix, A. De Arcangelis, M. Baker, B. Serrels, K.M. Hodivala-Dilke, Dual role of pericyte α6β1-integrin in tumour blood vessels. J. Cell Sci. 130, 1583–1595 (2017)
D. Sinha, L. Chong, J. George, H. Schlüter, S. Mönchgesang, S. Mills, J. Li, C. Parish, D. Bowtell, P. Kaur, Pericytes promote malignant ovarian cancer progression in mice and predict poor prognosis in serous ovarian cancer patients. Clin. Cancer Res. 22, 1813–1824 (2015)
A. Chavez-Gonzalez, B. Bakhshinejad, K. Pakravan, M.L. Guzman, S. Babashah, Novel strategies for targeting leukemia stem cells: Sounding the death knell for blood cancer. Cell. Oncol. 40, 1–20 (2017)
B. Leyh, A. Dittmer, T. Lange, J.W. Martens, J. Dittmer, Stromal cells promote anti-estrogen resistance of breast cancer cells through an insulin-like growth factor binding protein 5 (IGFBP5)/B-cell leukemia/lymphoma 3 (Bcl-3) axis. Oncotarget 6, 39307–39328 (2015)
R.D. Guardia, B. Lopez-Millan, J.R. Lavoie, C. Bueno, J. Castaño, M. Gómez-Casares, S. Vives, L. Palomo, M. Juan, J. Delgado, M.L. Blanco, J. Nomdedeu, A. Chaparro, J.L. Fuster, E. Anguita, M. Rosu-Myles, P. Menéndez, Detailed characterization of mesenchymal stem/stromal cells from a large cohort of AML patients demonstrates a definitive link to treatment outcomes. Stem Cell Reports 8, 1573–1586 (2017)
K.M. McAndrews, D.J. McGrail, N. Ravikumar, M.R. Dawson, Mesenchymal stem cells induce directional migration of invasive breast cancer cells through TGF-β. Sci. Rep. 5, 16941 (2015)
H.H. Wang, Y.L. Cui, N.G. Zaorsky, J. Lan, L. Deng, X.L. Zeng, Z.Q. Wu, Z. Tao, W.H. Guo, Q.X. Wang, L.J. Zhao, Z.Y. Yuan, Y. Lu, P. Wang, M.B. Meng, Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 375, 349–359 (2016)
T.L. Watts, R. Cui, P. Szaniszlo, V.A. Resto, D.W. Powell, I.V. Pinchuk, PDGF-AA mediates mesenchymal stromal cell chemotaxis to the head and neck squamous cell carcinoma tumor microenvironment. J. Transl. Med. 14, 337 (2016)
F. Ma, D. Chen, F. Chen, Y. Chi, Z. Han, X. Feng, X. Li, Z. Han, Human umbilical cord mesenchymal stem cells promote breast cancer metastasis by interleukin-8- and interleukin-6-dependent induction of CD44+/CD24- cells. Cell Transplant. 24, 2585–2599 (2015)
P.F. Yu, Y. Huang, C.L. Xu, L.Y. Lin, Y.Y. Han, W.H. Sun, G.H. Hu, A.B. Rabson, Y. Wang, Y.F. Shi, Downregulation of CXCL12 in mesenchymal stromal cells by TGFβ promotes breast cancer metastasis. Oncogene 36, 840–849 (2017)
A.C. Dudley, Tumor endothelial cells. Cold Spring Harb Perspect Med 2, a006536 (2012)
A. Scholz, P.N. Harter, S. Cremer, B.H. Yalcin, S. Gurnik, M. Yamaji, M. Di Tacchio, K. Sommer, P. Baumgarten, O. Bahr, J.P. Steinbach, J. Trojan, M. Glas, U. Herrlinger, D. Krex, M. Meinhardt, A. Weyerbrock, M. Timmer, R. Goldbrunner, M. Deckert, C. Braun, J. Schittenhelm, J.T. Frueh, E. Ullrich, M. Mittelbronn, K.H. Plate, Y. Reiss, Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol. Med. 8, 39–57 (2016)
M.-Y. Hsu, M.H. Yang, C.I. Schnegg, S. Hwang, B. Ryu, R.M. Alani, Notch3 signaling-mediated melanoma–endothelial crosstalk regulates melanoma stem-like cell homeostasis and niche morphogenesis. Lab. Invest. 97, 725–736 (2017)
E. Wieland, J. Rodriguez-Vita, S.S. Liebler, C. Mogler, I. Moll, S.E. Herberich, E. Espinet, E. Herpel, A. Menuchin, J. Chang-Claude, M. Hoffmeister, C. Gebhardt, H. Brenner, A. Trumpp, C.W. Siebel, M. Hecker, J. Utikal, D. Sprinzak, A. Fischer, Endothelial Notch1 activity facilitates metastasis. Cancer Cell 31, 355–367 (2017)
B. Balinisteanu, A.M. Cimpean, E. Melnic, M. Coculescu, R.A. Ceausu, M. Raica, Crosstalk between tumor blood vessels heterogeneity and hormonal profile of pituitary adenomas: Evidence and controversies. Anticancer Res. 34, 5413–5420 (2014)
