Abstract
Lignin is the main constituent of lignocellulosic biomasses, which have a significant untapped ability to replace ecologically unfavorable and non-renewable fossil fuels. The lignin is broken down by ligninolytic bacteria, which also use a peripheral pathway to transform heterogeneous lignin derivatives into central intermediates like protocatechuate or catechol. By undergoing ring cleavage through the -ketoadipate pathway, these intermediates become metabolites by producing acetyl-CoA for internal product biosynthesis, including the creation of triacylglycerols and polyhydroxyalkanoates. Expanding our understanding of ligninolytic microbial communities, strains, and enzymes through bioprospecting can help us better understand the metabolism of aromatics. The most viable idea for sustainable development is the valorization of lignin into biopolymers as well as other high-value goods. This process is now being used to generate a variety of biopolymers, including polyesters, epoxies, phenol resins, poly (lactic acids), poly hydroxyl alkanoates, and polyurethanes. Furthermore, lignin recalcitrance remained a possible barrier to efficient lignin valorization, prompting several efforts to design high-efficiency bioprocesses to produce specific polymer types as well as other important bioproducts.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lignin molecule is well-known as the 2nd most abundant infinite organically grown polymer (acts as the most active material) which allows plants to create hard chemical structures and protects hemicellulose and cellulose from hydrolysis in lignocellulosic biomass [1]. Lignocellulosic biomass, which is abundant regionally, demands minimal capital expenditure for bioconversion, minimizes greenhouse gas emissions, and generates job chances for rural habitats. Lignin (15–20%), cellulose (40–50%), and hemicelluloses (25–35%) make up about two-thirds of lignocellulosic [2]. Lignin is among the most prevalent biomolecules accessible for biorefining as a raw material. It is, nevertheless, among the most underutilized plant elements in biorefineries, where it is now largely employed to generate process electricity and steam [3]. The primary cause is lignin’s intrinsic obstinacy and structural variability, which makes the availability of important aromatic side chains difficult. Nonetheless, lignin valorization, or the transformation of lignin into fine chemicals, minerals, or fuels is regarded as critical for the effective realization of low-cost lignocellulosic biorefineries [4].
Speedy expansion and the need to feed an expanding population resulted in a massive volume of lignocellulosic biomass being produced. According to some estimates, each year approximately 709,200,000 metric tons of waste from wheat, 731 million tons of residues from rice crop [5], one hundred and forty billion metric tons of biomass trash, five thousand million metric tons of crop remains [6] are generated. The most common source of lignin is lignocellulosic biomass (Fig. 1). Softwood and grasses are largely composed of G (guaiacyl) unit of lignin with a concentration of > 95% and 35–80% respectively while hardwood is mainly composed of S (syringyl) unit of lignin with a concentration of 45–75% [7]. Total dry biomass can be produced on forest and agricultural sites. Despite this, pulp and paper companies create 30% of lignocellulosic material in form of lignin. Consequently, the sugar industry produces lignocellulosic waste, which contains between 27 and 30% lignin by dry weight. As per the current situation, biorefineries primarily use hemicellulose and cellulose lignocellulosic biomass components to make important goods such as levulinic acid, pyrones, and furfuryl alcohol, ethanol, furfural, and butanol, leaving lignin as a residual waste [8]. Pulp and paper sectors solely create 50–70 million tonnes of lignin every year, and the developing biofuel industries are expected to produce 62 million tonnes per year. According to some estimates, this might reach 225 million tonnes per year by 2030 [9]. In comparison to crude oil, one of the most plentiful and cheapest biomasses is lignocellulosic biomass.
Globally, large amounts of agro-industrial byproducts are produced each year, and they usually include 15–25% lignin [1]. In 2017, rice husks, barley straw, soybean hulls, wheat straw, maize stoves, and sugarcane bagasse, contributed nearly 3.9 Gt of biomass. As a result, certain new chemical pathways are suitable for producing great premium lignin molecules [3]. One of the most efficient bio-based refinery procedures now contains four essential distributions: enzymatic hydrolysis, material collection and stockpiling, pre-treatment, and fermenting of liberated sugar into biofuels [1]. Furthermore, lignin seems to be a significant renewable feedstock with a substantial quantity of cyclic monomers [10]. Hydrolytic enzymes are critical in the generation of biomass-derived sugars, bio-based products, and biochemicals by breaking down complex biomass into macromolecules and subsequently simple sugars [2]. Furthermore, developments in combining computational designing, analytical chemistry, and genetic engineering modeling aspects for lignin preprocessing have made lignin depolymerization and transformation simpler [11]. The aromaticity and side chains on the C-backbone of lignin have also been established to make it a more useful property than the other current biomass traits [1]. In a nutshell, 2 primary process concerns that define the importance and utility of lignin biorefineries are the convenience of taking out lignin through a decline in cross-linkages with other polymeric materials in biomass and depolymerization routes of intrinsically compound lignin structure.
Ligninocellulose Depolymerization Techniques
The biorefinery approach is focused on using biomass feedstocks to generate a wide variety of industrially relevant bioproducts, like chemicals and fuels while reducing dependency on fossil fuels. Former publications distinguished biorefineries depending on the processing amenability, feedstock, and bio-based outputs. Using a fixed bioprocessing capability, dried distillers’ grain, ethanol and carbon dioxide, are created from dry grains in the 1st method. Grain is used to create CO2, high-fructose syrup, ethanol, corn oil, dried distillers’ grain, and starch in the 2nd technique, which provides process flexibility. The use of integrated processing technologies and a wide range of feedstocks is an extra sophisticated idea in biorefinery, which is at a halt in the early stages of research and development. Biorefinery entails the biosynthesis of classic and novel fuels and chemicals by incorporating specifically created biomass conversion methods and technology. A primary goal of the biorefinery is to create innovative bioproducts that are not available from fossil sources, besides emulating petro-refinery. Nevertheless, to build effective bioconversion techniques that create optimal products, a thorough understanding of biomass chemistries is required [12].
Biorefineries use processed wood, cellulose, and hemicellulose to make a variety of goods. The majority of integrated biorefinery ideas have four primary sections: feedstock gathering and storage, pretreatment of yeast biomass, hydrolysis of enzymes, and fuel transformation. The majority of biologically based biorefinery designs depend on cellulose biomass, and roughly 60% more lignin is produced than is necessary. SDGs’ synergistic deployment and ligninolytic armory’s enzyme-assisted biological transformation perspective will serve as a prospective edge for producing numerous goods from a variety of bio-based resources. To depolymerize lignin, some fungal and bacterial species are created having powerful metabolic systems. These bacteria-restricted strains frequently lack desirable industrial features. With today’s microbes, for example, yield recovery and productivity are key challenges [13].
Nowadays, biorefineries are concentrating on generating fuels from feedstocks having high sugar content. Lignin valorization includes a specific effective method known as the hybrid biorefinery route, which has the advantages of increased lignin depolymerization effectiveness via certain chemical approaches and good selectivity of focused elevated value-added bio compounds or chemical products via green microbial methods [14]. Lignin has a market value of roughly $300 million [3]. Lignin is predicted to become the primary sustainable source for biofuel and chemical industries because of its economic performance. Furthermore, lignin can be used as an environmentally benign substitute for a variety of thermoplastics, petrochemicals, rubber additives, and medicines [15]. There are several roadblocks to the complete development and deployment of lignin-based biorefineries caused by a lack of new processing methods. Consequently, commercialized biochemical synthesis from lignin-based feedstocks must turn into a complicated technique.
Generally, lignin-originated compounds are a combination of lignin products created during depolymerization; nevertheless, extraction of active compounds is time-intensive, and solitary-derived yields are commercially low. Compounds such as alcohol, carboxyl, alkyl, ester and carbonyl, molecules are used as polymers, solvents, renewable fuels, and aromatic organic chemicals during the depolymerization of lignin. There are profitable avenues for converting lignin into worthy chemicals and fuels, which is one of the most significant issues related to lignin valorization. HDO (Hydrodeoxygenation) of lignin depolymerization intermediates is generally a critical and key improving step in prime focus modifications that influence the phenolic core. Lignin-derived hydro deoxygenation cyclohexane products, that are produced from methoxylated phenols via a synergistic act of rubidium, palladium, platinum (noble), or hydrogenation, proceeded by dehydration and demethylation may contribute as mid-range fuel additives [16]. Moreover, there is the possibility of certain harmful interactions among the generated intermediate compounds during the lignin conversion process. Even though lignin extraction and utilization technologies have been employed since the early 1900s, researchers are still looking for effective lignin valorization approaches. A reasonable association among diverse depolymerization demands the same lignin substrate and vice versa. When these prerequisites are met, depolymerization and fractionation processes could be evaluated across numerous explorations. In any instance, an additional challenge is a difference in analytical procedures that exist between various research, resulting in varying outcomes. As a result, identical evaluations that recall the recommended criteria and adopt a similar analytical method might give important attention to promote a suitable and reliable assessment of the various fractionations and depolymerization approaches. An optimum lignin valorization into synthetic goods necessitates the best usage of whole lignin from feedstock, which demands a mixture of an elevated return lignin confinement and depolymerization processes.
