Abstract
The vast majority of breast cancers are positive for estrogen receptor (ER) and depend on estrogens for growth. These tumors are treated with a variety of ER-targeted endocrine therapies, although eventual resistance remains a major clinical problem. Other steroid hormone receptors such as progesterone receptor (PR) and androgen receptor (AR) are emerging as additional prospective targets in breast cancer. The fundamental mechanism of action of these steroid receptors in gene regulation has been defined mainly by several breast cancer cell lines that were established in the late 1970s. More recently, breast cancer patient-derived xenografts (PDX) have been developed by multiple groups at institutions in several countries. These new models capture the large degree of heterogeneity between patients and within tumors and promise to advance our understanding of steroid hormone receptor positive breast cancer and endocrine resistance. Unfortunately, steroid hormone receptor positive breast cancers are much more difficult than their receptor negative counterparts to establish into sustainable PDX. Herein we discuss the derivation of steroid hormone receptor positive breast cancer PDX, several pitfalls in their genesis, and their utility in preclinical and translational steroid hormone receptor research.
Similar content being viewed by others
Introduction
Breast cancer remains the most commonly diagnosed cancer in women and the second leading cause of cancer related mortality in the USA [1]. Breast cancer has traditionally been divided into distinct histopathological subtypes based on expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Breast cancers devoid of all three markers are classified as triple negative (TN). More recently, breast cancer has been categorized into molecular subtypes based on gene expression profiling. The original and most widely used molecular panel utilizes 50 discriminator genes, termed PAM50 [2, 3]. This divides breast tumors into five groups: two ER positive groups termed Luminal A and Luminal B, and three ER− groups termed HER2+ER−, basal-like, and claudin-low [3]. The two luminal ER+ subtypes constitute 70–75 % of all cases. Luminal tumor growth is driven by the mitogenic actions of estrogens and, accordingly, estrogen-targeted endocrine therapies are the mainstay of neoadjuvant and adjuvant treatments. The selective ER modulator (SERM) tamoxifen has been used to treat breast cancer since the 1970s [4]. Other widely used endocrine therapies include aromatase inhibitors (AIs) (i.e., letrozole, anastrozole) that block the endogenous production of 17β-estradiol or selective ER degraders (SERDs) (i.e., fulvestrant (ICI)) that destabilize ER [5]. Some combination and duration of these endocrine agents can stabilize or eradicate luminal breast cancer in many cases. However, development of endocrine resistance, which can occur acutely or over many years, still manifests in ∼40 % of patients [6, 7]. Therefore, models that recapitulate the stages of luminal tumor progression, with the capacity to address genetic diversity and tumor heterogeneity, are direly needed to improve outcome.
The plethora of in vivo research in human breast cancer has relied on implantation and growth of established cell lines into solid tumors [8]. These tumors are, however, limited in their ability to recapitulate the innate heterogeneity observed in most primary breast cancers and thus models that represent patient tumors more closely have long been sought. To this end, direct grafting of patient breast tumor material into immune-compromised mice has been attempted continuously not long after such models became available [9, 10]. Breast cancer presents a particular challenge for development of such patient-derived xenografts (PDX), as each of the described subtypes has a different prospective origin and natural course of the disease. The relatively recent emergence of more severely immune-compromised mouse strains as well as improved transplant techniques has increased the feasibility and efficiency of utilizing direct patient breast tumor engraftment. As such, the number of groups developing breast cancer PDX banks has increased in the last several years. A major interest in their development is to utilize PDX as preclinical models for testing experimental therapeutics. However, PDX are also useful for the study of tumor heterogeneity, metastasis, and signaling pathway dependencies pre- and post- drug resistance. Luminal breast cancer PDX in particular will be valuable in dissecting the role of steroid hormone receptors in the context of solid tumors.
Steroid Hormone Receptors in Breast Cancer
Steroid hormone receptors belong to the nuclear receptor family of ligand activated transcription factors. There are six defined members which contain a conserved double zinc finger DNA binding domain and are activated by lipophilic ligands that are derived from cholesterol through the process of steroidogenesis [11, 12]. ERs are encoded by two separate genes, ER-alpha (ESR1/NR3A1) and ER-beta (ESR2/NR3A2), that bind to distinct palindromic DNA sequences termed estrogen response elements (EREs). The remaining members are each encoded by a single gene and bind ligand, including progesterone receptor (PR, encoded by PGR/NR3C3), androgen receptor (AR, encoded by AR/NR3C4), glucocorticoid receptor (GR, encoded by GCR/NR3C1), and mineralocorticoid receptor (MR, encoded by MCR/NR3C2). The latter four steroid receptors (PR, AR, GR, and MR) share a similar palindromic consensus DNA binding sequence. While estrogens are the main mitogens in breast cancer, progesterone and androgens can have autonomous context-dependent actions on tumor growth [13, 14] and, through their receptors PR and AR, can directly modulate the actions of ER [15, 16]. Early clinical studies found high dose synthetic progestins and sometimes androgens were partially efficacious in treating late stage breast cancer; progestins in some studies were equivocal to tamoxifen, which thereafter became standard of care [17–23]. There is currently renewed interest in targeting both PR and AR in breast cancer. However, the divergent roles of these receptors in driving breast cancer growth complicate their use and necessitate further study for patient selection [13, 14]. There is also recent interest in the role of GR in breast cancer, particularly the ER− subtypes [24–28]. PDX models that express each of these receptors in different combinations will be critical to evaluate new steroid receptor-targeted drugs and make selections for appropriate candidates for treatments. Unfortunately, most steroid hormone receptor positive breast cancers develop into xenografts much less efficiently than their receptor negative counterparts, which usually proliferate faster and do not require hormonal stimulation.
Maintenance of appropriate steroid hormone receptor expression between patient tumors and PDX is a critical step in capturing relevant models for research and preclinical drug testing. While ER-alpha is expressed in 70–75 % of breast cancers at initial diagnosis, there is widespread heterogeneity in the level of ER expression and fidelity of ER-alpha-mediated signaling among tumors. Currently, tumors with 1 % or greater ER immunoreactive cells are recommended as candidates for endocrine therapies [29]. Furthermore, complete loss of ER-alpha upon endocrine resistance is rare; tumors usually remain clinically ER-alpha+ (≥1 %) but often bypass ER-alpha signaling and utilize receptor kinase-driven pathways such as EGFR/HER2 or PI3K/AKT/mTOR [7]. ER-beta is expressed in a significant proportion of breast cancers (76 %); however, its role in breast cancer remains elusive and ER-alpha remains the major prognostic indicator and target [30–32]. PR is expressed in over half of ER+ tumors and has been used as a functional measure of ER [33], while AR is expressed in 70–80 % of all breast cancers [34–36]. GR has been measured less frequently in breast tumors; estimates indicate between 30 and 70 % of invasive breast cancers express GR (reviewed in [37]). MR to our knowledge has not been comprehensively measured in breast cancer, although mRNA levels were detected in TN MDA-MB-231 breast cancer cells [38]. In this review, we focus on ER-alpha (termed ER), PR, and AR expression in breast PDX models.
