Abstract
Cardiac sarcoidosis (CS) is an inflammatory disease with high morbidity and mortality, with a pathognomonic feature of non-caseating granulomatous inflammation. While 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is a well-established modality to image inflammation and diagnose CS, there are limitations to its specificity and reproducibility. Imaging focused on the molecular processes of inflammation including the receptors and cellular microenvironments present in sarcoid granulomas provides opportunities to improve upon FDG-PET imaging for CS. This review will highlight the current limitations of FDG-PET imaging for CS while discussing emerging new nuclear imaging molecular targets for the imaging of cardiac sarcoidosis.


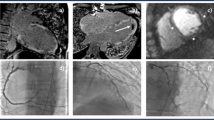
Source: Gormsen et al,47 EJNMMI Research 2016 (https://doi.org/10.1186/s13550-016-0207)

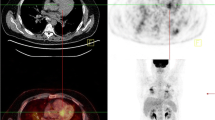
Source: Norikane et al,68 EJNMMI Research 2017 (https://doi.org/10.1186/s13550-017-0321-0)
Similar content being viewed by others
Abbreviations
- CS:
-
Cardiac sarcoidosis
- FDG:
-
Fluorodeoxyglucose
- PET:
-
Positron emission tomography
- FLT:
-
Fluorodeoxythymidine
- F-MISO:
-
Fluoromisonidazole
- SSTR:
-
Somatostatin receptor
- CXCR:
-
CXC chemokine receptor
References
Perry A, Vuitch F. Causes of death in patients with sarcoidosis: A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 1995;119:167‐72.
Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1304‐23. https://doi.org/10.1016/J.HRTHM.2014.03.043.
Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI–ASNC expert consensus document on the role of 18F-FDG PET/CT in cardiac sarcoid detection and therapy monitoring. J Nucl Cardiol 2017;24:1741‐58. https://doi.org/10.1007/s12350-017-0978-9.
Slart RHJA, Glaudemans AWJM, Lancellotti P, et al. A joint procedural position statement on imaging in cardiac sarcoidosis: From the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American. J Nucl Cardiol 2018;25:298‐319. https://doi.org/10.1007/s12350-017-1043-4.
Alvi RM, Young BD, Shahab Z, et al. Repeatability and optimization of FDG positron emission tomography for evaluation of cardiac sarcoidosis. JACC Cardiovasc Imaging 2019;12:1284‐7. https://doi.org/10.1016/j.jcmg.2019.01.011.
Mochizuki T, Tsukamoto E, Kuge Y, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med 2001;42:1551‐5.
Scholtens AM, Verberne HJ, Budde RPJ, Lam MGEH. Additional heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging. J Nucl Med 2016. https://doi.org/10.2967/jnumed.115.166884.
Osborne MT, Hulten EA, Murthy VL, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 2017;24:86‐99. https://doi.org/10.1007/s12350-016-0502-7.
Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging 2010;3:1219‐28. https://doi.org/10.1016/j.jcmg.2010.09.015.
Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18 F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004;45:1989‐98.
Varghese M, Smiley D, Bellumkonda L, Rosenfeld LE, Zaret B, Miller EJ. Quantitative interpretation of FDG PET for cardiac sarcoidosis reclassifies visually interpreted exams and potentially impacts downstream interventions. Sarcoidosis Vasc Diffus Lung Dis 2018;35:342.
Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol 2014;21:925‐39. https://doi.org/10.1007/s12350-014-9901-9.
McArdle BA, Birnie DH, Klein R, et al. Is there an association between clinical presentation and the location and extent of myocardial involvement of cardiac sarcoidosis as assessed by 18F-fluorodoexyglucose positron emission tomography? Circ Cardiovasc Imaging 2013. https://doi.org/10.1161/CIRCIMAGING.112.000289.
Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol 2009;16:801‐10. https://doi.org/10.1007/s12350-009-9110-0.
Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol 2017;24:413‐24. https://doi.org/10.1007/s12350-016-0490-7.