Cutting-edge technology allows us to utilize garbage while also developing a key global economy. Lignin (byproduct of the biorefining sectors, paper, and pulp) is gaining an extra notice than ever before. The aromaticity of lignin has significant market viability. Its use is both environmentally and commercially beneficial. Lignin has also been used as an unprocessed ingredient for artificial polymers like epoxy resin, polyester, polyurethane resin, and phenol–formaldehyde resin [17]. Lignin thus provides an exceptional environment for lignocellulosic biomass valorization and boosting lignin-based biorefineries. Lignin could be a font of a variety of cyclic molecules and chemicals. Chemically altered lignin could work as reinforcement material or filler in polymer blends and composites [18]. Aromatic and aliphatic acids, quinones, vanillic acid, aromatic aldehydes, cyclohexanol, DMSO, and –keto adipate were all produced when lignin was oxidized under varied oxidation circumstances [19]. Lignin depolymerization had been investigated utilizing mild oxidants (CuO) to create phenolic aldehydes in the presence of an alkaline media [3]. Aromatic hydrocarbons including catechol, phenol, resorcinol, cresols, substituted phenols, eugenol, benzene, and guaiacols can all be produced from lignin.
Essential features of lignocellulosic biomass made it a suitable substrate for developing a range of products with huge biotechnological and industrial value [20]. Sugar extraction from lignocellulose is a crucial step in converting these biomasses into important products. To maximize resource recovery and offer a cost-effective solution, several pretreatment myths have been suggested. Chemical, physicochemical, physical, and biological pretreatment procedures are used [21]. Because sugar separation from lignocellulose is such an important step in the process, choosing appropriate solutions is critical.
Pretreatment Approaches for Converting Lignocellulose in Valuable Products
The important stage in the breakdown of essential parts of biomass and subsequent alteration to important products is lignocellulosic biomass pretreatment [22]. It is resistant to decomposition because of its chemical and structural features, and the appropriate digesting process is defined by biomass type and chemical makeup. The basic objective of pretreatment is to produce hemicellulose, cellulose, and lignin more available to be transformed into sugar. Enzymatic hydrolysis, pretreatment, and alteration to end products were the three essential phases in biomass conversion to important products (Table 1) [23]. Pretreatment is a critical phase in the biomass conversion process, and it is also the most energy-intensive [23]. Several pretreatment technologies are currently offered, some of which are substantially competent and environmentally friendly. Pretreatment techniques are split up into three groups: chemical, physical, and biological. The pretreatment procedures are chosen based on several factors. For example, the chosen approach should not alter biomass size, other fractions such as hemicellulose must be preserved, the degraded product must be minimized, cost-effective ways that are proficient should be chosen, and techniques must use cost-effective pretreatment catalysts [24].
Catabolic Pathways Involved in Lignin Degradation
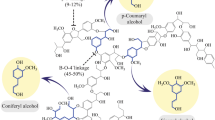
The following catabolic pathways are involved in the degradation of lignin-derived compounds (Fig. 2) [3].
Catabolic pathways involved in degradation of lignin derived compounds. Reprinted from Ref. [3] with permission from Oxford University Press and Copyright Clearance Center. License Number: 5422330178476
Beta-Aryl Ether Degradation Pathways
In Sphingomonas paucimobilis SYK-6, the bacterial catabolism routes for a number of lignin constituents have been thoroughly investigated [33]. The NAD-dependent dehydrogenase LigD catalyzes the first oxidation of the alpha-hydroxyl group to the corresponding ketone. Pseudomonas acidovorans has also been shown to degrade this model b-aryl ether molecule [34]. Using Pseudomonas putida and Rhodococcus jostii RHA1, the ketone product was found to be a by-product of the breakdown of lignocellulose. It may have formed through a b-etherase cleavage reaction or a b-elimination reaction [35]. The ketone product is next converted to vanillic acid by oxidation of the g-hydroxyl group to the carboxylic acid and a further C–C cleavage reaction similar to fatty acid b-oxidation. The creation of the methyl ketone acetovanillone, which is a recognised lignin breakdown byproduct and acts as a mediator for fungal laccases, may be explained by the decarboxylation of the carboxylic acid intermediate [36]. The fungus ascomycete strain 2BW-1 has also been shown to contain a b-etherase enzyme [37].
In S. paucimobilis SYK-6, a tetrahydrofolate-dependent demethylase named LigM catalyzes the demethylation of vanillic acid to protocatechuic acid, producing 5-methyl tetrahydrofolate as a byproduct [38]. In contrast, a non-heme iron-dependent demethylase enzyme catalyzes the identical demethylation process in Pseudomonas and Acinetobacter [39, 40]. Protocatechuic acid, the end result, is then used as a substrate for an oxidative ring cleavage, as will be discussed below. It is known that Phanerochaete chrysosporium in white-rot fungi metabolizes b-aryl ether model compounds mostly by Ca-Cb oxidative cleavage, producing vanillin as a byproduct [41]. The lignin peroxidase from P. chrysosporium is known to catalyze this oxidative cleavage process in vitro [42]. Vanillate dehydrogenase, which is produced by the bacteria S. paucimobilis SYK-6 and Pseudomonas sp. HR199 [4], converts vanillin into vanillic acid. 2-hydroxyacetaldehyde is a by-product of oxidative C–C breakage. Additionally, metabolites from the bacterial degradation of lignocellulose have been found to include oxalic acid [35].
Biphenyl Degradation Pathways
Through oxidation to 2,3-dihydroxybiphenyl and oxidative meta-cleavage, a number of soil bacteria may break down biphenyl and mildly chlorinated biphenyls [33]. One of the key elements of lignin is the biphenyl bond, which frequently exists between two guaiacyl units. An enzyme called LigX catalyzes the demethylation of one methoxy group [40]. Two decarboxylases, LigW, and LigW2 have been discovered in S. paucimobilis SYK-6. The catecholic product of LigX is then a substrate for oxidative meta-cleavage by the extradiol dioxygenase LigZ, and the ring fission product is then cleaved by C–C hydrolase LigY, resulting in 5-carboxyvanillic acid and 4-carboxy-2-hydroxypentadienoic [43].
Diarylpropane Degradation Pathways
In P. chysosporium, the Cα-Cβ link is oxidatively broken, producing aromatic aldehyde compounds by lignin peroxidase. An enzyme activity that in bacteria catalyzes the removal of formaldehyde and water from a model molecule called diarylpropane has been discovered and isolated from Pseudomonas paucimobilis TMY1009 [44]. Although the gene encoding this enzyme has not yet been discovered, the metabolism of the reaction’s end product, lignostilbene, has received considerable attention.
From Pseudomonas paucimobilis TMY1009, lignostilbene dioxygenase isozymes have been isolated, characterized, and the associated genes have been found [40]. The larger family of carotenoid cleavage dioxygenases, which is in charge of the manufacture of apocarotenoid signalling molecules in plants, shares sequence similarities with this enzyme [45].
Degradation of Phenylcoumarane and Pinoresinol
The white-rot fungus (Phanerochaete chrysposporium) degraded the alkylated phenylcoumarane by first oxidizing the side chain, then oxidizing the heterocyclic ring to furan, and eventually cleaving the oxidative Cα-Cβ bond [46]. Conversely, the Fusarium solani M-13–1 (filamentous fungus) broke down the phenolic phenylcoumarane through a direct Cα-Cβ bond, yielding 5-acetylvanillone. Although in S. paucimobilis (bacteria) SYK-6 has been shown to break down phenylcoumarane compounds in lignin [3], the responsible genes have yet to be identified.
The breakdown of the (lignin component) pinoresinol has also been analyzed in Fusarium solani M-13–1. The benzylic group was oxidized, resulting in the cleavage of the C–O bond and the formation of a monocyclic ketone intermediate. A carboxylic acid product and the associated lactone were produced through further aryl–alkyl oxidation. In bacteria, S. paucimobilis SYK-6 has been shown in bacteria to degrade pinoresinol compounds of lignin, but the genes that are responsible are still to be identified. Catabolic pathways for both heterocyclic components of lignin appear to begin with a-hydroxylation. It is thus worth noting that a-hydroxylation of phenylpropane molecules using iron porphyrin bio-inspired models has a chemical precedent [47].
Bacterial Degradation of Ferulic Acid
Ferulic acid is another phenolic component of lignocellulose (released by esterase enzymes found in fungi and bacteria) that is connected to hemicellulose through ester linkages [48]. Ferulic acid esterase has already been extracted from Aspergillus niger, [49] and esterase enzymes that can produce ferulic acid from hemicelluloses have also been observed in Pseudomonas fluorescens [50] and Streptomyces avermitilis [51]. In bacteria, ferulic acid is degraded by 2 catabolic pathways. Feruloyl CoA synthetase enzyme FerA in S. Paucimobilis SYK-6 works by converting ferulic acid into feruloyl CoA, after which it is transformed into vanillin and acetyl CoA by a feruloyl CoA hydratase [4]. Some bacteria have a decarboxylase enzyme that converts hydroxycinnamic acids into 4-vinyl aromatic compounds (it was discovered in Bacillus sp. BP-7) [52].
Oxidative Cleavage of Protocatechuic Acid
Vanillin or its oxidation product vanillic acid is produced by many catabolic pathways, and vanillin or vanillic acid is then transformed by demethylation to protocatechuic acid. Numerous soil bacteria have the ability to break down protocatechuic acid through oxidative ring breakage while utilizing catechol dioxygenase enzymes that are not heme iron reliant. Intradiol cleavage between the two phenolic hydroxyl groups, catalyzed by iron(III)-dependent dioxygenases, and extradiol cleavage next to the phenolic hydroxy groups, catalyzed by iron(II)-dependent dioxygenases, are the two types of oxidative cleavage processes that are analyzed [33]. A protocatechuate 4,5-dioxygenase enzyme named LigAB has been discovered in Sphingomonas paucimobilis SYK-6. Pyruvate and oxaloacetate are then produced by hydratase LigJ and aldolase LigK. Using the trisubstituted gallic acid and 3-methyl-gallic acid, which are both cleaved by catechol dioxygenases DesB and DesZ, two iron (II)-dependent dioxygenases, S. paucimobilis SYK-6 may also break down compounds of syringyl lignin [53]. Therefore, the routes for the degradation of guaiacyl and syringyl lignin components merge upon subsequent ring cleavage. Other bacteria use protocatechuate 3,4-dioxygenase to cleave protocatechuic acid into cis,cis-muconic acid, which is then metabolized through the β-keto-adipate pathway or ortho-cleavage pathway [3].