Recent Development of Steroid Hormone Receptor Positive Breast Cancer PDX Collections
To date, at least 10 laboratories have described establishment and maintenance of steroid hormone receptor positive PDX (Table 1). Here we focus on several reports that have emerged since 2007. In the first, Marangoni et al. utilized intact female nude mice supplemented with 17β-estradiol via the drinking water [39]. In the original report, 200 primary tumors were implanted into the interscapular region which yielded 18 patient-derived lines [39]. However, only one of these was ER+ (<1 % engraftment rate compared to 36 % for TN breast cancer). The same group recently updated their success rate; utilizing the same methods, they have now implanted 314 ER+ tumors and developed 8 ER+ lines (a 2.5 % engraftment rate) [40]. Bergamaschi et al. implanted 26 ER+ primary tumors into NOD.Cg-Prkdcscid (severe combined immunodeficient—SCID) mice on the dorsal flank with 17β-estradiol pellets [41]. This yielded a single ER+PR+ PDX, and one ER−PR− PDX (which originated from an ER−PR+ tumor), a 7.7 % overall engraftment rate, and an engraftment rate of 3.8 % for ER+ PDX. The ER+ PDX resembled the original patient tumor over multiple passages by histopathologic and gene expression comparisons. In 2011, DeRose et al. described derivation of five ER+PR+ breast cancer PDX lines [42]. These originated from implantation of 49 specimens from 42 patients into female nonobese diabetic NOD.CB17-Prkdcscid (NOD/SCID) mice with cleared mammary fat pads and commercial slow release estrogen pellets (an overall engraftment rate of 27 % for both ER+ and ER− PDX). Three of the lines originate from serial pleural effusions of the same patient and one was also HER2+. They also described that co-injection of mesenchymal stem cells increased growth and supported tumor vascularization [42]. Estrogen dependency was maintained in the five ER+ PDX lines.
Our group, Kabos et al., utilized female NOD.Cg-Prkdcscid- Il2rgtm1Wjl/SzJ (NSG) ovary-intact mice with silastic estrogen pellet supplementation and implanted 18 ER+ specimens into the intact mammary fat pads [43]. We described establishment of five ER+ PDX lines (3 ER+PR+, 2 ER+PR−); two of these emanated from liquid metastases (one pleural effusion and one ascites), and one was HER2 amplified. All of the PDX retained estrogen dependency to some degree. To date, we have implanted 74 ER+ specimens and generated 12 transplantable ER+ PDX lines, representing a 16 % engraftment rate. In 2013, Vaillant et al. report the implanting of 108 ER+ patient tumors into the inguinal mammary fat pad of NSG mice, resulting in the development of 13 ER+ PDX lines with variable PR expression, representing a 12 % take rate [44]. These authors report that PDX tumors of subtype Luminal B, when treated with tamoxifen plus small molecule Bcl antagonists, display therapeutic response above that observed with tamoxifen alone. Zhang et al. utilized a combination of SCID/Beige and NSG mice, with estrogen pellets supplied in most cases [45]. They established a total of 32 lines from 25 patients, 3 of which are ER+; one of these PDX is ER+PR+, one is ER+PR−, and one is ER+PR−HER2+ (representing a take rate of 9.4 %). Notably, they observed that clearing the mammary fat pads and humanizing with human fibroblasts did not improve take rate regardless of patient tumor subtype (from 21.4 % overall take rate across all subtypes, down to 3.4 % take rate with humanized glands). In 2013, Li et al. reported a collection emphasizing ER+ PDX, developed in female NSG mice [46]. They implanted a total of 54 ER+ specimens, the majority of these from advanced stage disease (pleural effusions and skin metastases) and developed 7 ER+ lines (a 13 % take rate). Notably, humanized fat pads significantly decreased the take rate of ER+ tumors (down to 3 %). They also noted that engrafted tumors have a high rate of p53 mutations (69 %). In 2015, Eirew et al. utilized NSG and NOD/Rag1−/−Il2rg−/− (NRG) mice to generate a collection of 15 breast cancer PDX [47]. They uniquely cultured breast cancer cells as organoids prior to implantation into mammary fat pads. For select ER+ specimens, animals received subcutaneous injections of 17β-estradiol in oil every 2 weeks. Their engraftment rate was 26 % (5/19) for ER+ tumors compared to 81 % for TN and 44 % for HER2+ER− tumors. Using single cell and deep genome sequencing, they determined that clonal selection occurs to some degree in all samples and continues through serial passaging. In 2016, Bruna et al., through collaboration with several institutions, reported the largest collection of ER+ PDX to date, in which 52 ER+ breast cancer PDX were developed in NRG and NSG mice from primary and metastatic patient tumors. Exogenous estrogens were not supplied during tumor development. Histological features, RNA expression, and copy number alterations were found to be stable through several passages. Breast cancer driver mutations were accurately represented in the PDX, although the rates of specific mutations were slightly lower than that seen in patients (i.e., PIK3CA in 27 % of ER+ PDX compared to 38–43 % of patients from METABRIC/TCGA databases).
A handful of reports utilizing PDX, in which PDX is not the primary research focus or in which PDX derivation is not described in detail, are not discussed herein. In addition, several other groups are in the process of developing breast cancer PDX banks that have not yet been reported which could increase the total number of generated ER+ lines. However, the number of established breast cancer PDX still weighs disproportionately towards the TN subtype and thus improvements are needed for capturing the wide genetic and histological variety of ER+ breast cancers, as discussed below for the mouse intraductal (MIND) method.
Methodological Considerations
Engraftment Rate of ER+ Breast Tumors
The biggest challenge to developing steroid hormone receptor positive PDX is a lower engraftment rate compared to receptor negative tumors. Estimated by several reports, this is on average less than 15 % for primary ER+ tumor samples, with potentially higher success for late stage metastases (Table 1), excluding the novel MIND method, which may yield higher success rates with difficult-to-engraft samples. This low engraftment rate is in comparison to TN breast cancers, in which primary tumors graft at 30–80 % [40, 45–47]. There are few HER2+ER− PDX models, prospectively due to their lower patient prevalence (∼15 % of all cases). Grade 3 tumors have higher engraftment rates than grade 1 or 2 tumors [45], prospectively due to a higher proliferative index. Therefore, existing ER+ breast cancer PDX favor the faster growing Luminal B subtype breast tumors. Luminal B tumors are more likely to become endocrine resistant, have a higher incidence of metastases, and have worse progression-free and overall survival than Luminal A tumors [49–51]. Luminal B tumors in general tend to have lower ER expression and increased intratumoral ER− cells which may also contribute to their better take rate. Where intrinsic molecular subtyping has been used, all ER+ PDX except one profile as Luminal B [41–43, 45, 46]. The one Luminal A PDX described by Petrillo et al. is, however, ER− [52]. Interestingly, primary tumor specimens that profile as Luminal A prior to implantation switch to Luminal B upon establishing a transplantable PDX [46]. This is likely due to selection pressure that favors relatively rapid proliferation and propagation (up to 6 months). Other histological breast tumor types such as lobular that tend to be highly ER+ are difficult to develop. Four mixed lobular/ductal breast cancer PDX have been described between two collections [42, 48]. Improved models for developing ER+ PDX, discussed below, may increase the efficiency of grafting such tumors.