Manabe O, Ohira H, Hirata K, et al. Use of 18 F-FDG PET/CT texture analysis to diagnose cardiac sarcoidosis. Eur J Nucl Med Mol Imaging 2019. https://doi.org/10.1007/s00259-018-4195-9.
Manabe O, Koyanagawa K, Hirata K, et al. Texture feature by FDG PET can predict events of cardiac sarcoidosis. J Nucl Med 2019;60:372.
Shelke AB, Aurangabadkar HU, Bradfield JS, Ali Z, Kumar KS, Narasimhan C. Serial FDG-PET scans help to identify steroid resistance in cardiac sarcoidosis. Int J Cardiol 2017;228:717‐22. https://doi.org/10.1016/j.ijcard.2016.11.142.
Muser D, Santangeli P, Castro SA, et al. Prognostic role of serial quantitative evaluation of 18F-fluorodeoxyglucose uptake by PET/CT in patients with cardiac sarcoidosis presenting with ventricular tachycardia. Eur J Nucl Med Mol Imaging 2018;45:1394‐404. https://doi.org/10.1007/s00259-018-4001-8.
Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014. https://doi.org/10.1007/s12350-013-9828-6.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983. https://doi.org/10.1038/jcbfm.1983.1.
Karakatsanis NA, Trivieri MG, Abgral R, et al. Direct 4D Patlak 18F-FDG PET/MR for the multi-parametric assessment of active cardiac sarcoidosis. In: 2017 IEEE nuclear science symposium and medical imaging conference, NSS/MIC 2017—Conference Proceedings. Institute of Electrical and Electronics Engineers Inc.; 2018. https://doi.org/10.1109/NSSMIC.2017.8532794.
Dweck MR, Abgral R, Trivieri MG, et al. Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 2018. https://doi.org/10.1016/j.jcmg.2017.02.021.
Lebasnier A, Legallois D, Bienvenu B, et al. Diagnostic value of quantitative assessment of cardiac 18F-fluoro-2-deoxyglucose uptake in suspected cardiac sarcoidosis. Ann Nucl Med 2018;32:319‐27. https://doi.org/10.1007/s12149-018-1250-3.
Nakajo M, Ojima S, Kawakami H, et al. Value of Patlak Ki images from 18F-FDG-PET/CT for evaluation of the relationships between disease activity and clinical events in cardiac sarcoidosis. Sci Rep 2021. https://doi.org/10.1038/s41598-021-82217-0.
Zubin Maslov P, Narula N, Narula J. Somatostatin receptor imaging in active cardiac sarcoidosis: Would less be enough? J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01824-7.
ten Bokum, Hofland, de Jong, et al. Immunohistochemical localization of somatostatin receptor sst2A in sarcoid granulomas. Eur J Clin Invest 1999;29:630-6. https://doi.org/10.1046/j.1365-2362.1999.00498.x.
Boy C, Heusner TA, Poeppel TD, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1–sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging 2011;38:1224‐36. https://doi.org/10.1007/s00259-011-1760-x.
Li X, Samnick S, Lapa C, et al. 68Ga-DOTATATE PET/CT for the detection of inflammation of large arteries: Correlation with18F-FDG, calcium burden and risk factors. EJNMMI Res 2012;2:52. https://doi.org/10.1186/2191-219X-2-52.
Rominger A, Saam T, Vogl E, et al. In vivo imaging of macrophage activity in the coronary arteries using 68Ga-DOTATATE PET/CT: Correlation with coronary calcium burden and risk factors. J Nucl Med 2010;51:193‐7. https://doi.org/10.2967/jnumed.109.070672.
Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by 68Ga-DOTATATE PET compared to [18F]FDG PET imaging. J Am Coll Cardiol 2017;69:1774‐91. https://doi.org/10.1016/j.jacc.2017.01.060.
Thackeray JT, Bankstahl JP, Wang Y, et al. Targeting post-infarct inflammation by PET imaging: Comparison of 68Ga-citrate and 68Ga-DOTATATE with 18F-FDG in a mouse model. Eur J Nucl Med Mol Imaging 2015. https://doi.org/10.1007/s00259-014-2884-6.