Biological Approach for Lignin Breakdown
For efficient depolymerization of lignin, several pre-treatment procedures (catalytic and thermochemical) have been stated. Such approaches, on the other hand, necessitate stringent reaction circumstances, together with temp and pressure. Furthermore, such technologies are frequently associated with significant environmental concerns and necessitate a significant amount of energy to carry out activities [33]. Several microorganisms have well-organized metabolic systems for mortifying lignin to cyclic compounds and then bioconverting the resultant products into energy through a variety of reaction pathways [3].
Lignin Depolymerization from Enzymes Produced by Fungi
Fungi are common microorganisms associated with lignin breakdown and depolymerization. The most often used basidiomycetes white-rot fungi for ligninolytic enzyme synthesis and lignin depolymerization aspects are basidiomycetes white-rot fungi [54]. When compared to bacteria, fungi have a higher rate of depolymerization and catalytic conversion [55]. White-rot fungi have been intensively investigated for a variety of industrial applications, including the production of biomethane, degradation of textile dye, biomass de-lignification, and removal of the phenolic chemical from pollutants, because of their high depolymerization and degradation capacity of lignin [56, 57]. Enzymes generated by white-rot fungus strains have been discovered to be extremely effective at degrading lignin [58]. Laccases, lignin peroxidases (LiPs), MnPs, and versatile peroxidases (VPs) are among the enzymes they create to depolymerize lignin. Pleurotus species manufacture various kinds of peroxidases to metabolize phenolic chemicals including methoxybenzene, and benzoic acid [53]. Pleurotus ostreatus and Trametes hirsute, two white-rot fungus strains, were investigated due to their capability to generate ligninolytic enzymes. The inclusion of fungal inoculates resulted in a twofold increase in fibrinolysis, as well as an increase in organic matter composability from 26.5 to 31.2%. The rate of lignin decomposition in tobacco stalks was improved by utilizing fungal ligninolytic enzymes, according to the study [59]. Coniophora puteana has been described as a laccase generator that may destroy cell walls in the presence of tannic acid [60]. Laccases and peroxidases work together to depolymerize the lignin polymer into aromatic compounds, which bacteria can then transform into high-valued commercial goods [61].
Linares et al. [62] used shock wave-induced acoustic cavitation to overexpress laccases and peroxidase enzymes in Phanerochaete chrysosporium to produce ligninolytic enzymes. Wheat bran and sugarcane bagasse were used to test the enzymes for potential delignification. When recombinant enzymes were used instead of native enzymes, outcomes showed a 25% increase in the depolymerization of lignin. Recombinant fungi were found to have 2.6-fold elevated activity of peroxidase and fourfold elevated activity of laccase than control fungi. Ligninolytic enzymes generated by Phanerochaete chrysosporium have been reported to depolymerize up to 99% of lignin in other research [63].
Improved fungal efficiency toward lignin depolymerization has been demonstrated in multiple routes, proteomic, and genomic studies. Lac I extracted from a strain of basidiomycetes (Phlebia brevispora) was reported. Gene was converted into Pichia pastoris for protein expression. Consequences showed that the enzyme created by lac I had a good solvent/salt tolerance, demonstrating that it might be used in a variety of biotechnological applications [64]. Jin et al. [65] stated the identification of the lac I gene from Ganoderma tsugae, which was found to have the ligninolytic capacity in knockout tests. The principal fungal enzymes concerned with the depolymerization of lignin are various peroxidases and laccases [66]. Penicillium chrysogenum and Aspergillus niger, two soft-rot fungal strains with the ability to degrade pine wood and sycamore, were found [67].
Lignin Depolymerization from Enzymes Produced by Bacteria
In comparison to fungus, bacterial strains are thought to be less efficient in ligninolytic. Only a few bacterial strains are capable of degrading lignin. Various bacteria have been shown to alter and depolymerize lignin into chemicals with remarkable efficiency. Rhodococcus jostii (RHA1 specie) had been widely explored for its lignin breakdown capabilities as well as lignin consumption and transformation to other value-added chemicals [68]. The metabolically re-routed mechanism was found in Rhodococcus jostii for breaking down lignin to produce aromatic dicarboxylic acids, which could be utilized to make bioplastics. Ortho-cleavage to -the keto-adipate pathway is extensively used in protocatechuic acid metabolism [69]. Two forms of DyPs were identified in Rhodococcus jostii (RAH1): DypA (periplasmic) and DypB (non-periplasmic). Peroxidases have substantially lower activity than other DyPs kinds, according to enzyme kinetics. When compared to pyrogallol or ABTS, DypA had a greatly enhanced (sixfold) decolorization capacity for Reactive Blue 4 dye. For ABTS and oxidized Mn(II), DypB had the highest misleading specificity. The work proposed a metabolic engineering technique to increase Rhodococcus jostii’s lignocellulose breakdown capacity (RAH1) [4]. Pseudomonas spp. can assimilate a wide range of cyclic compounds and frequently present in bacteria separated for their capability to nurture on cyclic substrates. They efficiently grow in minimal medium and have an inclusive genetic toolbox for metabolic engineering [70]. As a result, it is thought to be an excellent model organism for investigating lignin biotransformation and generating new industrial strains.
Previous studies have shown that Pseudomonas putida has a high lignin degradation capacity and can break down lignin into low molecular weight components. By changing lignin-rich fractions, bioconversion leads to the buildup of polyhydroxyalkanoates (PHA), competent unprocessed material for creating bioplastics [71, 72] using genome sequencing to extensively investigate lignin transforming enzymes in Pseudomonas putida strain NX-1. Peroxidases with dye decolorization capability were investigated, and they were found to be effective on dyes as well as lignin-derived aromatic compounds. The P. putida KT2440 strain helped researchers better understand aromatic compound uptake [73, 74].
Apart from the bacterial strains mentioned above, Amycolatopsis specie had been extensively reported for having elevated bioconversion of high-molecular-weight chemicals into compounds with low molecular weight and excellent lignin depolymerization capability when compared to other genera [75].To study the metabolism of aromatic chemicals in Amycolatopsis spp., a variety of metabolic engineering methods have been applied [76]. The vanillin dehydrogenase gene was extracted and cloned from the genome of Amycolatopsis (39,116 spp.). Vanillin production was improved after vanillin dehydrogenase was knocked out (up to 2.3 times). The bacteria lost their ability to use vanillin as a C-source for the production of energy after knocking out the vanillin dehydrogenase gene, so produced vanillin was competently stored in cells [77].
Depolymerizing lignin biomass could also produce value-added cis-muconic acid. Genome engineering was used to study the Amycolatopsis species for biomass conversion and important compound manufacturing, including biofuels [78]. Using pre-engineered Amycolatopsis, lignin depolymerization found to be capable of producing cis-muconic acid (39,116 spp.). Because the cis-muconic acid hydrolyzing enzyme (catB gene) was knocked out, cis-muconic acid cannot be collected in the cell. Even though the gene knockdown reduced the growth of the cell, the modified microbe accumulated more than three times the amount of original microbes. Furthermore, several ligninolytic bacteria have previously been discovered and their metabolic routes have been described, and there is yet a wide variety of organisms to be discovered. As a result, there is a pressing need to create effective screening approaches that allow for the discovery of novel microbial activity [79].
Other Microbial Enzymes for Lignin Depolymerization
Various bacterial and fungal strains have been found to produce enzymes with the ability to degrade lignin. The use of in vitro enzymes in ligninolytic processes can alleviate some of the drawbacks, such as direct contact and culturing time between the substrate and the microbe [80]. The use of a single enzyme to digest biomass containing lignin has been described in several studies. Further inquiry and exploration of molecular mechanisms will require multi-enzymatic complicated systems [81]. Non-specific cleavage is used by the majority of ligninolytic enzymes to break down cyclic compounds. Laccases and peroxidases are the two most common groups of in vitro enzymes. By oxidative cleavage, both enzymatic families depolymerize lignin. Rather than cleaving links or substrates, these enzymes target lignin molecules at random. MnP and LiP are the two most common forms of peroxidases. Furthermore, due to their wide applicability, two other kinds, DyP and VP, have also gained attention [13].
Manganese Peroxidases (MnPs)
The MnP (E.C1.11.1.13) enzyme is a glycosylated protein that needs H2O2 (oxidant) to begin catalysis [33]. It is found all over the world. MnP manufacturing is gaining popularity as a result of its numerous uses in the industrial and biological industries [82, 83]. It is a high-potential enzyme having a highly flexible nature that plays a key role in industrial bioprocesses. MnP enzyme is involved in the oxidation of non-phenolic and phenolic substances [84]. MnPs are extracellular enzymes that are generated by several white and brown-rot fungus taxa, as well as a few bacterial species. MnPs are regarded as the proficient biocatalysts for lignin depolymerization when conjugated with other enzymes such as laccases. MnP production was first discovered in Phanerochaete chrysosporium [85].
The ability to synthesize MnP in sufficient quantities is critical for developing an environmentally benign substitute to lower the chemical cost of producing industrial-scale biofuels. MnPs have a wide range of applications, including pollution bioremediation/biodegradation, bio-bleaching, bio-pulping, and more [86]. A variety of microbial taxa, including fungi, bacteria, and algae, have been found to produce MnP efficiently [87]. Extracellular MnP production has been reported from Phanerochaete chrysosporium, Ceriporiopsis subvermispora, Phanerochaete sordida, Physisporinus rivulosus, Dichomeris squalene and Phlebia radiata. MnP production was reported by utilizing Phanerochaete chrysosporium BKM-1767 under a variety of nutritional and climatic circumstances. To boost the biodegradation efficiency of xenobiotics, more research into MnP synthesis with improved inorganic/organic ions tolerance is needed [88].