The Estrogen Milieu for Establishing ER+ PDX
In most cases, establishment of ER+ breast cancer PDX requires exogenous estrogen supplementation. Multiple methods exist for in vivo delivery of estrogen. One method involves frequent (daily to weekly) injection of hormones dissolved in peanut (or similar) oil vehicle; this method, while efficacious, is labor intensive and imparts more stress on the animals. A second method is delivery via the drinking water [53]. While noninvasive, this method requires frequent preparation of fresh 17β-estradiol stocks and biweekly water changes, with some debate over bioavailability, though circulating levels induce uterine hypertrophy similarly to that observed with pellet supplementation [53]. The most common method of estrogen delivery is through slow release pellets. These are available commercially in several doses and release times from Innovative Research of America (Sarasota, FL) and can be inserted subcutaneously via trochar. Alternatively, some laboratories prepare their pellets in-house using commercially available hormone stocks; the most common method involves packaging hormones within silastic medical tubing [54]. With this method, the dose of hormones can be manipulated, is suitable for use with steroid hormones (17β-estradiol, progesterone, dihydrotestosterone), and lasts for 3–4 months. Circulating levels of steroid hormones can be monitored relative to dose using ELISAs or RIAs; 17β-estradiol is in the high physiological range, estimated at <100 pg/mL for silastic pellets (1 mg) [55]. By survey, most groups use intact animals for establishment of PDX with chronic estrogen supplementation. For experiments beyond initial passaging, animals may be ovariectomized to provide the cleanest hormone negative background where necessary.
One of the first routine experiments conducted upon successful engraftment of an ER+ tumor specimen is evaluation of its dependency on estrogen for growth. In our hands, tumors are partitioned into animals upon passage 1 or 2 that are supplemented with placebo or slow release silastic estrogen pellets to document the degree of estrogen dependency. In our experience, some highly ER+ tumors will not form tumors in ovary-intact mice without exogenous estrogen; there is little to no residual material at necropsy 3–4 months after implantation. Other PDX show partial growth in the absence of exogenous estrogen; these are usually late stage tumors or primary tumors with lower ER expression. In addition, we often monitor relative tumor growth in the presence of estrogen plus either progesterone or the androgen dihydrotestosterone. The tumors we have tested do not grow significantly larger than placebo with either progestins or androgens alone.
Maintaining Steroid Receptor Expression and Function in PDX
Another major hurdle in development of steroid receptor positive PDX is loss of receptors upon establishment and/or propagation. Ideally, receptor expression is initially monitored through routine parallel immunostaining comparing the PDX to the original tumor specimen. However, this is not always feasible due to limiting material and/or availability, or staining challenges inherent with fluid samples. From our own experience and from other reports, patient ER levels are initially captured in the majority of established ER+ PDX. Careful evaluation of ER staining at each passage is then necessary, and if lost, that PDX should be reevaluated for usefulness as a model. ER undergoes ligand-dependent down-regulation and this should be considered when evaluating ER expression in breast cancer PDX [56–59]. The constant low dose estrogen necessary to propagate ER+ tumors will also yield chronic cycling of the transcriptionally active ERs. Removal of estrogen for several weeks (estrogen withdrawal, EWD) can lead to an increase in ER levels (Fig. 1a). Similarly, tamoxifen treatment can stabilize ER protein and lead to an observed upregulation of ER in PDX. In our experience, PDX that appear to have low or absent ER levels will show an increase in detectable ER upon tamoxifen treatment, signifying a low but active level of ER (Fig. 1b). This is important as it mimics clinical tumors with lower ER that may be less dependent on estrogens and are clinically difficult to treat. Conversely, SERDs such as ICI will decrease ER levels in breast cancer (Fig. 1b). Therefore, it is always important to consider the hormonal context under which ER is measured in breast cancer PDX.
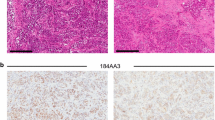
Context-dependent expression levels of ER, PR, and AR in breast cancer PDX. Formalin-fixed paraffin-embedded sections of select PDX breast tumors were processed for immunohistochemistry as previously described [43]. Tumors were stained with antibodies to ERα (Thermo-Fisher, clone SP1, 1:100), PR (DAKO, clone 1294, 1:500), or AR (DAKO, clone AR441, 1:500). a ER and PR expression fluctuate with estrogen levels. Sections of PDX UCD12 were stained for ER and PR from estrogen-treated tumors (E, silastic pellet) or from tumors that were withdrawn from estrogen for 3 weeks (EWD). b ER expression fluctuates with endocrine treatments. Sections of UCD4 were stained by IHC for ER under conditions of estrogen plus either vehicle (E) or tamoxifen (Tam, administered as 1 mg Tam dissolved in peanut oil IP 3× per week for 3 weeks), or under conditions of estrogen plus vehicle or ICI (5 mg ICI in peanut oil IP 1× per week for 5 weeks). c PR expression in two different TN breast cancer PDX (UCD18 and UCD111). d AR expression in HER2+ER− tumor UCD62 and TN tumor UCD138. Scale bars 300 μm
PR at present is the only other steroid receptor routinely measured in breast cancer. PR is present in over half of ER+ tumors [33]. It has been recently described that the PR locus is subject to copy number loss, which may account for its overall lower abundance relative to ER in breast cancers [15]. In addition, PR expression is particularly difficult to retain in PDX. PR expression in breast cancer is usually estrogen dependent. Thus, the chronic estrogen required to establish and propagate ER+ PDX should assist in maintaining PR expression where possible; likewise, EWD can lead to loss of PR expression (Fig. 1a). In our collection, 6/12 ER+ tumor lines retain PR expression (one undetermined), reflective of its clinical prevalence. However, in two of these, expression levels are <10 %, making it difficult to study PR action. ER+PR− PDX breast cancers have poorer prognosis overall than their ER+PR+ counterparts [60], and thus these models will be useful for studying this subset of the disease. We have noted that patient tumors that are characterized as TN occasionally generate PDX that express PR in <10 % of cells, in the absence of ER or supplemented estrogens (Fig. 1c).
