Abstract
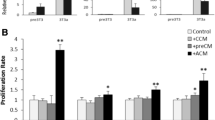
The objective of this study is to investigate interactions between adipocytes and breast cancer cells, and identify the responsible factors for the observed effects. In 27 breast cancer patients undergoing mastectomy, mammary adipose tissue was obtained from the breast quadrant bearing the tumor and corresponding non-tumoral quadrant. Isolated normal breast adipocytes (NBAs) and cancer-associated adipocytes (CAAs) were cultured in collagen gels to mimic the in vivo environment. Immunohistochemistry, qRT-PCR, and cell proliferation assays were performed to analyze adipocyte phenotypes. MCF7 and MDA-MB-231 breast cancer cell lines were co-cultured with adipocytes to detect phenotypic changes. Migration of MCF7 and MDA-MB-231 cells was assessed in NBA- and CAA-conditioned media. Cytokine levels in conditioned media were measured by cytokine array. Migration assays were repeated using conditioned media containing neutralizing antibodies. NBAs and CAAs lost their morphological phenotype in culture, acquiring a spindle-like shape, and CAAs showed higher cell proliferation, suggesting reversion to an immature phenotype. In co-cultures with MCF7 or MDA-MB-231 cells, NBAs exhibited increased cell proliferation, indicating acquisition of the immature phenotype of CAAs. MCF7 and MDA-MB-231 showed higher migration in a CAA-conditioned medium than in an NBA-conditioned medium. Cytokine array analysis of conditioned media revealed higher levels of interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) in the CAA-conditioned medium. Neutralization experiments using antibodies against IL-6 or MCP-1 showed abrogation of migration-enhancing effects of the CAA-conditioned medium. Adipocytes revert to an immature and proliferative phenotype in the presence of breast cancer cells, and promote cancer cell migration via adipokines including IL-6 and MCP-1.








Similar content being viewed by others
References
Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17(3):320–329
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239–252
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322
Calle EE, Thun MJ (2004) Obesity and cancer. Oncogene 23(38):6365–6378
Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B (2008) Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat 111(2):329–342
Dirat B, Bochet L, Escourrou G, Valet P, Muller C (2010) Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev 19:45–52
Rodbell M (1964) Metabolism of isolated fat cells I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239:375–380
Elsdale T, Bard J (1972) Collagen substrata for studies on cell behavior. J Cell Biol 54(3):626–637
Sugihara H, Yonemitsu N, Toda S, Miyabara S, Funatsumaru S, Matsumoto T (1988) Unilocular fat cells in three-dimensional collagen gel matrix culture. J Lipid Res 29(5):691–697
Moreno A, Akcakanat A, Munsell MF, Soni A, Yao JC, Meric-Bernstam F (2008) Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer 15(1):257–266
Flavell DM, Ireland H, Stephens JW, Hawe E, Acharya J, Mather H, Hurel SJ, Humphries SE (2005) Peroxisome proliferator-activated receptor alpha gene variation influences age of onset and progression of type 2 diabetes. Diabetes 54(2):582–586
Chen HC, Farese RV Jr (2002) Determination of adipocyte size by computer image analysis. J Lipid Res 43(6):986–989
Sugihara H, Yonemitsu N, Miyabara S, Toda S (1987) Proliferation of unilocular fat cells in the primary culture. J Lipid Res 28(9):1038–1045
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71(7):2455–2465
Kushiro K, Chu RA, Verma A, Nunez NP (2012) Adipocytes promote B16BL6 melanoma cell invasion and the epithelial-to-mesenchymal transition. Cancer Microenviron 5(1):73–82
Dethlefsen C, Hojfeldt G, Hojman P (2013) The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat 138(3):657–664
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM (2009) Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 28(33):2940–2947
Manabe Y, Toda S, Miyazaki K, Sugihara H (2003) Mature adipocytes, but not preadipocytes, promote the growth of breast carcinoma cells in collagen gel matrix culture through cancer-stromal cell interactions. J Pathol 201(2):221–228
Acknowledgments
This work was supported by MEXT KAKENHI Grant Number 26461942.
Conflict of Interest
All authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujisaki, K., Fujimoto, H., Sangai, T. et al. Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat 150, 255–263 (2015). https://doi.org/10.1007/s10549-015-3318-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3318-2