Lignin Peroxidases (LiPs)
LiP is a heme-containing monomeric protein that destroys lignin-containing biomass residues by oxidative degradation [89]. LiP was first studied in Phanerochaete chrysosporium’s extracellular matrix [90]. Following that, LiP isozymes from different microbial species such as Trametes versicolor, Phanerochaete sordida, Phlebia radiata, and others have been discovered [91]. Approximately 96% of all species produced MnPs. Daedaleopsis septentrionalis and Cylindrobasidium evolve, two novels, LiPs-producing fungal strains, were found in 26% of the total strains tested. The automated screening was used to identify ligninolytic enzymes in this study, which resulted in enhanced test conditions. To begin and catalyze phenolic and non-phenolic chemicals, LiP requires H2O2 [92]. Due to its larger redox potential than other enzymes in the same family that impart significant lignin disruption features, LiP is the most prevalent peroxidase type. Apart from depolymerizing lignin, LiP can also be used to generate bioplastics and other value-added chemicals due to its great delignification capacity [58, 93, 94]. LiPs have been shown to depolymerize and degrade lignin with great selectivity and yield when used alone or in conjunction with other ligninolytic enzymes [95].
Laccases
Laccase enzymes are oxidoreductases found in a variety of bacterial and fungal species. Species of brown-rot and white-rot fungus (with redox potentials up to 800 milli volts) have been reported to produce highly powerful and efficient laccases [66]. Laccases are blue copper phenoloxidases that utilize O2 as an electron acceptor to oxidize phenolic molecules [96]. It is termed the “greenest” enzyme because it has a basic obligation of utilizing O2 from the environment and converting it into the H2O as a by-product [97]. Laccases can be found all over the place in nature. They are involved in a variety of processes, including degradative and synthetic ones [66]. Their biological functions may differ from the synthesis and breakdown of lignin (in developed plants) and chitin (in arthropods) by diverse fungal and bacterial strains. More than sixty fungal strains with excellent laccase production capacity have been reported, according to a preliminary estimate. Laccase-producing fungal species include Dichomitus squalene, Lentinula edodes, Current maxima, Trametes versicolor, Pleurotus ostreatus, Phanerochaete chrysosporium, and Irpex lacteus [98,99,100].
Laccases have lower oxidative adaptability and require less catalysis. These enzymes catalyze a variety of chemical processes, such as aromatic compound oxyfunctionalization and ring cleavage, monomeric crosslinking, and polymer degradation [101]. Laccases are used in a variety of redox processes, including fiber alteration, ethanol generation, bioremediation, biofuel cell production, effluent treatment, and biosensors [102]. Furthermore, due to their ecologically friendly nature, fungal enzymes reduce pollution.
Ligninocellulose Depolymerization Products
The following valuable products are obtained from ligninocellulose depolymerization (Fig. 3).
Industrial Biocatalysts
The focus of current research is on developing sustainable methods for manufacturing enzymes to make overall bioprocess less costly [103, 104]. In several industries, enzymes have piqued interest as potential biocatalysts. The use of lignocellulosic waste biomass reduces the cost of raw materials (60%), making the entire industrial bioprocess more cost-effective [81]. Several investigations had reported the production of industrially important enzymes such as cellulases, laccases, xylanases, proteases, lipases, peroxidases, pectinases, and -galactosidases from agricultural-industrial lignocellulosic materials by microbial fermentation. Using delicious peels of citrus fruit as a substrate for growth, Pectinases were manufactured for the fruit juice business from the Schizophyllum commune [105]. A lipase yield of 1038.86 U/gds was attained from Penicillium fellutanum from a canola oilseed cake (solid-state culture) under optimal processing conditions [106]. Among a wide range of agricultural-industrial wastes tested, sesame, canola, cotton, and linseed oilseed cake, wheat and rice bran triggered the greatest lipolytic enzyme synthesis by P. notatum [107]. Laccase manufacture had produced by Myrothecium gramineum from cowpea pods. Xylanase was obtained from sugarcane straw under submerged fermentation conditions using Trichoderma reesei QM 9414[108].
Biologically Active Compounds
Pharmaceutical and food sectors value biological active compounds such as phytoconstituents and phenolics, which have antioxidant and health-promoting properties. Phenolic chemicals, such as tannins, flavonoids, phenolic acids, and others, are abundant in vegetables and [109]. Agricultural waste is a rich basis of bioactive compounds which could be extracted via microbial fermentation [110]. From solid-state bioprocessing of Aspergillus niger HT4 by-products, 8 distinct phenolics were discovered with the possible antioxidant ability [111]. Microbial transformation of apricot pomace resulted in a 30% increase in neochlorogenic acid, chlorogenic acid, rutin, and quercetin 3-acetyl-glucoside titer with A. niger and a 78% enhancement with Rhizopus oligosporus in a variety of phenolic compounds titer unambiguously, quercetin 3-acetyl-gluco, rutin, and neochlorogenic acid [112].
Biofuels
Known negative effects of traditional fuels, microbes-assisted biofuels manufactured from lignocellulosic substrates like rice straw, pods, molasses, bagasse, sweet potato, maize waste, and sugar beet waste have attracted enormous attention in the last 20 years. Biofuel production from edible food crops has been heavily criticized because the majority of the world’s population is previously suffering from severe food shortages and hunger. Investigating non-edible feedstocks for biofuels promises to be a viable research field, and the use of lignocellulosic biomasses is an appealing count to industry. Agro-industrial leftovers include an elevated number of carbohydrates, lipids, and amino acids which could be used as substitute resources for biofuel production [113]. S. cerevisiae cultured in yeast extract, D-glucose, and Bacto peptone-based fermentation medium produced bioethanol from date palm waste [114]. Fermentative bioprocess produced 15% ethanol under ideal conditions.
Biodiesel
Trans-esterification of fats and oils from plants, animals, and microorganisms produces biodiesel, which is a variety of fatty acid alkyl esters with various chain lengths [115, 116]. Because a major amount of the molecular structure of the feedstock is preserved in the ensuing bioproduct, feedstocks for biodiesel had a significant impact on product attributes. Enzyme-assisted production of biodiesel has several distinct advantages, including low energy requirements, efficient bioconversion of free fatty acid-containing oils, pure glycerol by-products, and easy product recovery and reprocessing [115, 117]. In presence of endogenous lipolytic enzymes, triglycerides in certain microalgal cells are transformed to FFA in favorable settings [118]. Membrane lipids of these cells also produce a significant amount of polar lipids in glycolipids and phospholipids form. Microalgae lipids appear to be potential feedstocks for biodiesel production, although they are high in phospholipids and FFA.
Biogas
Another bioenergy-raised area that may be made from bioresources such as hydrogen and methane is biogas. Several biological methods for producing biohydrogen have been proposed; however, the type of synthesis chosen may have an impact on the yield. Biohydrogen has been produced using a variety of fermentative microbial strains (Clostridium, Enterobacter, and Bacillus) [119, 120]. Species of Bacillus and Thermotogales were dominating bacteria yielding both H2 and CH4 in mesophilic fermentation settings of food waste [121]. In thermophilic circumstances, however, Desulfotomaculum geothermicum and Thermoanaerobacterium thermosaccharolytium were dominating strains, ensuring the formation of sole hydrogen.
Along with the growing population of human beings, the production of agricultural waste is expected to rise [122]. Agro-waste is primarily made up of bagasse, crop peelings, and animal manure, all of which contain a large number of carbohydrates that could be converted to fermentable sugars through the production of biogas. Many additional industrial waste materials had also been identified as a potential source for the synthesis of biogas, including effluent from oilseed mills, a slurry of rice, and cassava waste [123,124,125].
Biopolymers
Many polymeric compounds can be produced by fungi and bacteria from lignocellulosic wastes, including polysaccharides (endo and Exo), polyphosphates, and polyhydroxyalkanoates [126]. Bioconversion of lignocelluloses to commercially important biopolymers such as xanthan, chitosan, cellulose, pullulan, and others has been studied extensively. Paper mill wastewater and apple pomace are suitable substrates for Penicillium citrinumin and Gongronella butleri to synthesize chitosan in a cost-effective and environmentally friendly manner [127, 128]. Pullulan was synthesized in a stirred tank bioreactor utilizing Aureobasidium pullulans P56 and pre-treated beet molasses in a study.
Biosurfactants
Biosurfactants are biomolecules with significant benefits over artificial complements in terms of selectivity, biodegradability, and toxicity. They are divided into many types, such as lipoproteins, lipopeptides, and glycolipids, and have a wide variety of dietary, medical, cleansing, bioremediation, and cosmetic applications. They could be used as a food preservative to improve the product’s rheological properties; however, their high production costs limit their use on a broader scale [129]. Paneibacillus sp. D9 produced lipopeptide-type biosurfactant using sunflower oil and discarded coconut as substrates for fermentation, according to Jimoh and Lin [130]. Serratia nematodiphila has proven synthesis of a glycolipid biosurfactant with emulsifying and antibacterial properties utilizing rice straw [131].