AR is present in 70–80 % of all breast tumors, with varying expression across the different subtypes. AR is found in approximately 80 % of ER+ tumors, over half of HER2+ER− tumors, and 10 % of TN breast tumors [34–36, 61, 62]. At present, AR is not routinely measured in clinical samples. Therefore, retention of AR expression in PDX needs to be compared to the primary patient specimen by parallel immunohistochemistry (IHC) where feasible. In our collection, AR is expressed in >1 % of cells in 7/11 ER+ PDX (one undetermined). Expression levels are highly variable similar to ER and PR; some lines contain very low AR (<5 %), making them less than ideal for most studies. Our one HER2+ER− PDX line is AR+ and, where measured, 2/6 TN PDX lines are AR+ (Fig. 1d). Each of these types of PDX models will be valuable as AR is being investigated as a putative target in ER+ and ER− tumor subtypes [63–65]. Unlike ER and PR which reside predominantly in the nucleus in both the absence and presence of ligand, AR is usually cytoplasmic and is shuttled to the nucleus with addition of an androgenic ligand. This is recapitulated in breast cancer PDX.
In contrast to most breast cancer cell lines, steroid receptor positive PDX generally retain the inherent intratumoral heterogeneity associated with patient tumors. For ER in particular, this includes a range of intensities and levels of receptors, ranging from <5 to near 100 % positive cells. This natural mosaic of ER expression underscores the challenge of treating ER+ tumors. It also may allow for the study of mixed populations, for example, ER+ and ER− cells within the same tumor microenvironment. However, heterogeneity can impede the use of molecular technology to study steroid receptor action. Gene regulation, for example, can be washed out by the prevalence of receptor negative cells. Furthermore, techniques such as chromatin immunoprecipitation followed by sequencing (ChIP-seq) and rapid immunoprecipitation followed by mass spectrometry of endogenous proteins (RIME) [66] for steroid receptors are not currently feasible on many PDX tumors, which require high concentrations of receptors for sufficient immunoprecipitations. Primary patient specimens are often too limited in size for these techniques such that expansion as PDX is attractive for such studies in the event that high receptor levels are maintained.
Newer Techniques That Improve Engraftment Rates of ER+ Tumors
Several adaptations to implantation methods have been tested to improve take rate, including choice of mouse strain, use of cleared mammary fat pads plus/minus humanization of the mammary gland, and co-injection of mesenchymal cells. Nude mice (NU/J) have the highest remaining murine immunity (retain B cells) and thus display the lowest engraftment efficiency for ER+ tumors (2.5 %) [40], and therefore most groups have moved to strains with more severely immune-compromised systems such as SCID/Bg (CB17.Cg-PrkdcscidLystbg-J/Crl), NOD/SCID, and NSG. Once PDX are established in these mice, it is feasible to backtransplant them to other strains. For example, TN PDX established in NSG mice can be backtransplanted efficiently to nude mice [67]. We have also backtransplanted ER+ PDX from NSG to B6-RAG1 (B6.129S7-Rag1tm1Mom/J) mice successfully with no observable loss of receptor expression or tumor phenotype. Humanizing the mammary gland does not improve and may in fact decrease take rates [45, 46], while co-injection of human mesenchymal stem cells can accelerate tumor vascularization and growth [42, 68].
An implantation technique termed MIND (mouse intraductal) was developed by Behbod et al. for in vivo study of ductal carcinoma in situ (DCIS) [69]. In this method, cells are injected directly into the milk ducts and form structures within the mammary fat pad. This technique was adapted by Sflomos et al. for breast cancer cell lines and primary tumor specimens using ovary-intact (low estrogen) SCID/Bg or NSG mice [70, 71]. MCF7 cells implanted into the fat pads using traditional techniques displayed a higher expression of basal and epithelial-mesenchymal transition (EMT) genes including SLUG compared to the MIND method of implantation. Furthermore the MIND-implanted tumor cells had higher ER expression levels, suggesting that the MIND method maintains a more luminal-like phenotype in tumors.
To establish MIND PDX, primary patient tumors were dissociated into single cell suspensions and 5000–10,000 cells injected into the ducts. Engrafted cells retained the original ER and PR expression levels, and resulted in the generation of seven ER+PR+ and three ER+PR− PDX lines (Table 1). MIND engraftment rates with ER+ primary patient samples were higher (17–100 % take rate, depending on patient sample) than is generally observed for intrascapular or mammary fat pad implantations of ER+ patient samples (2.5–27 %, from multiple collections summarized in Table 1) [71]. Importantly, lower grade tumors (1 and 2), Luminal A tumors, and lobular tumors, subtypes that historically have proven difficult to engraft, all established growths with the MIND method, and PDX tumors maintained both histopathological similarity and proliferative indices as primary tumors. Growth kinetics of luminal-subtype tumors are frequently altered while establishing PDX due to selection pressure, as a clonal population of fast-growing cells may not reflect the growth kinetics of the original tumor; hence, the predominance of Luminal B PDX. Thus, the MIND approach could allow for a more robust representation of the spectrum of ER+ tumors. One disadvantage is that the quantity of material may be limiting for larger scale experiments (i.e., dispersal into multiple animals for preclinical drug testing or proteomics-based experiments).
Caveats and Limitations of ER+ Breast Cancer PDX
There are several caveats inherent to all PDX that also apply to steroid hormone receptor positive breast cancer PDX. These include acquisition of proliferating murine stroma, development of transferable murine malignancies, viral contamination, and potential genetic drift over passaging. Murine stroma that propagates with whole fragments of tumors can become activated and spontaneously immortalized such that it proliferates faster than steroid hormone receptor positive breast cancer epithelial cells. In such cases, the murine cells take over the tumor within one to two passages. Loss of hormone receptor expression, loss of human cytokeratin expression, and gain of a squamous spindly appearance all indicate a problem. If this occurs, it may be possible to reconstitute the line from earlier passages, but research on the current specimen should be discontinued.
Use of immunocompromised mice brings additional considerations. NOD/SCID animals in particular are susceptible to lymphoma [72]. This can passage with the PDX leading to rapid growth of solid masses of murine cells and necessary euthanasia of the animals prior to PDX regrowth. In contrast, NSG mice are less susceptible to lymphoma. In our experience, while we have had occasional development of lymphoma using NOD/SCID mice for long incubation periods (∼6 months), we have not observed this phenomenon in NSG mice. Viral contamination is another recurring issue that may arise while propagating tumors in mice [73]. The most problematic viral contamination is lactate dehydrogenase elevating virus (LDEV). This is a common murine viral contaminant that can be found in commercially available basement membrane extract (BME) preparations that are frequently used when implanting tumor cell suspensions or solid tumor pieces [73]. The virus can take several passages before physical signs appear, at which time animals develop partial or full paralysis. The virus is transferred via direct tissue/fluids and infects murine macrophages [74]; plasma, liver, and spleen tissue from infected mice display the highest LDEV titer [75].
