Abstract
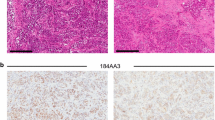
Resistance to endocrine therapy is a major complication of luminal breast cancer and studies of the biological features of hormonal resistance are limited by the lack of adequate preclinical models. The aim of this study is to establish and characterize a panel of primary human luminal breast carcinoma xenografts, and to evaluate their response to endocrine therapies. Four hundred and twenty-three tumor fragments obtained directly from patients have been grafted in the interscapular fatpad of Swiss nude mice. After stable engraftment with estradiol supplementation, xenografted tumors have been validated by conventional pathology and immunohistochemistry examination, and additional molecular studies. In vivo tumor growth and response to different endocrine treatments were evaluated. We have engrafted 423 tumors including 314 ER+ tumors, and 8 new luminal breast cancer xenografts have been obtained (2.5%). Tumor take was much lower for luminal tumors than for non-luminal tumors (2.5 vs. 24.7%, P < 0.0001), and was associated with two independent criteria, i.e., ER status (P < 0.0001) and a high grade tumor (P = 0.05). Histological and immunohistochemical analyses performed on patient’s tumors and xenografts showed striking similarities in the tumor morphology as well as in the expression level of ER, PR, and HER2. Response to hormone therapy, evaluated in 6 luminal models, showed different sensitivities, thus exhibiting heterogeneity similar to what is observed in the clinic. We have established a panel of primary human luminal breast cancer xenografts, recapitulating the biological and clinical behaviors of patient tumors, and therefore suitable for further preclinical experiments.





Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, (2010). CA Cancer J Clin 60(5):277–300
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784
Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF (2010) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol 11(12):1135–1141
Musgrove EA, Sutherland RL (2009) Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9(9):631–643
Guix M, de Matos Granja N, Meszoely I, Adkins TB, Wieman BM, Frierson KE, Sanchez V, Sanders ME, Grau AM, Mayer IA, Pestano G, Shyr Y, Muthuswamy S, Calvo B, Krontiras H, Krop IE, Kelley MC, Arteaga CL (2008) Short preoperative treatment with erlotinib inhibits tumor cell proliferation in hormone receptor-positive breast cancers. J Clin Oncol 26(6):897–906
Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M (2009) Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol 27(33):5538–5546
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, Revil C, Jones A (2009) Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol 27(33):5529–5537
Haddad TC, Yee D (2008) Of mice and (wo)men: is this any way to test a new drug? J Clin Oncol 26(6):830–832
Talmadge JE, Singh RK, Fidler IJ, Raz A (2007) Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170(3):793–804
Vargo-Gogola T, Rosen JM (2007) Modelling breast cancer: one size does not fit all. Nat Rev Cancer 7(9):659–672
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW (2006) A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10(6):515–527
de Plater L, Lauge A, Guyader C, Poupon MF, Assayag F, de Cremoux P, Vincent-Salomon A, Stoppa-Lyonnet D, Sigal-Zafrani B, Fontaine JJ, Brough R, Lord CJ, Ashworth A, Cottu P, Decaudin D, Marangoni E (2010) Establishment and characterisation of a new breast cancer xenograft obtained from a woman carrying a germline BRCA2 mutation. Br J Cancer 103(8):1192–1200
Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, de Plater L, Guyader C, De Pinieux G, Judde JG, Rebucci M, Tran-Perennou C, Sastre-Garau X, Sigal-Zafrani B, Delattre O, Dieras V, Poupon MF (2007) A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 13(13):3989–3998
Zafrani B, Aubriot MH, Mouret E, De Cremoux P, De Rycke Y, Nicolas A, Boudou E, Vincent-Salomon A, Magdelenat H, Sastre-Garau X (2000) High sensitivity and specificity of immunohistochemistry for the detection of hormone receptors in breast carcinoma: comparison with biochemical determination in a prospective study of 793 cases. Histopathology 37(6):536–545
Balaton A (1999) Recommendations for the immunohistochemistry of the hormonal receptors on paraffin sections in breast cancer. Update 1999. Group for Evaluation of Prognostic Factors using Immunohistochemistry in Breast Cancer (GEFPICS-FNCLCC). Ann Pathol 19(4):336–343
Vincent-Salomon A, MacGrogan G, Couturier J, Arnould L, Denoux Y, Fiche M, Jacquemier J, Mathieu MC, Penault-Llorca F, Rigaud C, Roger P, Treilleux I, Vilain MO, Mathoulin-Pelissier S, Le Doussal V (2003) Calibration of immunohistochemistry for assessment of HER2 in breast cancer: results of the French multicentre GEFPICS study. Histopathology 42(4):337–347
Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, Erban JK, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Ljung BM, Mankoff DA, Marcom PK, Mayer IA, McCormick B, Pierce LJ, Reed EC, Sachdev J, Smith ML, Somlo G, Ward JH, Wolff AC, Zellars R (2011) Invasive breast cancer. J Natl Compr Canc Netw 9(2):136–222