Bioplastics
At present, bioplastic manufacturing sectors have huge cost-competitiveness difficulty when compared to petro-based plastics, that is much less expensive. These findings have piqued researchers’ interest in using agro-based fermentative feedstocks to make cheaper bioplastics using fungal and bacterial strains. Sugar refinery waste was exploited to produce PHA by a Pseudomonas aeruginosa submerged fermentative bioprocess [132]. PHB bioplastics were produced and accumulated from Ralstonia eutropha fermentation using hemp hurds biomass [133], Bacillus sp. fermentation using sugarcane bagasse [134], Lactobacillus acidophilus fermentation using date molasses [135], and Cupriavidus necator fermentation using cashew apple juice [136].
Biocontrol Agents
By using microbial-assisted fermentative processes, agriculture lignocellulosic residues are being investigated to produce new biocontrol agents like bioinsecticides and biopesticides. A recent study used rice husk as a lignocellulosic substrate to produce bioinsecticide from Trichoderma harzianum and Beauveria bassiana in a packed bed bioreactor. Furthermore, cassava dregs and palm kernel cake blends were revealed to be promising substrates for generating bioinsecticides from Bacillus thuringiensis subsp. aizawai [137].
Polyurethane Products
Polyurethanes are exceptional polymeric compounds that are among the most flexible polymers for the purposes they are designed for. Due to its ability to replace fossil fuel-based rigid polyurethane foam (RPUF) components, the valorization of lignin in RPUF has been the goal of research. However, the overall viability of RPUF is determined by a variety of factors, such as processability, cost-effectiveness, and performance retention throughout the foam’s service life [138]. Furthermore, lignin can be consolidated as a polyol in polyurethane production and thus structurally changed before use [139]. Furthermore, the hydroxyl (–OH) functional lignin polymer can efficiently substitute polyols in the manufacture of polyurethane [140].
Vanillin
Since 1937, vanillin has been economically extracted from lignin, and in 2011, the global market for vanillin was predicted to be 16,000 metric tonnes with a value of $230 million. Around 20% of produced vanillin comes through the lignin valorization process, with the remaining 80% coming from crude petroleum [141]. Vanillin is one of the most widely used flavoring agents in the world [63], and it is made from lignin via an oxidative catalytic mechanism rather than a hydrogenolysis pathway [77].
The Techno-Economic Analysis of Lignin Valorization
Bioproducts made from biological lignin have not been produced at commercial, demonstration, or pilot scales. In order to reduce the technical risk for scale-up and boost cost competitiveness across the board in the next-generation biorefinery, techno-economic analysis is currently used to not only identify key cost drivers of lignin valorization but also to prioritize research directions and project the economic impacts of the coproduction integration of lignin-based products through process design. As depicted in Fig. 4, several lignin scenarios that may be utilized to create goods with additional value were found. After burning lignin-rich wastes, scenario 1 of the NREL 2011 design case [142] plans to use 23.9% of the total energy for processing steam or heat, 52.5% for electricity consumption, and 23.6% for revenue-generating sales to the grid. In case 2, only 23.6% of the lignin leftovers would be turned into lignin-based coproducts, with the remainder being burned to provide electricity and steam for the plant and no extra electricity being sold to the grid. The manufacturing of lignin-based coproducts would get 76.1% of the lignin leftovers under scenario 3, while the remaining 23.9% would be burned to generate plant steam and heat [143].
In case three, no lignin-derived electricity would be produced, therefore plant electricity would have to be obtained from the grid. Additional operation units and processing steps in the integrated new biorefinery design will result in higher operating and total capital costs when the coproduction facility is envisioned as a bolt-on process into a lignocellulosic ethanol plant [142]. The results of the techno-economic study demonstrated that the economic viability of lignin valorization in biorefineries depended on high yields of lignin conversion to value-added products and low costs of product separation. Thus, the development of biorefineries that turn plant biomass into fuel ethanol will result in the production of significantly more lignin; nevertheless, in order to create a viable biorefinery, efforts must still be made to convert this lignin into coproducts of higher value.
Future Perspectives
Current lignin-based developments are still mostly bound by low fuel and energy consumption. The commercialization of lignin in the same way as an originator of chosen macromolecular materials and aromatic compounds will necessitate more exploration and improvement. Concerning the evaluation of natural lignin configuration, there are still obstacles in the treatment of lignin depolymerization, and the applications of end products. Even though such a zone is being seriously considered. However, major efforts in terms of powerful collaboration amongst many fields in science and engineering are required. There is also an issue of feedstock variation from one region to the next, as well as from one growing season to the next. Despite these challenges, lignin is able to be used as a substitute for fossil-based feedstock in a variety of applications. Furthermore, by establishing strong approaches that push for fossil fuel substitutes, the government can play a significant part in stimulating commercialization. Lignin valorization is expected to progress and thrive rapidly, according to a multidisciplinary and collaborative team. Achieving optimal lignin valorization in chemicals needs combining an efficient lignin separation and depolymerization activity. Likewise, the sustainability of lignin depolymerization is determined by the depolymerization technique and the chemical makeup of the lignin substrate. Because of multifaceted features of lignin, that can strongly affect the involved reaction pathways in lignin valorization, not only is progressive in cost-effective approaches at a standstill, but implicated processes also significantly distort the structural individuality of the lignin composition. As a result, the yield for current and future biorefineries, as well as certain high-value-added platform chemicals, has been constrained.
Conclusions
Pulp and paper mills, power plants, and biorefineries all use lignin biomass to generate energy and value-added chemicals. Traditional lignin valorization methods limit its use as a low-quality solid fuel in biorefinery businesses to generate power and heat. Its valorization could open up new frontiers in biofuel and value-added chemical synthesis because of its high aromatic characteristics and large accessibility of renewable feedstock. Furthermore, an integrated biorefinery with lignin conversion will allow for a cost-effective, long-term, and diverse business based on lignocellulosic biomass. Less reactivity, highly asymmetrical polymer formation, and robust hydrogen interactions during diverse industrial processes are the key obstacles to efficient lignin utilization. The subsequent product recovery is mostly governed by the fractionation procedure. The main concern is that the amount of lignin produced will much outnumber the existing global marketplace for lignin utilized in specialty stocks. To turn lignin into value-added goods, new ideas and technologies are required.
References
Ponnusamy VK, Nguyen DD, Dharmaraja J, Shobana S, Banu JR, Saratale RG, Chang SW, Kumar G (2019) A review on lignin structure, pretreatments, fermentation reactions and biorefinery potential. Bioresour Technol 271:462–472. https://doi.org/10.1016/j.biortech.2018.09.070
Thankappan S, Kandasamy S, Joshi B, Sorokina KN, Taran OP, Uthandi S (2018) Bioprospecting thermophilic glycosyl hydrolases, from hot springs of Himachal Pradesh, for biomass valorization. AMB Exp 8:1–15. https://doi.org/10.1186/s13568-018-0690-4
Masai E, Katayama Y, Fukuda M (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci Biotechnol Biochem 71:1–15. https://doi.org/10.1271/bbb.60437
Becker J, Wittmann C (2019) A field of dreams: lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol Adv 37:107360. https://doi.org/10.1016/j.biotechadv.2019.02.016
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioproc 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Schutyser W, Renders T, Van den Bosch S, Koelewijn S-F, Beckham G, Sels BF (2018) Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem Soc Rev 47(852):908. https://doi.org/10.1039/C7CS00566K