P. Ghiabi, J. Jiang, J. Pasquier, M. Maleki, N. Abu-Kaoud, N. Halabi, B.S. Guerrouahen, S. Rafii, A. Rafii, Breast cancer cells promote a notch-dependent mesenchymal phenotype in endothelial cells participating to a pro-tumoral niche. J. Transl. Med. 13, 27 (2015)
R. Wilson, C. Espinosa-Diez, N. Kanner, N. Chatterjee, R. Ruhl, C. Hipfinger, S.J. Advani, J. Li, O.F. Khan, A. Franovic, S.M. Weis, S. Kumar, L.M. Coussens, D.G. Anderson, C.C. Chen, D.A. Cheresh, S. Anand, MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nat. Commun. 7, 13597 (2016)
K. Gupta, R. Metgud, J. Gupta, Evaluation of stromal myofibroblasts in oral leukoplakia, oral submucous fibrosis, and oral squamous cell carcinoma - an immunohistochemical study. J. Cancer Res. Ther. 11, 893–898 (2015)
M. Chaudhary, A.R. Gadbail, G. Vidhale, M.P. Mankar, S.M. Gondivkar, M. Gawande, S. Patil, Comparison of myofibroblasts expression in oral squamous cell carcinoma, verrucous carcinoma, high risk epithelial dysplasia, low risk epithelial dysplasia and normal oral mucosa. Head Neck Pathol. 6, 305–313 (2012)
M. Mehdipour, M. Shahidi, S. Manifar, S. Jafari, F. Mashhadi Abbas, M. Barati, H. Mortazavi, M. Shirkhoda, A. Farzanegan, Z. Elmi Rankohi, Diagnostic and prognostic relevance of salivary microRNA-21, −125a, −31 and -200a levels in patients with oral lichen planus - a short report. Cell. Oncol. 41, 329–334 (2018)
R. Tamamura, H. Nagatsuka, C.H. Siar, N. Katase, I. Naito, Y. Sado, N. Nagai, Comparative analysis of basal lamina type IV collagen α chains, matrix metalloproteinases-2 and -9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem. 115, 113–119 (2013)
H.-X. Fan, H.-X. Li, D. Chen, Z.-X. Gao, J.-H. Zheng, Changes in the expression of MMP2, MMP9, and ColIV in stromal cells in oral squamous tongue cell carcinoma: Relationships and prognostic implications. J. Exp. Clin. Cancer Res. 31, 90 (2012)
P. Le Bars, P. Piloquet, A. Daniel, B. Giumelli, Immunohistochemical localization of type IV collagen and laminin (alpha1) in denture stomatitis. J. Oral Pathol. Med. 30, 98–103 (2001)
F.B. Galateau-Salle, R.E. Luna, K. Horiba, M.N. Sheppard, T. Hayashi, M.V. Fleming, T.V. Colby, W. Bennett, C.C. Harris, W.G. Stetler-Stevenson, L. Liotta, V.J. Ferrans, W.D. Travis, Matrix metalloproteinases and tissue inhibitors of metalloproteinases in bronchial squamous preinvasive lesions. Hum. Pathol. 31, 296–305 (2000)
M. Sathyakumar, G. Sriram, T.R. Saraswathi, B. Sivapathasundharam, Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral precancerous lesion-leukoplakia. J. Oral Maxillofac. Pathol. 16, 343–348 (2012)
S. Ramsridhar, M. Narasimhan, Immunohistochemical evaluation of mast cells in leukoplakia and oral squamous cell carcinoma. J. Clin. Diagn. Res. 10, ZC100–ZC103 (2016)
M. Kinra, K. Ramalingam, A. Sarkar, F. Rehman, K.L. Girish, Comparison of mast cell count and mast cell density in normal mucosa, oral leukoplakia, oral lichen planus, oral submucous fibrosis and oral squamous cell carcinoma–a study on 50cases. JPSI 1, 4–11 (2012)
S. Nayak, M.M. Goel, A. Makker, V. Bhatia, S. Chandra, S. Kumar, S.P. Agarwal, Fibroblast growth factor (FGF-2) and its receptors FGFR-2 and FGFR-3 may be putative biomarkers of malignant transformation of potentially malignant oral lesions into oral squamous cell carcinoma. PLoS One 10, e0138801 (2015)
P.A. Kenny, M.J. Bissell, Tumor reversion: Correction of malignant behavior by microenvironmental cues. Int. J. Cancer 107, 688–695 (2003)
S.P. Kerkar, N.P. Restifo, Cellular constituents of immune escape within the tumor microenvironment. Cancer Res. 72, 3125–3130 (2012)
H. Qin, C. Zhao, Y. Sun, J. Ren, X. Qu, Metallo-supramolecular complexes enantioselectively eradicate cancer stem cells in vivo. J. Am. Chem. Soc. 139, 16201–16209 (2017)
V. Pautu, D. Leonetti, E. Lepeltier, N. Clere, C. Passirani, Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol. Res. 126, 31–53 (2017)