McLaughlin LA, Dickmann LJ, Wolf CR, Henderson CJ (2008) Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug Metab Dispos 36(7):1322–1331
Fiebig HH, Schuchhardt C, Henss H, Fiedler L, Lohr GW (1984) Comparison of tumor response in nude mice and in the patients. Behring Inst Mitt 74:343–352
Giesemann T, Krumbach R, Schüler J, Vuaroqueaux V, Hofmann M, Liu N, Haegebarth A, Beckers T, Fiebig H (2010) Patient-derived breast cancer xenografts: Molecular characteristics and growth properties. In: 22th EORTC-NCI-AACR Conference, Berlin. Abstract 634
Bergamaschi A, Hjortland GO, Triulzi T, Sorlie T, Johnsen H, Ree AH, Russnes HG, Tronnes S, Maelandsmo GM, Fodstad O, Borresen-Dale AL, Engebraaten O (2009) Molecular profiling and characterization of luminal-like and basal-like in vivo breast cancer xenograft models. Mol Oncol 3(5–6):469–482
Luo M, Fan H, Nagy T, Wei H, Wang C, Liu S, Wicha MS, Guan JL (2009) Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res 69(2):466–474
Sakakibara T, Xu Y, Bumpers HL, Chen FA, Bankert RB, Arredondo MA, Edge SB, Repasky EA (1996) Growth and metastasis of surgical specimens of human breast carcinomas in SCID mice. Cancer J Sci Am 2(5):291–300
Visonneau S, Cesano A, Torosian MH, Miller EJ, Santoli D (1998) Growth characteristics and metastatic properties of human breast cancer xenografts in immunodeficient mice. Am J Pathol 152(5):1299–1311
Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, Abbott RM, Hoog J, Dooling DJ, Koboldt DC, Schmidt H, Kalicki J, Zhang Q, Chen L, Lin L, Wendl MC, McMichael JF, Magrini VJ, Cook L, McGrath SD, Vickery TL, Appelbaum E, Deschryver K, Davies S, Guintoli T, Crowder R, Tao Y, Snider JE, Smith SM, Dukes AF, Sanderson GE, Pohl CS, Delehaunty KD, Fronick CC, Pape KA, Reed JS, Robinson JS, Hodges JS, Schierding W, Dees ND, Shen D, Locke DP, Wiechert ME, Eldred JM, Peck JB, Oberkfell BJ, Lolofie JT, Du F, Hawkins AE, O’Laughlin MD, Bernard KE, Cunningham M, Elliott G, Mason MD, Thompson DM Jr, Ivanovich JL, Goodfellow PJ, Perou CM, Weinstock GM, Aft R, Watson M, Ley TJ, Wilson RK, Mardis ER (2010) Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464(7291):999–1005
Just L, Timmer M, Tinius J, Stahl F, Deiwick A, Nikkhah G, Bader A (2003) Identification of human cells in brain xenografts and in neural co-cultures of rat by in situ hybridisation with Alu probe. J Neurosci Methods 126(1):69–77
Fisher CR, Graves KH, Parlow AF, Simpson ER (1998) Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95(12):6965–6970
Toda K, Takeda K, Okada T, Akira S, Saibara T, Kaname T, Yamamura K, Onishi S, Shizuta Y (2001) Targeted disruption of the aromatase P450 gene (Cyp19) in mice and their ovarian and uterine responses to 17beta-oestradiol. J Endocrinol 170(1):99–111
Acknowledgments
We are especially indebted to all the patients who consented to give their tumors for animal experiments. We would also like to thank all the members of the surgical oncology, and tumor biology teams who devoted much time to the patient tumor sampling. We deeply thank O. Chouchane and A. Nicolas for the IHC pictures. The Institut Curie in vivo experiment platform members should be gratefully acknowledged for their invaluable contribution to the mouse care. We are also indebted to C. Tran-Perennou and C. Barbaroux who performed the qPCR analyses. This work was supported by the Translational Research Department of Institut Curie. Dr. Paul Cottu is supported by a Grant from the Institut Curie Foundation.
Conflict of interest
All authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Clinical characteristics of the 8 patients with xenografts (DOC 41 kb)
Supplementary Table 2
Actual mean volumes in each treatment groups in all reported experiments. (XLS 19 kb)
Supplementary Fig. 6
Extensive results of endocrine therapy testing on luminal xenografts. The figures show the response curves to endocrine therapies in tested models. In all figures, error bars were omitted for the sake of legibility. a Sensitivity to tamoxifen of the HBCx-3 model. b Extended therapeutic characterization of HBCx-3. The experiment is at a too early stage for P values. c Therapeutic characterization of HBCx-22. Specific P values are: tamoxifen <0.001; fulvestrant <0.001; d Extended therapeutic characterization of HBCx-22. Specific P values for endocrine treatments curves versus control are: ovariectomy = 0.19; ovariectomy + letrozole = 0.02; letrozole = 0.04; tamoxifen = 0.01; 4-OH-tamoxifen = 0.03. e Detailed view of selected groups from (d). f Therapeutic characterization of HBCx-34. Specific P values are: ovariectomy <0.001; fulvestrant <0.001; tamoxifen = 0.03 (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Cottu, P., Marangoni, E., Assayag, F. et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat 133, 595–606 (2012). https://doi.org/10.1007/s10549-011-1815-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1815-5