Schutyser W, Renders T, Van den Bossche G, Van den Bosch S, Koelewijn S-F, Ennaert T, Sels B (2017) Catalysis in lignocellulosic biorefineries: the case of lignin conversion. Nanotechnology in Catalysis: Applications in the Chemical Industry, Energy Development, and Environment Protection, 537–584. https://doi.org/10.1002/9783527699827.ch23
Bilal M, Vilar DS, Eguiluz KIB, Ferreira LFR, Bhatt P, Iqbal HM (2021) Biochemical conversion of lignocellulosic waste into renewable energy. In Advanced Technology for the Conversion of Waste into Fuels and Chemicals (pp. 147–171). Woodhead Publishing. https://doi.org/10.1016/B978-0-12-823139-5.00007-1
Mandlekar N, Cayla A, Rault F, Giraud S, Salaün F, Malucelli G, Guan J-P (2018) An overview on the use of lignin and its derivatives in fire retardant polymer systems. Lignin-Trends Appl 9:207–231. https://doi.org/10.5772/intechopen.72963
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118:801–838. https://doi.org/10.1021/acs.chemrev.7b00203
Sun Z, Fridrich B, De Santi A, Elangovan S, Barta K (2018) Bright side of lignin depolymerization: toward new platform chemicals. Chem Rev 118:614–678. https://doi.org/10.1021/acs.chemrev.7b00588
Amoah J, Kahar P, Ogino C, Kondo A (2019) Bioenergy and biorefinery: feedstock, biotechnological conversion, and products. Biotechnol J 14:1800494. https://doi.org/10.1002/biot.201800494
Bilal M, Qamar SA, Yadav V, Cheng H, Khan M, Adil SF, Taherzadeh MJ, Iqbal HM (2021) Exploring the potential of ligninolytic armory for lignin valorization-a way forward for sustainable and cleaner production. J Clean Prod 326:129420. https://doi.org/10.1016/j.jclepro.2021.129420
Wu W, Dutta T, Varman AM, Eudes A, Manalansan B, Loqué D, Singh S (2017) Lignin valorization: two hybrid biochemical routes for the conversion of polymeric lignin into value-added chemicals. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-07895-1
Wang S, Dai G, Yang H, Luo Z (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86. https://doi.org/10.1016/j.pecs.2017.05.004
Bosch S, Koelewijn S-F, Renders T, Bossche G, Vangeel T, Schutyser W, Sels B (2020) Catalytic strategies towards lignin‑derived chemicals. In: Serrano L, Luque R, Sels B (eds) Lignin chemistry. Topics in Current Chemistry Collections. Springer, Cham. https://doi.org/10.1007/978-3-030-00590-0_6
Dou X, Hasa I, Saurel D, Vaalma C, Wu L, Buchholz D, Bresser D, Komaba S, Passerini S (2019) Hard carbons for sodium-ion batteries: structure, analysis, sustainability, and electrochemistry. Mater Today 23:87–104. https://doi.org/10.1016/j.mattod.2018.12.040
Gan D, Xing W, Jiang L, Fang J, Zhao C, Ren F, Fang L, Wang K, Lu X (2019) Plant-inspired adhesive and tough hydrogel based on Ag-lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat Commun 10:1–10. https://doi.org/10.1038/s41467-019-09351-2
Soltanian S, Aghbashlo M, Almasi F, Hosseinzadeh-Bandbafha H, Nizami A-S, Ok YS, Lam SS, Tabatabaei M (2020) A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Conver Manag 212:112792. https://doi.org/10.1016/j.enconman.2020.112792
González-González RB, Iqbal HM, Bilal M, Parra-Saldívar R (2022) (Re)-thinking the bio-prospect of lignin biomass recycling to meet sustainable development goals and circular economy aspects. Curr Opin Green Sust Chem 38:100699. https://doi.org/10.1016/j.cogsc.2022.100699
Li X, Xu R, Yang J, Nie S, Liu D, Liu Y, Si C (2019) Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind Crops Prod 130:184–197. https://doi.org/10.1016/j.indcrop.2018.12.082
Bilal M, Iqbal HM (2022) Nanoengineered ligninolytic enzymes for sustainable lignocellulose biorefinery. Curr Opin Green Sust Chem 38:100697. https://doi.org/10.1016/j.cogsc.2022.100697
Bilal M, Iqbal H (2020) Ligninolytic enzymes mediated ligninolysis: an untapped biocatalytic potential to deconstruct lignocellulosic molecules in a sustainable manner. Catal Lett 150(2):524–543. https://doi.org/10.1007/s10562-019-03096-9
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioproc 4:1–19. https://doi.org/10.1186/s40643-017-0137-9
Becker J, Kuhl M, Kohlstedt M, Starck S, Wittmann C (2018) Metabolic engineering of Corynebacterium glutamicum for the production of cis, cis-muconic acid from lignin. Microb Cell Fact 17:1–14. https://doi.org/10.1186/s12934-018-0963-2
Kohlstedt M, Starck S, Barton N, Stolzenberger J, Selzer M, Mehlmann K, Schneider R, Pleissner D, Rinkel J, Dickschat JS (2018) From lignin to nylon: cascaded chemical and biochemical conversion using metabolically engineered Pseudomonas putida. Metab Eng 47:279–293. https://doi.org/10.1016/j.ymben.2018.03.003
Sharma RK, Mukhopadhyay D, Gupta P (2019) Microbial fuel cell-mediated lignin depolymerization: a sustainable approach. J Chem Technol Biotechnol 94:927–932. https://doi.org/10.1002/jctb.5841
Li X, Xu Z, Cort JR, Qian W-J, Yang B (2021) Lipid production from non-sugar compounds in pretreated lignocellulose hydrolysates by Rhodococcus jostii RHA1. Biomass Bioenergy 145:105970. https://doi.org/10.1016/j.biombioe.2021.105970
Wang J, Tanaka Y, Ohno H, Jia J, Mori T, Xiao T, Yan B, Kawagishi H, Hirai H (2019) Biotransformation and detoxification of the neonicotinoid insecticides nitenpyram and dinotefuran by Phanerochaete sordida YK-624. Environ Poll 252:856–862. https://doi.org/10.1016/j.envpol.2019.06.022
Wang Z, Li N, Pan X (2019) Transformation of ammonia fiber expansion (AFEX) corn stover lignin into microbial lipids by Rhodococcus opacus. Fuel 240:119–125. https://doi.org/10.1016/j.fuel.2018.11.081
Liu Q, Luo L, Zheng L (2018) Lignins: biosynthesis and biological functions in plants. Int J Mol Sci 19:335. https://doi.org/10.3390/ijms19020335
Liu Z-H, Xie S, Lin F, Jin M, Yuan JS (2018) Combinatorial pretreatment and fermentation optimization enabled a record yield on lignin bioconversion. Biotechnol Biofuels 11:1–20. https://doi.org/10.1186/s13068-018-1021-3
Chio C, Sain M, Qin W (2019) Lignin utilization: a review of lignin depolymerization from various aspects. Renew Sustain Energy Rev 107:232–249. https://doi.org/10.1016/j.rser.2019.03.008
Chauhan PS (2020) Role of various bacterial enzymes in complete depolymerization of lignin: a review. Biocatal Agric Biotechnol 23:101498. https://doi.org/10.1016/j.bcab.2020.101498
Bugg TD, Williamson JJ, Alberti F (2021) Microbial hosts for metabolic engineering of lignin bioconversion to renewable chemicals. Renew Sustain Energy Rev 152. https://doi.org/10.1016/j.rser.2021.111674
Martínez A, Camarero S, Ruiz-Dueñas F, Martínez M (2018) Biological lignin degradation. Lignin Valoriz: Emerg Approaches 19:199. https://doi.org/10.1039/9781788010351-00199
Ali SS, Elsamahy T, Koutra E, Kornaros M, El-Sheekh M, Abdelkarim EA, Zhu D, Sun J (2021) Degradation of conventional plastic wastes in the environment: a review on current status of knowledge and future perspectives of disposal. Sci Total Environ 771:144719. https://doi.org/10.1016/j.scitotenv.2020.144719
Levy-Booth DJ, Hashimi A, Roccor R, Liu L-Y, Renneckar S, Eltis LD, Mohn WW (2021) Genomics and metatranscriptomics of biogeochemical cycling and degradation of lignin-derived aromatic compounds in thermal swamp sediment. ISME J 15:879–893. https://doi.org/10.1038/s41396-020-00820-x
Batie CJ, Ballou DP, Correll CC (2019) Phthalate dioxygenase reductase and related flavin-iron-sulfur containing electron transferases. In: Editor(ed) Chemistry and biochemistry of flavoenzymes, edn. CRC Press, pp 543–556
Kamimura N, Takahashi K, Mori K, Araki T, Fujita M, Higuchi Y, Masai E (2017) Bacterial catabolism of lignin-derived aromatics: new findings in a recent decade: update on bacterial lignin catabolism. Environ Microbiol Rep 9:679–705. https://doi.org/10.1111/1758-2229.12597
Asina F, Brzonova I, Kozliak E, Kubátová A, Ji Y (2017) Microbial treatment of industrial lignin: successes, problems and challenges. Renew Sustain Energy Rev 77:1179–1205. https://doi.org/10.1016/j.rser.2017.03.098
Chan JC, Paice M, Zhang X (2020) Enzymatic oxidation of lignin: challenges and barriers toward practical applications. ChemCatChem 12:401–425. https://doi.org/10.1002/cctc.201901480
Xu Z, Lei P, Zhai R, Wen Z, Jin M (2019) Recent advances in lignin valorization with bacterial cultures: microorganisms, metabolic pathways, and bio-products. Biotechnol Biofuels 12:1–19. https://doi.org/10.1186/s13068-019-1376-0
Presley GN, Werner AZ, Katahira R, Garcia DC, Haugen SJ, Ramirez KJ, Giannone RJ, Beckham GT, Michener JK (2021) Pathway discovery and engineering for cleavage of a β-1 lignin-derived biaryl compound. Metab Eng 65:1–10. https://doi.org/10.1016/j.ymben.2021.02.003
Toews DP, Hofmeister NR, Taylor SA (2017) The evolution and genetics of carotenoid processing in animals. Trends Genet 33:171–182. https://doi.org/10.1016/j.tig.2017.01.002
Abdelaziz OY, Brink DP, Prothmann J, Ravi K, Sun M, García-Hidalgo J, Sandahl M, Hulteberg CP, Turner C, Lidén G (2016) Biological valorization of low molecular weight lignin. Biotechnol Adv 34:1318–1346. https://doi.org/10.1016/j.biotechadv.2016.10.001
Kamimura N, Sakamoto S, Mitsuda N, Masai E, Kajita S (2019) Advances in microbial lignin degradation and its applications. Curr Opin Biotechnol 56:179–186. https://doi.org/10.1016/j.copbio.2018.11.011