Nobashi T, Nakamoto Y, Kubo T, et al. The utility of PET/CT with 68Ga-DOTATOC in sarcoidosis: Comparison with 67Ga-scintigraphy. Ann Nucl Med 2016;30:544‐52. https://doi.org/10.1007/s12149-016-1095-6.
Lapa C, Reiter T, Kircher M, et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: An initial comparison to cardiac MRI. Oncotarget 2016;7:77807‐14. https://doi.org/10.18632/oncotarget.12799.
Breeman WAP, De Blois E, Sze Chan H, Konijnenberg M, Kwekkeboom DJ, Krenning EP. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: Current status of research, clinical applications, and future perspectives. Semin Nucl Med 2011;41:314‐21. https://doi.org/10.1053/j.semnuclmed.2011.02.001.
Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 2010. https://doi.org/10.1007/s00259-010-1512-3.
Walker RC, Smith GT, Liu E, Moore B, Clanton J, Stabin M. Measured human dosimetry of 68Ga-DOTATATE. J Nucl Med 2013. https://doi.org/10.2967/jnumed.112.114165.
Shastry M, Kayani I, Wild D, et al. Distribution pattern of 68Ga-DOTATATE in disease-free patients. Nucl Med Commun 2010;31:1025‐32. https://doi.org/10.1097/MNM.0b013e32833f635e.
Soultanidis G, Robson PM, Tsoumpas C, et al. Assessment of optimal circulation time for 68Ga-DOTATATE PET/MR imaging of cardiac sarcoidosis: Preliminary data. J Nucl Med 2020. https://jnm.snmjournals.org/content/61/supplement_1/1338.
Sandström M, Velikyan I, Garske-Román U, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med 2013;54:1755‐9. https://doi.org/10.2967/jnumed.113.120600.
Poeppel TD, Binse I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med 2011;52:1864‐70. https://doi.org/10.2967/jnumed.111.091165.
Bravo PE, Bajaj N, Padera RF, et al. Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: A pilot study. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01782-0.
Rinne P, Hellberg S, Kiugel M, et al. Comparison of somatostatin receptor 2-targeting PET tracers in the detection of mouse atherosclerotic plaques. Mol Imaging Biol 2016. https://doi.org/10.1007/s11307-015-0873-1.
Pettinato C, Sarnelli A, Di Donna M, et al. 68Ga-DOTANOC: Biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2008;35:72‐9. https://doi.org/10.1007/s00259-007-0587-y.
Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: Characterization of uptake in normal organs and tumor lesions. QJ Nucl Med Mol Imaging 2010;54:61‐7.
Krausz Y, Freedman N, Rubinstein R, et al. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: Comparison with 111In-DTPA-octreotide (OctreoScan®). Mol Imaging Biol 2011;13:583‐93. https://doi.org/10.1007/s11307-010-0374-1.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52. https://doi.org/10.1186/s13550-016-0207-6.
Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 DOTA-NOC PET/CT as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun 2018. https://doi.org/10.1097/mnm.0000000000000869.
Schreiter NF, Brenner W, Nogami M, et al. Cost comparison of 111In-DTPA-octreotide scintigraphy and 68Ga-DOTATOC PET/CT for staging enteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2012;39:72‐82. https://doi.org/10.1007/s00259-011-1935-5.
Hennrich U, Benešová M. [68Ga]Ga-DOTA-TOC: The first FDA-approved 68Ga-radiopharmaceutical for PET imaging. Pharmaceuticals 2020;13:38. https://doi.org/10.3390/ph13030038.
Kroiss A, Putzer D, Decristoforo C, et al. 68Ga-DOTA-TOC uptake in neuroendocrine tumour and healthy tissue: Differentiation of physiological uptake and pathological processes in PET/CT. Eur J Nucl Med Mol Imaging 2013;40:514‐23. https://doi.org/10.1007/s00259-012-2309-3.
