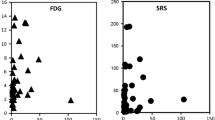
Abstract
Purpose
Dynamic PET studies with68Ga-DOTATOC were performed in patients with non-small cell lung cancer (NSCLC) to assess the somatostatin receptor 2 (SSTR2) expression. Furthermore, dynamic18F-fluorodeoxyglucose (FDG) studies were performed in the same patients to compare the SSTR2 expression with the tumour viability.
Methods
The study population comprised nine patients, examined with both tracers on two different days within 1 week. Standardised uptake values (SUVs) were calculated and a two-tissue compartment model was applied to the data. Furthermore, a non-compartment model based on the fractal dimension (FD) was applied to the data.
Results
The DOTATOC uptake was generally lower than the FDG uptake. Moderately enhanced DOTATOC uptake was noted in seven of the nine tumours. All kinetic parameters exceptk 4 were lower for DOTATOC than for FDG. The mean SUV was 2.018 for DOTATOC, in comparison to 5.683 for FDG. In particular,k 3 was highly variable for DOTATOC and showed an overlap with the normal lung tissue. The fractional blood volumeV B was relatively low for both tracers, not exceeding 0.3. The highest significant logarithmic correlation was found for the FD of the two tracers (r=0.764,p=0.017). The logarithmic correlation for SUVs was also significant (r=0.646,p=0.060), as was that forV B (r=0.629,p=0.069). In contrast, none of the eight metastases which were positive on FDG PET showed any DOTATOC uptake.
Conclusion
The results demonstrated moderate68Ga-DOTATOC uptake in primary NSCLC but did not provide any evidence for SSTR2 expression in metastases. This may be caused by loss of the gene expression in metastases as compared with the primary tumours.







Similar content being viewed by others
References
Mena E, Camacho V, Estorch M, Fuertes J, Flotats A, Carrió I.99mTc-depreotide scintigraphy of bone lesions in patients with lung cancer. Eur J Nucl Med Mol Imaging 2004;31:1399–1404
Szereday Z, Schally AV, Szepeshazi K, Bajo AM, Hebert F, Halmos G, et al. Effective treatment of H838 human non-small cell lung carcinoma with a targeted cytotoxic somatostatin analog, AN-238. Int J Oncol 2003;22:1141–1146
Bohuslavizki KH, Brenner W, Braunsdorf WE, Behnke A, Tinnemeyer S, Hugo HH, et al. Somatostatin receptor scintigraphy in the differential diagnosis of meningioma. Nucl Med Commun 1996;17:302–310
Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumor-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 1999;7:1974–1981
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–648
Burger C, Buck A. Requirements and implementations of a flexible kinetic modeling tool. J Nucl Med 1997;38:1818–1823
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A Java environment for medical image data analysis: initial application for brain PET quantitation. Med Inform 1998;23:207–214
Miyazawa H, Osmont A, Petit-Taboue MC, Tillet I, Travere JM, Young AR, et al. Determination of18F-fluoro-2-deoxy-D-glucose rate constants in the anesthetized baboon brain with dynamic positron tomography. J Neurosci Methods 1993;50:263–272
Sokoloff L, Smith CB. Basic principles underlying radioisotopic methods for assay of biochemical processes in vivo. In: Greitz T, Ingvar DH, Widén L, editors. The metabolism of the human brain studied with positron emission tomography. New York: Raven Press; 1983; p.123–148
Ohtake T, Kosaka N, Watanabe T, Yokoyama I, Moritan T, Masuo M, et al. Noninvasive method to obtain input function for measuring glucose utilization of thoracic and abdominal organs. J Nucl Med 1991;32:1432–1438
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Mikolajczyk K, Lehnert T, Bernd L, et al. On the fractal nature of dynamic positron emission tomography (PET) studies. World J Nucl Med 2003;2:306–313
Kelly RF, Tran T, Holmstrom A, Murar J, Segurola RJ Jr. Accuracy and cost-effectiveness of (18F)-2-fluoro-deoxy-D-glucose-positron emission tomography scan in potentially resectable non-small cell lung cancer. Chest 2004;125:1413–1423
Dwamena BA, Sonnad SS, Angobaldo JO, Wahl RL. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s—meta-analytic comparison of PET and CT. Radiology 1999;213:530–536
Fujita T, Yamaji Y, Sato M, Murao K, Takahara J. Gene expression of somatostatin receptor subtypes, SSTR 1 and SSTR 2, in human lung cancer cell lines. Life Sci 1994;55:1797–1806
Blum J, Handmaker H, Lister-James J, Rinne N. A multicenter trial with a somatostatin analog99mTc depreotide in the evaluation of solitary pulmonary nodules. Chest 2000;117:1232–1238
Kahn D, Menda Y, Kernstine K, Bushnell D, McLaughlin K, Miller S, et al The utility of99mTc depreotide compared with F-18-fluorodeoxyglucose positron emission tomography and surgical staging in patients with suspected non-small cell lung cancer. Chest 2004;125:494–501
Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide (DOTA-TOC) kinetics in patients with meningiomas. J Nucl Med 2005;46:763–769
Koukouraki S, Strauss LG, Georgoulias V, Schuhmacher J, Haberkorn U, Karkavitsas N, et al. Pharmacokinetic studies of 68Ga-DOTATOC PET in patients with metastatic neuroendocrine tumours planned for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging 2006 Jan 17:1–7 [Epub ahead of print]. DOI 10.1007/s00259-005-0006-1
Dimitrakopoulou-Strauss A, Strauss LG, Burger C. Quantitative PET studies in pretreated melanoma patients: a comparison of 6-(18F)-fluoro-L-dopa with 18F-FDG and 15O-water using compartment and noncompartment analysis. J Nucl Med 2001;42:248–256
Acknowledgements
This study was supported by DFG grants HA2901/3-1 and 3-2 from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimitrakopoulou-Strauss, A., Georgoulias, V., Eisenhut, M. et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using68Ga-DOTATOC PET and comparison with18F-FDG PET. Eur J Nucl Med Mol Imaging 33, 823–830 (2006). https://doi.org/10.1007/s00259-005-0063-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0063-5