Shahidi F, Yeo J (2016) Insoluble-bound phenolics in food. Molecules 21:1216. https://doi.org/10.3390/molecules21091216
Dilokpimol A, Mäkelä MR, Aguilar-Pontes MV, Benoit-Gelber I, Hildén KS, de Vries RP (2016) Diversity of fungal feruloyl esterases: updated phylogenetic classification, properties, and industrial applications. Biotechnol Biofuels 9:1–18. https://doi.org/10.1186/s13068-016-0651-6
Liu S, Soomro L, Wei X, Yuan X, Gu T, Li Z, Wang Y, Bao Y, Wang F, Wen B (2021) Directed evolution of feruloyl esterase from Lactobacillus acidophilus and its application for ferulic acid production. Bioresour Technol 332:124967. https://doi.org/10.1016/j.biortech.2021.124967
Tamayo-Cabezas J (2019) Biocatalytic acylation of carbohydrates to produce feruloylated oligosaccharides and carbohydrate fatty acid esters as potential functional ingredients: novel approaches for efficient enzymatic synthesis by carboxylic. McGill University (Canada)
Xu W, Fu S, Yang Z, Lu J, Guo R (2018) Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour Technol 259:18–23. https://doi.org/10.1016/j.biortech.2018.02.046
Janusz G, Pawlik A, Sulej J, Świderska-Burek U, Jarosz-Wilkołazka A, Paszczyński A (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41:941–962. https://doi.org/10.1093/femsre/fux049
Singh AK, Bilal M, Iqbal HM, Meyer AS, Raj A (2021) Bioremediation of lignin derivatives and phenolics in wastewater with lignin modifying enzymes: Status, opportunities and challenges. Sci Total Environ 777:145988. https://doi.org/10.1016/j.scitotenv.2021.145988
Spence EM, Calvo-Bado L, Mines P, Bugg TD (2021) Metabolic engineering of Rhodococcus jostii RHA1 for production of pyridine-dicarboxylic acids from lignin. Microb Cell Fact 20:1–12. https://doi.org/10.1186/s12934-020-01504-z
Zhuo R, Fan F (2021) A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci Total Environ 778:146132. https://doi.org/10.1016/j.scitotenv.2021.146132
Pecková V, Legerská B, Chmelová D, Horník M, Ondrejovič M (2021) Comparison of efficiency for monoazo dye removal by different species of white-rot fungi. Int J Environ Sci Technol 18:21–32. https://doi.org/10.1007/s13762-020-02806-w
Asgher M, Nasir I, Khalid N, Qamar SA (2020) Development of biocomposites based on bacterial cellulose reinforced delignified rice husk-PVA plasticized with glycerol. J Polym Res 27:1–11. https://doi.org/10.1007/s10965-020-02314-y
Yang L, Yuan H, Yang Y, Wang R, Wang C, Wei X, Chen S, Yu J, Ma X (2020) Enhanced lignin degradation in tobacco stalk composting with inoculation of white-rot fungi Trametes hirsuta and Pleurotus ostreatus. Waste Biomass Valoriz 11:3525–3535. https://doi.org/10.1007/s12649-019-00692-z
Hyde KD, Xu J, Rapior S, Jeewon R, Lumyong S, Niego AGT, Abeywickrama PD, Aluthmuhandiram JV, Brahamanage RS, Brooks S (2019) The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers 97:1–136. https://doi.org/10.1007/s13225-019-00430-9
Kameshwar AKS, Barber R, Qin W (2018) Comparative modeling and molecular docking analysis of white, brown and soft rot fungal laccases using lignin model compounds for understanding the structural and functional properties of laccases. J Mol Graph Model 79:15–26. https://doi.org/10.1016/j.jmgm.2017.10.019
Linares NC, Fernández F, Loske AM, Gómez-Lim MA (2018) Enhanced delignification of lignocellulosic biomass by recombinant fungus Phanerochaete chrysosporium overexpressing laccases and peroxidases. Microb Physiol 28:1–13. https://doi.org/10.1159/000485976
Chen Z, Wan C (2017) Biological valorization strategies for converting lignin into fuels and chemicals. Renew Sustain Energy Rev 73:610–621. https://doi.org/10.1016/j.rser.2017.01.166
Fonseca MI, Molina MA, Winnik D, Busi MV, Fariña JI, Villalba LL, Zapata PD (2018) Isolation of a laccase-coding gene from the lignin-degrading fungus Phlebia brevispora BAFC 633 and heterologous expression in Pichia pastoris. J Appl Microbiol 124:1454–1468. https://doi.org/10.1111/jam.13720
Jin W, Li J, Feng H, You S, Zhang L, Norvienyeku J, Hu K, Sun S, Wang Z (2018) Importance of a laccase gene (Lcc1) in the development of Ganoderma tsugae. Int J Mol Sci 19:471. https://doi.org/10.3390/ijms19020471
Janusz G, Pawlik A, Świderska-Burek U, Polak J, Sulej J, Jarosz-Wilkołazka A, Paszczyński A (2020) Laccase properties, physiological functions, and evolution. Int J Mol Sci 21:966. https://doi.org/10.3390/ijms21030966
Salem MZ, Mansour MM, Elansary HO (2019) Evaluation of the effect of inner and outer bark extracts of sugar maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J Wood Chem Technol 39:136–147. https://doi.org/10.1080/02773813.2018.1547763
Spence EM, Scott HT, Dumond L, Calvo-Bado L, di Monaco S, Williamson JJ, Persinoti GF, Squina FM, Bugg TD (2020) The hydroxyquinol degradation pathway in Rhodococcus jostii RHA1 and Agrobacterium species is an alternative pathway for degradation of protocatechuic acid and lignin fragments. Appl Environ Microbiol 86:e01561-e1620. https://doi.org/10.1128/AEM.01561-20
Sun Z, Bottari G, Afanasenko A, Stuart MC, Deuss PJ, Fridrich B, Barta K (2018) Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat Catal 1:82–92. https://doi.org/10.1038/s41929-017-0007-z
Nikel PI, de Lorenzo V (2018) Pseudomonas putida as a functional chassis for industrial biocatalysis: from native biochemistry to trans-metabolism. Metab Eng 50:142–155. https://doi.org/10.1016/j.ymben.2018.05.005
Xu Z, Pan C, Li X, Hao N, Zhang T, Gaffrey MJ, Pu Y, Cort JR, Ragauskas AJ, Qian W-J (2021) Enhancement of polyhydroxyalkanoate production by co-feeding lignin derivatives with glycerol in Pseudomonas putida KT2440. Biotechnol Biofuels 14:1–23. https://doi.org/10.1186/s13068-020-01861-2
Xu Z, Xu M, Cai C, Chen S, Jin M (2021) Microbial polyhydroxyalkanoate production from lignin by Pseudomonas putida NX-1. Bioresour Technol 319:124210. https://doi.org/10.1016/j.biortech.2020.124210
Ravi K, García-Hidalgo J, Gorwa-Grauslund MF, Lidén G (2017) Conversion of lignin model compounds by Pseudomonas putida KT2440 and isolates from compost. Appl Microbiol Biotechnol 101:5059–5070. https://doi.org/10.1007/s00253-017-8211-y
Khan MU, Ahring BK (2019) Lignin degradation under anaerobic digestion: influence of lignin modifications-a review. Biomass Bioenergy 128:105325. https://doi.org/10.1016/j.biombioe.2019.105325
Barton N, Horbal L, Starck S, Kohlstedt M, Luzhetskyy A, Wittmann C (2018) Enabling the valorization of guaiacol-based lignin: Integrated chemical and biochemical production of cis, cis-muconic acid using metabolically engineered Amycolatopsis sp ATCC 39116. Metab Eng 45:200–210. https://doi.org/10.1016/j.ymben.2017.12.001
Bugg TD, Williamson JJ, Rashid GM (2020) Bacterial enzymes for lignin depolymerisation: new biocatalysts for generation of renewable chemicals from biomass. Curr Opin Chem Biol 55:26–33. https://doi.org/10.1016/j.cbpa.2019.11.007
Banerjee G, Chattopadhyay P (2019) Vanillin biotechnology: the perspectives and future. J Sci Food Agric 99:499–506. https://doi.org/10.1002/jsfa.9303
López-Mondéjar R, Algora C, Baldrian P (2019) Lignocellulolytic systems of soil bacteria: a vast and diverse toolbox for biotechnological conversion processes. Biotechnol Adv 37:107374. https://doi.org/10.1016/j.biotechadv.2019.03.013
Gonçalves CC, Bruce T, Silva CDOG, Fillho EXF, Noronha EF, Carlquist M, Parachin NS (2020) Bioprospecting microbial diversity for lignin valorization: dry and wet screening methods. Front Microbiol 11:1081. https://doi.org/10.3389/fmicb.2020.01081
Li P, Chen Q, Wang TC, Vermeulen NA, Mehdi BL, Dohnalkova A, Browning ND, Shen D, Anderson R, Gómez-Gualdrón DA (2018) Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chem 4:1022–1034. https://doi.org/10.1016/j.chempr.2018.03.001
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of lignocellulosic biomass for biofuels and bioproducts: an overview. Waste Biomass Valoriz 10:235–251. https://doi.org/10.1007/s12649-017-0059-y
Bilal M, Rasheed T, Iqbal HM, Yan Y (2018) Peroxidases-assisted removal of environmentally-related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci Total Environ 644:1–13. https://doi.org/10.1016/j.scitotenv.2018.06.274
Bilal M, Iqbal HM (2019) Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatal Agric Biotechnol 20:101205. https://doi.org/10.1016/j.bcab.2019.101205
Bilal M, Asgher M, Iqbal HM, Hu H, Wang W, Zhang X (2017) Bio-catalytic performance and dye-based industrial pollutants degradation potential of agarose-immobilized MnP using a Packed Bed Reactor System. Int J Biol Macromol 102:582–590. https://doi.org/10.1016/j.ijbiomac.2017.04.065
Huang S, Huang D, Qitang W, Meifang H, Xiaoyan T, Jian Z (2020) Effect of environmental C/N ratio on activities of lignin-degrading enzymes produced by Phanerochaete chrysosporium. Pedosphere 30:285–292. https://doi.org/10.1016/S1002-0160(17)60391-6