Lee R, Kim J, Paeng JC, et al. Measurement of 68Ga-DOTATOC uptake in the thoracic aorta and its correlation with cardiovascular risk. Nucl Med Mol Imaging 2018;52:279‐86. https://doi.org/10.1007/s13139-018-0524-y.
Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2007;34:1617‐26. https://doi.org/10.1007/s00259-007-0450-1.
Afshar-Oromieh A, Giesel FL, Linhart HG, et al. Detection of cranial meningiomas: Comparison of 68Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging 2012;39:1409‐15. https://doi.org/10.1007/s00259-012-2155-3.
Pizarro C, Kluenker F, Dabir D, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail 2018;5:249‐61. https://doi.org/10.1002/ehf2.12243.
Nyuyki F, Plotkin M, Graf R, et al. Potential impact of 68Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging 2010;37:310‐8. https://doi.org/10.1007/s00259-009-1270-2.
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using68Ga-DOTATOC PET and comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2006;33:823‐30. https://doi.org/10.1007/s00259-005-0063-5.
Lapa C, Reiter T, Li X, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT—A comparison to cardiac MRI. Int J Cardiol 2015;194:44‐9. https://doi.org/10.1016/j.ijcard.2015.05.073.
Shields AF. PET imaging with 18F-FLT and thymidine analogs: Promise and pitfalls. J Nucl Med 2003;44:1432‐4.
Been LB, Suurmeijer AJH, Cobben DCP, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: Current status and opportunities. Eur J Nucl Med Mol Imaging 2004;31:1659‐72. https://doi.org/10.1007/s00259-004-1687-6.
Oh SJ, Mosdzianowski C, Chi DY, et al. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol 2004;31:803‐9. https://doi.org/10.1016/j.nucmedbio.2004.01.008.
Vesselle H, Grierson J, Peterson LM, Muzi M, Mankoff DA, Krohn KA. 18F-fluorothymidine radiation dosimetry in human PET imaging studies. J Nucl Med 2003;44:1482‐8.
Frings V, De Langen AJ, Smit EF, et al. Repeatability of metabolically active volume measurements with 18F-FDG and 18F-FLT PET in non-small cell lung cancer. J Nucl Med 2010;51:1870‐7. https://doi.org/10.2967/jnumed.110.077255.
Herrmann K, Buck AK, Schuster T, et al. Predictive value of initial18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med 2011;52:690‐6. https://doi.org/10.2967/jnumed.110.084566.
Cobben DCP, Van Der Laan BFAM, Maas B, et al. 18F-FLT PET for visualization of laryngeal cancer: Comparison with 18F-FDG PET. J Nucl Med 2004;45:226‐31.
Troost EGC, Bussink J, Hoffmann AL, Boerman OC, Oyen WJG, Kaanders JHAM. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med 2010. https://doi.org/10.2967/jnumed.109.069310.
Yap CS, Czernin J, Fishbein MC, et al. Evaluation of thoracic tumors with 18F-fluorothymidine and 18F-fluorodeoxyglucose-positron emission tomography. Chest 2006;129:393‐401. https://doi.org/10.1378/chest.129.2.393.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Dobashi H, Nishiyama Y. Comparative evaluation of 18F-FLT and 18F-FDG for detecting cardiac and extra-cardiac thoracic involvement in patients with newly diagnosed sarcoidosis. EJNMMI Res 2017;7:69. https://doi.org/10.1186/s13550-017-0321-0.
Ye YX, Calcagno C, Binderup T, et al. Imaging macrophage and hematopoietic progenitor proliferation in atherosclerosis. Circ Res 2015;117:835‐45. https://doi.org/10.1161/CIRCRESAHA.115.307024.
Tan YY, Liang J, Liu DF, et al. 18F-FLT PET/CT imaging in a Wister rabbit inflammation model. Exp Ther Med 2014;8:69‐72. https://doi.org/10.3892/etm.2014.1687.