G. Valenti, H.M. Quinn, G.J.J.E. Heynen, L. Lan, J.D. Holland, R. Vogel, A. Wulf-Goldenberg, W. Birchmeier, Cancer stem cells regulate cancer-associated fibroblasts via activation of hedgehog signaling in mammary gland tumors. Cancer Res. 77, 2134–2147 (2017)
C. Zang, J. Eucker, P. Habbel, C. Neumann, C.-O. Schulz, N. Bangemann, L. Kissner, H. Riess, H. Liu, Targeting multiple tyrosine kinase receptors with Dovitinib blocks invasion and the interaction between tumor cells and cancer-associated fibroblasts in breast cancer. Cell Cycle 14, 1291–1299 (2015)
F. Mpekris, P. Papageorgis, C. Polydorou, C. Voutouri, M. Kalli, A.P. Pirentis, T. Stylianopoulos, Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J. Control. Release 261, 105–112 (2017)
Y. Du, Q. Long, L.I.N. Zhang, Y. Shi, X. Liu, X. Li, B.I.N. Guan, Y. Tian, X. Wang, L.E.I. Li, D. He, Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int. J. Oncol. 47, 2064–2072 (2015)
K. Li, H. Kang, Y. Wang, T. Hai, G. Rong, H. Sun, Letrozole-induced functional changes in carcinoma-associated fibroblasts and their influence on breast cancer cell biology. Med. Oncol. 33, 64 (2016)
K.P.L. Fabian, N. Chi-Sabins, J.L. Taylor, R. Fecek, A. Weinstein, W.J. Storkus, Therapeutic efficacy of combined vaccination against tumor pericyte-associated antigens DLK1 and DLK2 in mice. Oncoimmunology 6, e1290035 (2017)
C. Wang, Y. Li, H. Chen, J. Zhang, J. Zhang, T. Qin, C. Duan, X. Chen, Y. Liu, X. Zhou, J. Yang, Inhibition of CYP4A by a novel flavonoid FLA-16 prolongs survival and normalizes tumor vasculature in glioma. Cancer Lett. 402, 131–141 (2017)
Q. Zhou, Y. Zhou, X. Liu, Y. Shen, GDC-0449 improves the antitumor activity of nano-doxorubicin in pancreatic cancer in a fibroblast-enriched microenvironment. Sci. Rep. 7, 13379 (2017)
C.J. Hanley, M. Mellone, K. Ford, S.M. Thirdborough, T. Mellows, S.J. Frampton, D.M. Smith, E. Harden, C. Szyndralewiez, M. Bullock, F. Noble, K.A. Moutasim, E.V. King, P. Vijayanand, A.H. Mirnezami, T.J. Underwood, C.H. Ottensmeier, G.J. Thomas, Targeting the myofibroblastic cancer-associated fibroblast phenotype through inhibition of NOX4. J. Natl. Cancer Inst. 110, 109–120 (2018). https://doi.org/10.1093/jnci/djx121
M. Gabasa, R. Ikemori, F. Hilberg, N. Reguart, J. Alcaraz, Nintedanib selectively inhibits the activation and tumour-promoting effects of fibroblasts from lung adenocarcinoma patients. Br. J. Cancer 117, 1128–1138 (2017)
Y. Wei, T.J. Kim, D.H. Peng, D. Duan, D.L. Gibbons, M. Yamauchi, J.R. Jackson, C.J. Le Saux, C. Calhoun, J. Peters, R. Derynck, B.J. Backes, H.A. Chapman, Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Invest. 127, 3675–3688 (2017)
M. Chen, X. Lei, C. Shi, M. Huang, X. Li, B. Wu, Z. Li, W. Han, B. Du, J. Hu, Q. Nie, W. Mai, N. Ma, N. Xu, X. Zhang, C. Fan, A. Hong, M. Xia, L. Luo, A. Ma, H. Li, Q. Yu, H. Chen, D. Zhang, W. Ye, Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J. Clin. Invest. 127, 3689–3701 (2017)
Acknowledgements
The authors acknowledge financial support from DST-SERB, Government of India, New Delhi, India (SERB/LS-1028/2013) and Dr. D.Y. Patil Vidyapeeth, Pune, India (DPU/05/01/2016).
Funding
This study was funded by DST-SERB, Government of India, New Delhi, India (SERB/LS-1028/2013) and Dr. D.Y. Patil Vidyapeeth, Pune, India (DPU/05/01/2016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval for this study is not required.
Informed consent
Informed consent for this study is not required.
Rights and permissions
About this article
Cite this article
Nilendu, P., Sarode, S.C., Jahagirdar, D. et al. Mutual concessions and compromises between stromal cells and cancer cells: driving tumor development and drug resistance. Cell Oncol. 41, 353–367 (2018). https://doi.org/10.1007/s13402-018-0388-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-018-0388-2