Two methods have been described to eliminate LDEV. The first includes FACS to eliminate all murine cells from a single cell preparation, followed by reimplantation of human tumor cells into new animals [76]. However, this requires serial sorting, and unless the sort is near 100 % efficient, the virus will still transfer. A second, older method, albeit one still recommended by Charles River, involves passage through nude rats which eliminate the murine macrophages. The sample can then be evaluated for loss of LDEV via qPCR and reimplanted into mice. The tumor would likewise need to be monitored for appreciable morphological changes. While this approach may be feasible for TN tumors, in our experience the continuous estrogen supplementation required to maintain ER+ tumor samples can lead to the appearance of spontaneous rat tumors that interfere with the slower growing human ER+ PDX. Most present sources of BME are LDEV-free which has mostly eliminated this problem (i.e., Cultrex®, Trevigen, Gaithersburg, MD).
The stability of the breast cancer PDX phenotype over passage is an important consideration and potential caveat that has not been entirely resolved in the field. Eirew et al. described that the major selection of patient-derived breast cancer cells fit for propagation as a PDX occurs in many cases during initial establishment, although some drift occurs through every subsequent passage [47]. Where measured, some PDX collections appear to retain copy number variations and SNPs compared to the primary patient sample over multiple passages, with occasional gain in activating SNPs [41, 42, 45]. Gene expression measured via Affymetrix analysis and proteome analysis via RPPA were found to be relatively stable measured every fifth passage [45]. Bruna et al. extensively monitored genomic and molecular fidelity using single nucleotide variations (SNV), copy number alterations, gene methylation, and gene expression profiling, and concluded that PDX tumors maintain high correlation with patient tumors across serial passages [48]. Deep sequencing identified single nucleotide variations accumulate out to passage 8 in ER+ PDX and were predominantly in non-cancer associated genes [46], a conclusion recently confirmed by Bruna et al. [48]. In the MIND model, PDX tumors did not accumulate de novo mutations over passage and thus may be more genetically stable [71].
While no consensus passage number exists at which PDX are reliable to utilize, commercially contracted PDX studies usually restrict the use of their models to within a few passages removed from the original patient sample. However, this is not always practical for steroid receptor positive breast cancer PDX which often grow slower and can take a few passages to establish and expand prior to performing experiments. Therefore, for preclinical studies on developmental therapeutics, the lowest possible passage should be used. Careful replenishing of frozen stocks and reimplantation of lower passages can prolong experimental use with the caveat that lower passage stocks will eventually be depleted. With diligent monitoring via IHC and other methods capable of detecting gene expression changes, ER+ PDX can be reasonably used for four to five passages for many types of studies. To minimize the usage of limited stock, Bruna et al. recently reported an ex vivo culture method wherein cell lines derived from PDX breast cancer tumors could be used for high throughput screening of single agents and combination therapy [48], which allows for pre-validation of therapeutic agents to minimize inefficient use of limited PDX stock. It is unknown at this time if the precise method of breast cancer PDX establishment and maintenance influences therapeutic response.
Steroid Hormone Receptor Positive PDX: New Avenues for Investigation
Gene Expression Profiling of Steroid Hormone Receptor Positive PDX
Steroid hormone receptor positive PDX offer new opportunities for defining hormone-dependent gene regulation. ER and PR regulated genes in breast cancer have been mainly defined by the immortalized cell lines MCF7 and T47D, respectively [77–81]. In addition, several clinical trials utilizing endocrine therapies have generated datasets reporting estrogen-dependent genes that are deposited in publically available databases [82–84]. Our group evaluated gene expression in PDX plus/minus estrogen, tamoxifen, and EWD [43]. In comparing three ER+ PDX to MCF7 cells, there were many similar estrogen-regulated genes such as GREB1, IGF1R, and PGR. Not surprisingly, each ER+ PDX contains tumor-unique estrogen-regulated genes. For example, in PDX UCD4 (PE4 in the original manuscript), estrogen highly up-regulated a group of genes located on the X chromosome termed cancer testis antigens (CT45), occasionally observed in multiple tumor types that are candidates for immunotherapies [85]. In our experience, progestin regulated genes are more variable among PDX. A preliminary comparison of overlap between PR regulated genes in PDX compared to those described in T47D cells noted only a few consistently PR regulated genes (i.e., TAT and CXCL13) (unpublished data). This coincides with the emerging functional heterogeneity that PR appears to convey in ER+ breast cancers. Assessment of PR regulated genes may pinpoint those tumors in which PR acts as an ER agonist vs. antagonist.
Endocrine Resistance and ER Mutations
There are limited models to study endocrine resistance in ER+ breast cancers. The most commonly used models are tamoxifen-resistant MCF7 breast cancer cell lines, which have proved valuable in investigating mechanisms of tamoxifen insensitivity [86, 87]. To generate similar endocrine-resistant PDX, samples must be implanted from pre-existing resistant tumors, or resistance manifested utilizing a chronic in vivo treatment protocol similar to that utilized for MCF7 cells in vitro. This strategy was described for a primary breast tumor implanted in nude mice over 3 years of tamoxifen treatment [88]. The Washington University collection contains ER+ PDX lines that were developed from late stage cancers that were endocrine resistant [46]. Interestingly, four of their ER+ PDX lines contain ER variants: an ESR1/YAP1 fusion protein resulting from a translocation event (WHIM18); amplification of the ESR1 promoter and coding region (WHIM16); an ESR1-E380Q point mutation in the ligand binding domain (WHIM24); and an ESR1-Y537S point mutation in the ligand binding domain (WHIM20) [46]. In our collection, UCD4, developed from a pleural effusion, harbors the ESR1-D538G mutation, and UCD65 displays ESR1 amplification. ER mutations are emerging as more prevalent than originally thought, likely due to improved sequencing and detection technologies [89, 90]. Naturally occurring ER mutant PDX models will be useful for testing novel ER mutant targeting drugs under development, particularly newer generation SERDs. In addition, companion cell lines could be generated from such tumors, albeit with no mutation negative control. These types of models will compliment CRISPR/Cas9-generated ER mutants in breast cancer cell lines. Mutations in PR and AR have not yet been described in breast cancer, likely since mutations are primarily driven by pressuring the ER signaling axis.
One inherent limitation of breast cancer PDX is the lack of an intact immune system. Immune cells and associated inflammation affect multiple components of tumor biology. Humanized models are being developed in which irradiated mice are reconstituted with human cells, including T cells, myeloid cells, and hematopoietic progenitor/stem cells, resulting in mice regaining partial immunity [91–93]. As the technology becomes more refined and available, PDX may be established and/or transplanted into animals with human-like immune systems. This may facilitate processes such as metastasis, which occur but are inefficient in immune-compromised hosts.