Lee TS, Ahn SH, Moon BS, et al. Comparison of 18F-FDG, 18F-FET and 18F-FLT for differentiation between tumor and inflammation in rats. Nucl Med Biol 2009;36:681‐6. https://doi.org/10.1016/j.nucmedbio.2009.03.009.
Kim SK, Im HJ, Kim W, Kim TS, Hwangbo B, Kim HJ. F-18 fluorodeoxyglucose and F-18 fluorothymidine positron emission tomography/computed tomography imaging in a case of neurosarcoidosis. Clin Nucl Med 2010. https://doi.org/10.1097/RLU.0b013e3181c7c149.
Rayamajhi SJ, Mittal BR, Maturu VN, et al. 18F-FDG and18F-FLT PET/CT imaging in the characterization of mediastinal lymph nodes. Ann Nucl Med 2015;30:207‐16. https://doi.org/10.1007/s12149-015-1047-6.
Norikane T, Yamamoto Y, Maeda Y, Noma T, Nishiyama Y. 18F-FLT PET imaging in a patient with sarcoidosis with cardiac involvement. Clin Nucl Med 2015. https://doi.org/10.1097/RLU.0000000000000653.
Martineau P, Pelletier-Galarneau M, Juneau D, Leung E, Nery P, DeKemp R, et al. FLT-PET for the assessment of sarcoidosis including cardiac and CNS involvement: Comparison to FDG-PET. J Nucl Med. http://jnm.snmjournals.org/content/60/supplement_1/226. Accessed 6 Apr 2020.
Martineau P, Pelletier-Galarneau M, Juneau D, et al. Imaging cardiac sarcoidosis with FLT-PET compared with FDG/perfusion-PET: A prospective pilot study. JACC Cardiovasc Imaging 2019. https://doi.org/10.1016/j.jcmg.2019.06.020.
Gómez V, Gispert JD, Amador V, Llop J. New method for routine production of l-[methyl-11C]methionine:in loop synthesis. J Label Compd Radiopharm 2008;51:83‐6. https://doi.org/10.1002/jlcr.1483.
Deloar HM, Fujiwara T, Nakamura T, et al. Estimation of internal absorbed dose of l-[methyl-11C]methionine using whole-body positron emission tomography. Eur J Nucl Med 1998;25:629‐33. https://doi.org/10.1007/s002590050265.
Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11 C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 2008;49:694‐9. https://doi.org/10.2967/jnumed.107.048082.
Okasaki M, Kubota K, Minamimoto R, et al. Comparison of 11C–4′-thiothymidine, 11C-methionine, and 18F-FDG PET/CT for the detection of active lesions of multiple myeloma. Ann Nucl Med 2015;29:224‐32. https://doi.org/10.1007/s12149-014-0931-9.
Yamada Y, Uchida Y, Tatsumi K, et al. Fluorine-18-fluorodeoxyglucose and carbon-11-methionine evaluation of lymphadenopathy in sarcoidosis. J Nucl Med 1998;39:1160‐6.
Jacobs AH, Thomas A, Kracht LW, et al. 18F-fluoro-l-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J Nucl Med 2005;46:1948‐58.
Ullrich RT, Kracht L, Brunn A, et al. Methyl-l-11 C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med 2009;50:1962‐8. https://doi.org/10.2967/jnumed.109.065904.
Thackeray JT, Bankstahl JP, Wang Y, Wollert KC, Bengel FM. Targeting amino acid metabolism for molecular imaging of inflammation early after myocardial infarction. Theranostics 2016;6:1768‐79. https://doi.org/10.7150/thno.15929.
Taki J, Wakabayashi H, Inaki A, et al. 14-Cmethionine uptake as a potential marker of inflammatory processes after myocardial ischemia and reperfusion. J Nucl Med 2013. https://doi.org/10.2967/jnumed.112.112060.
Maya Y, Werner RA, Schütz C, et al. 11C-methionine PET of myocardial inflammation in a rat model of experimental autoimmune myocarditis. J Nucl Med 2016. https://doi.org/10.2967/jnumed.116.174045.
Zhao S, Kuge Y, Kohanawa M, et al. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT. J Nucl Med 2007;49:135‐41. https://doi.org/10.2967/jnumed.107.044578.
Ng D, Jacobs M, Mantil J. Combined C-11 methionine and F-18 FDG PET imaging in a case of neurosarcoidosis. Clin Nucl Med 2006;31:373‐5. https://doi.org/10.1097/01.rlu.0000222735.19513.bb.
Hain SF, Beggs AD. C-11 methionine uptake in granulomatous disease. Clin Nucl Med 2004. https://doi.org/10.1097/01.rlu.0000134997.91642.56.
Hsieh HJ, Lin SH, Lin KH, Lee CY, Chang CP, Wang SJ. The feasibility of 11C-methionine-PET in diagnosis of solitary lung nodules/masses when compared with 18F-FDG-PET. Ann Nucl Med 2008;22:533‐8. https://doi.org/10.1007/s12149-007-0142-8.
Spreckelmeyer S, Schulze O, Brenner W. Fully-automated production of [68Ga]Ga-PentixaFor on the module Modular Lab-PharmTracer. EJNMMI Radiopharm Chem 2020. https://doi.org/10.1186/s41181-020-0091-2.
Herrmann K, Lapa C, Wester HJ, et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J Nucl Med 2015;56:410‐6. https://doi.org/10.2967/jnumed.114.151647.
Rausch I, Beitzke D, Li X, et al. Accuracy of PET quantification in [68Ga]Ga-pentixafor PET/MR imaging of carotid plaques. J Nucl Cardiol 2020. https://doi.org/10.1007/s12350-020-02257-3.
Herhaus P, Habringer S, Vag T, et al. Response assessment with the CXCR4-directed positron emission tomography tracer [68Ga]pentixafor in a patient with extranodal marginal zone lymphoma of the orbital cavities. EJNMMI Res 2017;7:1‐4. https://doi.org/10.1186/s13550-017-0294-z.
Hyafil F, Pelisek J, Laitinen I, et al. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the radiotracer 68Ga-Pentixafor for PET. J Nucl Med 2017. https://doi.org/10.2967/jnumed.116.179663.
Weiberg D, Thackeray JT, Daum G, et al. Clinical molecular imaging of chemokine receptor CXCR4 expression in atherosclerotic plaque using 68 Ga-pentixafor PET: Correlation with cardiovascular risk factors and calcified plaque burden. J Nucl Med 2018;59:266‐72. https://doi.org/10.2967/jnumed.117.196485.
Derlin T, Sedding DG, Dutzmann J, et al. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [68Ga]pentixafor PET/CT. Eur J Nucl Med Mol Imaging 2018;45:1934‐44. https://doi.org/10.1007/s00259-018-4076-2.
Thackeray JT, Derlin T, Haghikia A, et al. Molecular imaging of the chemokine receptor CXCR4 after acute myocardial infarction. JACC Cardiovasc Imaging 2015. https://doi.org/10.1016/j.jcmg.2015.09.008.
Reiter T, Kircher M, Schirbel A, et al. Imaging of C-X-C motif chemokine receptor CXCR4 expression after myocardial infarction with [68 Ga]pentixafor-PET/CT in correlation with cardiac MRI. JACC Cardiovasc Imaging 2018. https://doi.org/10.1016/j.jcmg.2018.01.001.
Su H, Spinale FG, Dobrucki LW, et al. Noninvasive targeted imaging of matrix metalloproteinase activation in a murine model of postinfarction remodeling. Circulation 2005;112:3157‐67. https://doi.org/10.1161/CIRCULATIONAHA.105.583021.
Thorn SL, Barlow SC, Feher A, et al. Application of hybrid matrix metalloproteinase-targeted and dynamic 201Tl single-photon emission computed tomography/computed tomography imaging for evaluation of early post-myocardial infarction remodeling. Circ Cardiovasc Imaging 2019. https://doi.org/10.1161/CIRCIMAGING.119.009055.