Conclusions
The recent upsurge in the development of breast cancer PDX has naturally led to generation of ER+ PDX; at least 116 transplantable lines exist worldwide. Many of the ER+ PDX co-express PR and AR, and several are also HER2+/amplified. While the process of ER+ PDX derivation is still relatively inefficient (<20 %), as technologies improve, these collections may allow for a more robust representation of ER+ breast cancers. In particular, utilization of the MIND approach may capture tumor types not amenable to solid specimen transplant into mammary fat pads. Breast cancer PDX retain the inherent heterogeneity in ER, PR, and AR expression, making them attractive for preclinical studies aimed at testing drug efficacy in a spectrum of the disease. Furthermore, primary tumor-derived PDX that were untreated or undertreated offer an advantage for testing new endocrine agents. In addition to anti-estrogen-targeted drugs, these may help delineate tumors that would respond to therapies with natural steroid hormones such as progesterone and androgens vs. those that could be stimulated by such treatments. In conclusion, steroid hormone receptor positive breast cancer PDX allow an unprecedented opportunity to study endocrine resistance and steroid receptor action in solid tumor models that more closely represent human breast cancer biology.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752
Prat A, Perou CM (2011) Deconstructing the molecular portraits of breast cancer. Mol Oncol 5(1):5–23
Jordan VC (2006) Tamoxifen (Ici46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 147(Suppl 1):S269–S276
Obiorah I, Jordan VC (2011) Progress in endocrine approaches to the treatment and prevention of breast cancer. Maturitas 70(4):315–321
Musgrove EA, Sutherland RL (2009) Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9(9):631–643
Osborne CK, Schiff R (2011) Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 62:233–247
Clarke R (1996) Human breast cancer cell line xenografts as models of breast cancer. The immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Res Treat 39(1):69–86
Outzen HC, Custer RP (1975) Growth of human normal and neoplastic mammary tissues in the cleared mammary fat pad of the nude mouse. J Natl Cancer Inst 55(6):1461–1466
Rae-Venter B, Reid LM (1980) Growth of human breast carcinomas in nude mice and subsequent establishment in tissue culture. Cancer Res 40(1):95–100
Gronemeyer H, Gustafsson JA, Laudet V (2004) Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 3(11):950–964
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM (1995) The nuclear receptor superfamily: the second decade. Cell 83(6):835–839
Hagan CR, Lange CA (2014) Molecular determinants of context-dependent progesterone receptor action in breast cancer. BMC Med 12:32
McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD (2014) Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer 21(4):T161–T181
Mohammed H, Russell IA, Stark R, Rueda OM, Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A, Menon S, Hadfield J, Pugh M, Raj GV, Brown GD, D’Santos C, Robinson JL, Silva G, Launchbury R, Perou CM, Stingl J, Caldas C, Tilley WD, Carroll JS (2015) Progesterone receptor modulates eralpha action in breast cancer. Nature 523(7560):313–317
Singhal H, Greene ME, Tarulli G, Zarnke AL, Bourgo RJ, Laine M, Chang YF, Ma S, Dembo AG, Raj GV, Hickey TE, Tilley WD, Greene GL (2016) Genomic agonism and phenotypic antagonism between estrogen and progesterone receptors in breast cancer. Sci Adv 2(6):e1501924
Stoll BA (1967) Progestin therapy of breast cancer: comparison of agents. Br Med J 3(5561):338–341
van Veelen H, Willemse PH, Tjabbes T, Schweitzer MJ, Sleijfer DT (1986) Oral high-dose medroxyprogesterone acetate versus tamoxifen. a randomized crossover trial in postmenopausal patients with advanced breast cancer. Cancer 58(1):7–13
Muss HB, Cruz JM (1992) High-dose progestin therapy for metastatic breast cancer. Ann Oncol 3(Suppl 3):15–20
Muss HB, Case LD, Atkins JN, Bearden JD 3rd, Cooper MR, Cruz JM, Jackson DV Jr, O’Rourke MA, Pavy MD, Powell BL et al (1994) Tamoxifen versus high-dose oral medroxyprogesterone acetate as initial endocrine therapy for patients with metastatic breast cancer: a piedmont oncology association study. J Clin Oncol 12(8):1630–1638
Fels E (1944) Treatment of breast cancer with testosterone propionate. J Clin Endocrinol 4(121)
Adair FE, Herrmann JB (1946) The use of testosterone propionate in the treatment of advanced carcinoma of the breast. Ann Surg 123:1023–1035
Herrmann JB, Adair FE (1946) The effect of testosterone propionate on carcinoma of the female breast with soft tissue metastases. J Clin Endocrinol Metab 6(6):769–775
Pan D, Kocherginsky M, Conzen SD (2011) Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res 71(20):6360–6370
Kach J, Conzen SD, Szmulewitz RZ (2015) Targeting the glucocorticoid receptor in breast and prostate cancers. Sci Transl Med 7(305):305ps319
Abduljabbar R, Negm OH, Lai CF, Jerjees DA, Al-Kaabi M, Hamed MR, Tighe PJ, Buluwela L, Mukherjee A, Green AR, Ali S, Rakha EA, Ellis IO (2015) Clinical and biological significance of glucocorticoid receptor (Gr) expression in breast cancer. Breast Cancer Res Treat 150(2):335–346
Skor MN, Wonder EL, Kocherginsky M, Goyal A, Hall BA, Cai Y, Conzen SD (2013) Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin Cancer Res 19(22):6163–6172
Regan Anderson TM, Ma SH, Raj GV, Cidlowski JA, Helle TM, Knutson TP, Krutilina RI, Seagroves TN, Lange CA (2016) Breast tumor kinase (Brk/Ptk6) is induced by hif, glucocorticoid receptor, and Pelp1-mediated stress signaling in triple-negative breast cancer. Cancer Res 76(6):1653–1663
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Tong D, Schuster E, Seifert M, Czerwenka K, Leodolte S, Zeillinger R (2002) Expression of estrogen receptor beta isoforms in human breast cancer tissues and cell lines. Breast Cancer Res Treat 71(3):249–255
Haldosen LA, Zhao C, Dahlman-Wright K (2014) Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 382(1):665–672
Fuqua SA, Schiff R, Parra I, Moore JT, Mohsin SK, Osborne CK, Clark GM, Allred DC (2003) Estrogen receptor beta protein in human breast cancer: correlation with clinical tumor parameters. Cancer Res 63(10):2434–2439
McGuire WL (1978) Hormone receptors: their role in predicting prognosis and response to endocrine therapy. Semin Oncol 5(4):428–433
Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM (2011) Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the nurses’ health study. Mod Pathol 24(7):924–931
Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, Henzen-Logmans SC (1996) The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer 32A(9):1560–1565
Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R (2010) Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23(2):205–212
Vilasco M, Communal L, Mourra N, Courtin A, Forgez P, Gompel A (2011) Glucocorticoid receptor and breast cancer. Breast Cancer Res Treat 130(1):1–10
Leo JC, Guo C, Woon CT, Aw SE, Lin VC (2004) Glucocorticoid and mineralocorticoid cross-talk with progesterone receptor to induce focal adhesion and growth inhibition in breast cancer cells. Endocrinology 145(3):1314–1321
Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, de Plater L, Guyader C, De Pinieux G, Judde JG, Rebucci M, Tran-Perennou C, Sastre-Garau X, Sigal-Zafrani B, Delattre O, Dieras V, Poupon MF (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 13(13):3989–3998
Cottu P, Marangoni E, Assayag F, de Cremoux P, Vincent-Salomon A, Guyader C, de Plater L, Elbaz C, Karboul N, Fontaine JJ, Chateau-Joubert S, Boudou-Rouquette P, Alran S, Dangles-Marie V, Gentien D, Poupon MF, Decaudin D (2012) Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat 133(2):595–606
Bergamaschi A, Hjortland GO, Triulzi T, Sorlie T, Johnsen H, Ree AH, Russnes HG, Tronnes S, Maelandsmo GM, Fodstad O, Borresen-Dale AL, Engebraaten O (2009) Molecular profiling and characterization of luminal-like and basal-like in vivo breast cancer xenograft models. Mol Oncol 3(5–6):469–482
DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL (2011) Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17(11):1514–1520
Kabos P, Finlay-Schultz J, Li C, Kline E, Finlayson C, Wisell J, Manuel CA, Edgerton SM, Harrell JC, Elias A, Sartorius CA (2012) Patient-derived luminal breast cancer xenografts retain hormone receptor heterogeneity and help define unique estrogen-dependent gene signatures. Breast Cancer Res Treat 135(2):415–432
Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ, Mann GB, Visvader JE, Lindeman GJ (2013) Targeting Bcl-2 with the Bh3 mimetic Abt-199 in estrogen receptor-positive breast cancer. Cancer Cell 24(1):120–129
Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, Landis MD, Wiechmann L, Schiff R, Giuliano M, Wong H, Fuqua SW, Contreras A, Gutierrez C, Huang J, Mao S, Pavlick AC, Froehlich AM, Wu MF, Tsimelzon A, Hilsenbeck SG, Chen ES, Zuloaga P, Shaw CA, Rimawi MF, Perou CM, Mills GB, Chang JC, Lewis MT (2013) A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res 73(15):4885–4897
Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, He X, Liu S, Hoog J, Lu C, Ding L, Griffith OL, Miller C, Larson D, Fulton RS, Harrison M, Mooney T, McMichael JF, Luo J, Tao Y, Goncalves R, Schlosberg C, Hiken JF, Saied L, Sanchez C, Giuntoli T, Bumb C, Cooper C, Kitchens RT, Lin A, Phommaly C, Davies SR, Zhang J, Kavuri MS, McEachern D, Dong YY, Ma C, Pluard T, Naughton M, Bose R, Suresh R, McDowell R, Michel L, Aft R, Gillanders W, DeSchryver K, Wilson RK, Wang S, Mills GB, Gonzalez-Angulo A, Edwards JR, Maher C, Perou CM, Mardis ER, Ellis MJ (2013) Endocrine-therapy-resistant Esr1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep 4(6):1116–1130
Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, Gelmon K, Chia S, Mar C, Wan A, Laks E, Biele J, Shumansky K, Rosner J, McPherson A, Nielsen C, Roth AJ, Lefebvre C, Bashashati A, de Souza C, Siu C, Aniba R, Brimhall J, Oloumi A, Osako T, Bruna A, Sandoval JL, Algara T, Greenwood W, Leung K, Cheng H, Xue H, Wang Y, Lin D, Mungall AJ, Moore R, Zhao Y, Lorette J, Nguyen L, Huntsman D, Eaves CJ, Hansen C, Marra MA, Caldas C, Shah SP, Aparicio S (2015) Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518(7539):422–426
Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, Pogrebniak K, Sandoval J, Cassidy JW, Tufegdzic-Vidakovic A, Sammut SJ, Jones L, Provenzano E, Baird R, Eirew P, Hadfield J, Eldridge M, McLaren-Douglas A, Barthorpe A, Lightfoot H, O’Connor MJ, Gray J, Cortes J, Baselga J, Marangoni E, Welm AL, Aparicio S, Serra V, Garnett MJ, Caldas C (2016) A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell 167(1):260–274, e222
Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, de Azambuja E, Viale G, Sotiriou C, Piccart M (2014) Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol 32(25):2794–2803
Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO (2009) Ki67 index, Her2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101(10):736–750
Creighton CJ (2012) The molecular profile of luminal B breast cancer. Biologics 6:289–297
Petrillo LA, Wolf DM, Kapoun AM, Wang NJ, Barczak A, Xiao Y, Korkaya H, Baehner F, Lewicki J, Wicha M, Park JW, Spellman PT, Gray JW, van’t Veer L, Esserman LJ (2012) Xenografts faithfully recapitulate breast cancer-specific gene expression patterns of parent primary breast tumors. Breast Cancer Res Treat 135(3):913–922
Levin-Allerhand JA, Sokol K, Smith JD (2003) Safe and effective method for chronic 17beta-estradiol administration to mice. Contemp Top Lab Anim Sci 42(6):33–35
Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S (1999) Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci U S A 96(5):2520–2525
Sartorius CA, Shen T, Horwitz KB (2003) Progesterone receptors a and B differentially affect the growth of estrogen-dependent human breast tumor xenografts. Breast Cancer Res Treat 79(3):287–299
Borras M, Hardy L, Lempereur F, el Khissiin AH, Legros N, Gol-Winkler R, Leclercq G (1994) Estradiol-induced down-regulation of estrogen receptor. Effect of various modulators of protein synthesis and expression. J Steroid Biochem Mol Biol 48(4):325–336
Cidlowski JA, Muldoon TG (1978) The dynamics of intracellular estrogen receptor regulation as influenced by 17beta-estradiol. Biol Reprod 18(2):234–246
Horwitz KB, McGuire WL (1978) Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem 253(22):8185–8191
Read LD, Greene GL, Katzenellenbogen BS (1989) Regulation of estrogen receptor messenger ribonucleic acid and protein levels in human breast cancer cell lines by sex steroid hormones, their antagonists, and growth factors. Mol Endocrinol 3(2):295–304
Cui X, Schiff R, Arpino G, Osborne CK, Lee AV (2005) Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23(30):7721–7735
Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ (2003) Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 120(5):725–731
Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, Duss S, Nicoulaz AL, Brisken C, Fiche M, Delorenzi M, Iggo R (2005) Identification of molecular apocrine breast tumours by microarray analysis. Oncogene 24(29):4660–4671
Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, Gomez F, Medicherla S, Alfaro IE, McCullagh E, Jedlicka P, Torkko KC, Thor AD, Elias AD, Protter AA, Richer JK (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16(1):R7
Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M (2011) Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 20(1):119–131
Ciupek A, Rechoum Y, Gu G, Gelsomino L, Beyer AR, Brusco L, Covington KR, Tsimelzon A, Fuqua SA (2015) Androgen receptor promotes tamoxifen agonist activity by activation of Egfr in Eralpha-positive breast cancer. Breast Cancer Res Treat 154(2):225–237
Mohammed H, Taylor C, Brown GD, Papachristou EK, Carroll JS, D’Santos CS (2016) Rapid immunoprecipitation mass spectrometry of endogenous proteins (Rime) for analysis of chromatin complexes. Nat Protoc 11(2):316–326
Tentler JJ, Ionkina AA, Tan AC, Newton TP, Pitts TM, Glogowska MJ, Kabos P, Sartorius CA, Sullivan KD, Espinosa JM, Eckhardt SG, Diamond JR (2015) P53 family members regulate phenotypic response to aurora kinase A inhibition in triple-negative breast cancer. Mol Cancer Ther 14(5):1117–1129
Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G (2013) Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther 4(3):70
Behbod F, Kittrell FS, LaMarca H, Edwards D, Kerbawy S, Heestand JC, Young E, Mukhopadhyay P, Yeh HW, Allred DC, Hu M, Polyak K, Rosen JM, Medina D (2009) An intraductal human-in-mouse transplantation model mimics the subtypes of ductal carcinoma in situ. Breast Cancer Res 11(5):R66
Sflomos G, Shamsheddin M, Brisken C (2015) An ex vivo model to study hormone action in the human breast. J Vis Exp 95:e52436
Sflomos G, Dormoy V, Metsalu T, Jeitziner R, Battista L, Scabia V, Raffoul W, Delaloye JF, Treboux A, Fiche M, Vilo J, Ayyanan A, Brisken C (2016) A preclinical model for Eralpha-positive breast cancer points to the epithelial microenvironment as determinant of luminal phenotype and hormone response. Cancer Cell 29(3):407–422
Leiter LH (1993) The nod mouse: a model for analyzing the interplay between heredity and environment in development of autoimmune disease. ILAR J 35(1):4–14
Baker DG (1998) Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 11(2):231–266
Kowalchyk K, Plagemann PG (1985) Cell surface receptors for lactate dehydrogenase-elevating virus on subpopulation of macrophages. Virus Res 2(3):211–229
Plagemann PGWG KF, Swim HE, Chan KKW (1963) Plasma lactic acid dehydrogenase-elevating agent of mice: distribution in tissues and effect on lactic dehydrogenase isozyme patterns. Can J Microbiol 9(1):75–86
Liu H, Bockhorn J, Dalton R, Chang YF, Qian D, Zitzow LA, Clarke MF, Greene GL (2011) Removal of lactate dehydrogenase-elevating virus from human-in-mouse breast tumor xenografts by cell-sorting. J Virol Methods 173(2):266–270
Clarke CL, Graham JD (2012) Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS ONE 7(4):e35859
Creighton CJ, Cordero KE, Larios JM, Miller RS, Johnson MD, Chinnaiyan AM, Lippman ME, Rae JM (2006) Genes regulated by estrogen in breast tumor cells in vitro are similarly regulated in vivo in tumor xenografts and human breast tumors. Genome Biol 7(4):R28
Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS (2003) Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144(10):4562–4574
Jacobsen BM, Schittone SA, Richer JK, Horwitz KB (2005) Progesterone-independent effects of human progesterone receptors (Prs) in estrogen receptor-positive breast cancer: Pr isoform-specific gene regulation and tumor biology. Mol Endocrinol 19(3):574–587
Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB (2002) Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277(7):5209–5218
Dunbier AK, Ghazoui Z, Anderson H, Salter J, Nerurkar A, Osin P, A’Hern R, Miller WR, Smith IE, Dowsett M (2013) Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin Cancer Res 19(10):2775–2786
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, Parker JS, Luo J, DeSchryver K, Allred DC, Esserman LJ, Unzeitig GW, Margenthaler J, Babiera GV, Marcom PK, Guenther JM, Watson MA, Leitch M, Hunt K, Olson JA (2011) Randomized phase Ii neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline Pam50-based intrinsic subtype—Acosog Z1031. J Clin Oncol 29(17):2342–2349
Miller WR, Larionov AA, Renshaw L, Anderson TJ, White S, Murray J, Murray E, Hampton G, Walker JR, Ho S, Krause A, Evans DB, Dixon JM (2007) Changes in breast cancer transcriptional profiles after treatment with the aromatase inhibitor, letrozole. Pharmacogenet Genomics 17(10):813–826
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5(8):615–625
Fagan DH, Uselman RR, Sachdev D, Yee D (2012) Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res 72(13):3372–3380
Lykkesfeldt AE, Madsen MW, Briand P (1994) Altered expression of estrogen-regulated genes in a tamoxifen-resistant and Ici 164,384 and Ici 182,780 sensitive human breast cancer cell line, Mcf-7/Tamr-1. Cancer Res 54(6):1587–1595
Naundorf H, Becker M, Lykkesfeldt AE, Elbe B, Neumann C, Buttner B, Fichtner I (2000) Development and characterization of a tamoxifen-resistant breast carcinoma xenograft. Br J Cancer 82(11):1844–1850
Fuqua SA, Gu G, Rechoum Y (2014) Estrogen receptor (Er) alpha mutations in breast cancer: hidden in plain sight. Breast Cancer Res Treat 144(1):11–19
Oesterreich S, Davidson NE (2013) The search for Esr1 mutations in breast cancer. Nat Genet 45(12):1415–1416
Holzapfel BM, Wagner F, Thibaudeau L, Levesque JP, Hutmacher DW (2015) Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells 33(6):1696–1704
Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, Gan G, McGettigan B, Fernandez P, Padilla-Just N, Varella-Garcia M, Song JI, Bowles DW, Schedin P, Tan AC, Roop DR, Wang XJ, Refaeli Y, Jimeno A (2016) Xactmice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 35(3):290–300
Zhou Q, Facciponte J, Jin M, Shen Q, Lin Q (2014) Humanized Nod-Scid Il2rg−/− mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett 344(1):13–19
Acknowledgments
This work was supported by National Institutes of Health grants NIH 2R01 CA140985 (CAS) and NIH R21 CA194477 (CAS). The authors wish to apologize to those whose work was not referenced due to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Matthews, S.B., Sartorius, C.A. Steroid Hormone Receptor Positive Breast Cancer Patient-Derived Xenografts. HORM CANC 8, 4–15 (2017). https://doi.org/10.1007/s12672-016-0275-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-016-0275-0