Boutagy N, Mikush N, Wang X, Hawley C, Liu C, Sinusas A. Early detection of doxorubicin-induced cardiotoxicity using the matrix metalloproteinase (MMP) targeted radiotracer, 99mTc-RP805. J Nucl Med 2016;57:1640.
Jung JJ, Razavian M, Challa AA, et al. Multimodality and molecular imaging of matrix metalloproteinase activation in calcific aortic valve disease. J Nucl Med 2015;56:933‐8. https://doi.org/10.2967/jnumed.114.152355.
Xu Z, Li XF, Zou H, Sun X, Shen B. 18F-fluoromisonidazole in tumor hypoxia imaging. Oncotarget 2017;8:94969‐79. https://doi.org/10.18632/oncotarget.21662.
Lee ST, Scott AM. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin Nucl Med 2007;37:451‐61. https://doi.org/10.1053/j.semnuclmed.2007.07.001.
Oh SJ, Dae YC, Mosdzianowski C, et al. Fully automated synthesis of [18F]fluoromisonidazole using a conventional [18F]FDG module. Nucl Med Biol 2005;32:899‐905. https://doi.org/10.1016/j.nucmedbio.2005.06.003.
Koh WJ, Rasey JS, Evans ML, et al. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992;22:199‐212. https://doi.org/10.1016/0360-3016(92)91001-4.
Watanabe S, Shiga T, Hirata K, et al. Biodistribution and radiation dosimetry of the novel hypoxia PET probe [18F]DiFA and comparison with [18F]FMISO. EJNMMI Res 2019;9:1‐11. https://doi.org/10.1186/s13550-019-0525-6.
Toyonaga T, Hirata K, Yamaguchi S, et al. 18F-fluoromisonidazole positron emission tomography can predict pathological necrosis of brain tumors. Eur J Nucl Med Mol Imaging 2016;43:1469‐76. https://doi.org/10.1007/s00259-016-3320-x.
Sato J, Kitagawa Y, Yamazaki Y, et al. Advantage of FMISO-PET over FDG-PET for predicting histological response to preoperative chemotherapy in patients with oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging 2014. https://doi.org/10.1007/s00259-014-2810-y.
Read SJ, Hirano T, Abbott DF, et al. Identifying hypoxic tissue after acute ischemic stroke using PET and 18F-fluoromisonidazole. Neurology 1998;51:1617‐21. https://doi.org/10.1212/WNL.51.6.1617.
Belton M, Brilha S, Manavaki R, et al. Hypoxia and tissue destruction in pulmonary TB. Thorax 2016;71:1145‐53. https://doi.org/10.1136/thoraxjnl-2015-207402.
Zhao S, Kuge Y, Zhao Y, et al. Hyoxia imaging with 18F-fluoromisonidazole (FMISO) for differentiating tumors from granulomas: A comparison with FDG in experimental rat models using small animal PET. J Nucl Med 2010;51:1103.
Manabe O, Hirata K, Shozo O, et al. 18F-fluoromisonidazole (FMISO) PET may have the potential to detect cardiac sarcoidosis. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-016-0495-2.
Furuya S, Naya M, Manabe O, et al. 18F-FMISO PET/CT detects hypoxic lesions of cardiac and extra-cardiac involvement in patients with sarcoidosis. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01976-6.
Mather SJ, Ellison D. Reduction-mediated technetium-99m labeling of monoclonal antibodies. J Nucl Med 1990;31:692‐7.
Galli F, Lanzolla T, Pietrangeli V, et al. In vivo evaluation of TNF-alpha in the lungs of patients affected by sarcoidosis. Biomed Res Int 2015. https://doi.org/10.1155/2015/401341.
Yalcin H, Elboga U, Kalender E. Overview of 99mTc-anti-TNF-α scintigraphy: Diagnostic applications. Rep Med Imaging 2013;7:1. https://doi.org/10.2147/RMI.S39098.
Roimicher L, Lopes FPPL, de Souza SAL, et al. 99mTc-anti-TNF-scintigraphy in RA: A comparison pilot study with MRI and clinical examination. Rheumatology 2011;50:2044‐50. https://doi.org/10.1093/rheumatology/ker234.
Smith WHT, Nair RU, Adamson D, Kearney MT, Ball SG, Balmforth AJ. Somatostatin receptor subtype expression in the human heart: Differential expression by myocytes and fibroblasts. J Endocrinol 2005. https://doi.org/10.1677/joe.1.06082.
Gandhi R, Cawthorne C, Craggs LJL, et al. Cell proliferation detected using [18F]FLT PET/CT as an early marker of abdominal aortic aneurysm. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01946-y.
Bollineni VR, Kramer GM, Jansma EP, Liu Y, Oyen WJG. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur J Cancer 2016;55:81‐97. https://doi.org/10.1016/j.ejca.2015.11.018.
Borchert T, Beitar L, Langer LBN, et al. Dissecting the target leukocyte subpopulations of clinically relevant inflammation radiopharmaceuticals. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01929-z.
Wester HJ, Keller U, Schottelius M, et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics 2015. https://doi.org/10.7150/thno.11251.
Honda Y, Nagai T, Ikeda Y, et al. Myocardial immunocompetent cells and macrophage phenotypes as histopathological surrogates for diagnosis of cardiac sarcoidosis in Japanese. J Am Heart Assoc 2016. https://doi.org/10.1161/JAHA.116.004019.
Antoniou KM, Soufla G, Proklou A, et al. Different activity of the biological axis VEGF-Flt-1 (fms-like tyrosine kinase 1) and CXC chemokines between pulmonary sarcoidosis and idiopathic pulmonary fibrosis: A bronchoalveolar lavage study. Clin Dev Immunol 2009. https://doi.org/10.1155/2009/537929.
Lawal IO, Popoola GO, Mahapane J, et al. [68Ga]Ga-pentixafor for pet imaging of vascular expression of CXCR-4 as a marker of arterial inflammation in HIV-infected patients: A comparison with18F[FDG] pet imaging. Biomolecules 2020;10:1629. https://doi.org/10.3390/biom10121629.
Torraca V, Tulotta C, Ewa Snaar-Jagalska B, Meijer AH. The chemokine receptor CXCR4 promotes granuloma formation by sustaining a mycobacteria-induced angiogenesis programme. Sci Rep 2017;7:1‐11. https://doi.org/10.1038/srep45061.
González AA, Segura AM, Horiba K, et al. Matrix metalloproteinases and their tissue inhibitors in the lesions of cardiac and pulmonary sarcoidosis: An immunohistochemical study. Hum Pathol 2002;33:1158‐64. https://doi.org/10.1053/hupa.2002.129423.
Elkington PTG, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax 2006. https://doi.org/10.1136/thx.2005.051979.
Rajendran JG, Krohn KA. F-18 fluoromisonidazole for imaging tumor hypoxia: Imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 2015;45:151‐62. https://doi.org/10.1053/j.semnuclmed.2014.10.006.
Piotrowski WJ, Kiszałkiewicz J, Pastuszak-Lewandoska D, et al. Expression of Hif-1A/VEGF/ING-4 axis in pulmonary sarcoidosis. Adv Exp Med Biol 2015. https://doi.org/10.1007/5584_2015_144.
Zhou Y, Yu J, Liu M, Li H, Yang Z, Wahl R. A machine learning-based parametric imaging algorithm for noninvasive quantification of dynamic [68Ga]DOTATATE PET-CT. J Nucl Med 2019;60:1186.
Disclosures
Drs. Jakob Park and Bryan Young have no disclosures. Dr. Edward Miller serves as a consultant for Eidos, Pfizer, and Roivant and has received grant support from Eidos, Pfizer, and Alnylam.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J., Young, B.D. & Miller, E.J. Potential novel imaging targets of inflammation in cardiac sarcoidosis. J. Nucl. Cardiol. 29, 2171–2187 (2022). https://doi.org/10.1007/s12350-021-02838-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02838-w