Saleem R, Khurshid M, Ahmed S (2018) Laccases, manganese peroxidases and xylanases used for the bio-bleaching of paper pulp: an environmental friendly approach. Protein Pept Lett 25:180–186. https://doi.org/10.2174/0929866525666180122100133
Chowdhary P, Yadav A, Kaithwas G, Bharagava R N (2017) Distillery wastewater: a major source of environmental pollution and its biological treatment for environmental safety. In: Editor(ed) Green technologies and environmental sustainability, edn. Springer, pp 409–435. https://doi.org/10.1007/978-3-319-50654-8_18
Chowdhary P, Shukla G, Raj G, Ferreira LFR, Bharagava RN (2019) Microbial manganese peroxidase: a ligninolytic enzyme and its ample opportunities in research. SN Appl Sci 1:1–12. https://doi.org/10.1007/s42452-018-0046-3
Singh AK, Bilal M, Iqbal HM, Raj A (2021) Lignin peroxidase in focus for catalytic elimination of contaminants-a critical review on recent progress and perspectives. Int J Biol Macromol 177:58–82. https://doi.org/10.1016/j.ijbiomac.2021.02.032
Khan R, Bhawana P, Fulekar M (2013) Microbial decolorization and degradation of synthetic dyes: a review. Rev Environ Sci Bio/Technol 12:75–97. https://doi.org/10.1007/s11157-012-9287-6
Mäkinen MA, Risulainen N, Mattila H, Lundell TK (2018) Transcription of lignocellulose-decomposition associated genes, enzyme activities and production of ethanol upon bioconversion of waste substrate by Phlebia radiata. Appl Microbiol Biotechnol 102:5657–5672. https://doi.org/10.1007/s00253-018-9045-y
Datta R, Kelkar A, Baraniya D, Molaei A, Moulick A, Meena RS, Formanek P (2017) Enzymatic degradation of lignin in solil: a review. Sustainability 9:1163. https://doi.org/10.3390/su9071163
Miyauchi S, Rancon A, Drula E, Hage H, Chaduli D, Favel A, Grisel S, Henrissat B, Herpoël-Gimbert I, Ruiz-Dueñas FJ (2018) Integrative visual omics of the white-rot fungus Polyporus brumalis exposes the biotechnological potential of its oxidative enzymes for delignifying raw plant biomass. Biotechnol Biofuels 11:1–14. https://doi.org/10.1186/s13068-018-1198-5
Xie P, Fan L, Huang L, Zhang C (2020) An innovative co-fungal treatment to poplar bark sawdust for delignification and polyphenol enrichment. Ind Crops Prod 157:112896. https://doi.org/10.1016/j.indcrop.2020.112896
Pham LTM, Seo H, Kim K-J, Kim YH (2018) In silico-designed lignin peroxidase from Phanerochaete chrysosporium shows enhanced acid stability for depolymerization of lignin. Biotechnol Biofuels 11:1–13. https://doi.org/10.1186/s13068-018-1324-4
Cannatelli MD, Ragauskas AJ (2017) Two decades of laccases: advancing sustainability in the chemical industry. Chem Rec 17:122–140. https://doi.org/10.1002/tcr.201600033
Kohli K, Prajapati R, Sharma BK (2019) Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 12:233. https://doi.org/10.3390/en12020233
Youn H-D, Hah YC, Kang S-O (1995) Role of laccase in lignin degradation by white-rot fungi. FEMS Microbiol Lett 132:183–188. https://doi.org/10.1111/j.1574-6968.1995.tb07831.x
Asgher M, Wahab A, Bilal M, Iqbal H (2018) Delignification of lignocellulose biomasses by alginate-chitosan immobilized laccase produced from Trametes versicolor IBL-04. Waste Biomass Valoriz 9:2071–2079. https://doi.org/10.1007/s12649-017-9991-0
Yesilada O, Birhanli E, Geckil H (2018) Bioremediation and decolorization of textile dyes by white rot fungi and laccase enzymes. In: Editor(ed) Mycoremediation and environmental sustainability, edn. Springer, pp 121–153 https://doi.org/10.1007/978-3-319-77386-5_5
Raveendran S, Parameswaran B, Ummalyma SB, Abraham A, Mathew AK, Madhavan A, Rebello S, Pandey A (2018) Applications of microbial enzymes in food industry. Food Technol Biotechnol 56:16. https://doi.org/10.17113/ftb.56.01.18.5491
Agrawal K, Chaturvedi V, Verma P (2018) Fungal laccase discovered but yet undiscovered. Bioresour Bioproc 5:1–12. https://doi.org/10.1186/s40643-018-0190-z
Asgher M, Wahab A, Bilal M, Iqbal HMN (2016) Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal Agric Biotechnol 6:195–201. https://doi.org/10.1016/j.bcab.2016.04.003
Bilal M, Wang Z, Cui J, Ferreira LFR, Bharagava RN, Iqbal HM (2020) Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers-a drive towards greener and eco-friendlier biocatalytic systems. Sci Total Environ 722:137903. https://doi.org/10.1016/j.scitotenv.2020.137903
Mehmood T, Saman T, Irfan M, Anwar F, Ikram MS, Tabassam Q (2019) Pectinase production from Schizophyllum commune through central composite design using citrus waste and its immobilization for industrial exploitation. Waste Biomass Valoriz 10:2527–2536. https://doi.org/10.1007/s12649-018-0279-9
Amin M, Bhatti HN, Sadaf S, Bilal M (2021) Optimization of lipase production by response surface methodology and its application for efficient biodegradation of polyester vylon-200. Catal Lett 151:3603–3616. https://doi.org/10.1007/s10562-021-03603-x
Boratyński F, Szczepańska E, Grudniewska A, Gniłka R, Olejniczak T (2018) Improving of hydrolases biosythesis by solid-state fermentation of Penicillium camemberti on rapeseed cake. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-28412-y
Campioni TS, de Azevedo CAF, de Figueiredo F, da Silva D, de Oliva NP (2020) Xylanases and cellulases biosynthesis by selected fungi in a simple and economic bio system using sugarcane straw. Int J Environ Agri Biotechnol 5:217–230. https://doi.org/10.22161/ijeab.51.31
Kalli E, Lappa I, Bouchagier P, Tarantilis PA, Skotti E (2018) Novel application and industrial exploitation of winery by-products. Bioresour Bioproc 5:1–21. https://doi.org/10.1186/s40643-018-0232-6
Kumar V, Ahluwalia V, Saran S, Kumar J, Patel AK, Singhania RR (2021) Recent developments on solid-state fermentation for production of microbial secondary metabolites: challenges and solutions. Bioresour Technol 323:124566. https://doi.org/10.1016/j.biortech.2020.124566
Buenrostro-Figueroa J, Velázquez M, Flores-Ortega O, Ascacio-Valdés J, Huerta-Ochoa S, Aguilar C, Prado-Barragán L (2017) Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Proc Biochem 62:16–23. https://doi.org/10.1016/j.procbio.2017.07.016
Dulf FV, Vodnar DC, Dulf E-H, Pintea A (2017) Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem Cent J 11:1–10. https://doi.org/10.1186/s13065-017-0323-z
Luo H, Liu Z, Xie F, Bilal M, Peng F (2021) Lignocellulosic biomass to biobutanol: Toxic effects and response mechanism of the combined stress of lignin-derived phenolic acids and phenolic aldehydes to Clostridium acetobutylicum. Ind Crops Prod 170:113722. https://doi.org/10.1016/j.indcrop.2021.113722
Ahmad A, Naqvi SA, Jaskani MJ, Waseem M, Ali E, Khan IA, Faisal Manzoor M, Siddeeg A, Aadil RM (2021) Efficient utilization of date palm waste for the bioethanol production through Saccharomyces cerevisiae strain. Food Sci Nutrit 9:2066–2074. https://doi.org/10.1002/fsn3.2175
Christopher LP, Kumar H, Zambare VP (2014) Enzymatic biodiesel: challenges and opportunities. Appl Energy 119:497–520. https://doi.org/10.1016/j.apenergy.2014.01.017
Ail SS, Dasappa S (2016) Biomass to liquid transportation fuel via Fischer Tropsch synthesis-technology review and current scenario. Renew Sustain Energy Rev 58:267–286. https://doi.org/10.1016/j.rser.2015.12.143
Adachi D, Hama S, Nakashima K, Bogaki T, Ogino C, Kondo A (2013) Production of biodiesel from plant oil hydrolysates using an Aspergillus oryzae whole-cell biocatalyst highly expressing Candida antarctica lipase B. Bioresour Technol 135:410–416. https://doi.org/10.1016/j.biortech.2012.06.092
Singh D, Sharma D, Soni S, Sharma S, Sharma PK, Jhalani A (2020) A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 262:116553. https://doi.org/10.1016/j.fuel.2019.116553
Fabiano B, Perego P (2002) Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int J Hydrog Energy 27:149–156. https://doi.org/10.1016/S0360-3199(01)00102-1
Kotay SM, Das D (2007) Microbial hydrogen production with Bacillus coagulans IIT-BT S1 isolated from anaerobic sewage sludge. Bioresour Technol 98:1183–1190. https://doi.org/10.1016/j.biortech.2006.05.009
Zhou M, Yan B, Wong JW, Zhang Y (2018) Enhanced volatile fatty acids production from anaerobic fermentation of food waste: a mini-review focusing on acidogenic metabolic pathways. Bioresour Technol 248:68–78. https://doi.org/10.1016/j.biortech.2017.06.121
Lemaire A, Limbourg S (2019) How can food loss and waste management achieve sustainable development goals? J Clean Prod 234:1221–1234. https://doi.org/10.1016/j.jclepro.2019.06.226
Jarunglumlert T, Prommuak C, Putmai N, Pavasant P (2018) Scaling-up bio-hydrogen production from food waste: feasibilities and challenges. Int J Hydrog Energy 43:634–648. https://doi.org/10.1016/j.ijhydene.2017.10.013
Tsang YF, Kumar V, Samadar P, Yang Y, Lee J, Ok YS, Song H, Kim K-H, Kwon EE, Jeon YJ (2019) Production of bioplastic through food waste valorization. Environ Int 127:625–644. https://doi.org/10.1016/j.envint.2019.03.076
Arranz-Piera P, Kemausuor F, Darkwah L, Edjekumhene I, Cortés J, Velo E (2018) Mini-grid electricity service based on local agricultural residues: feasibility study in rural Ghana. Energy 153:443–454. https://doi.org/10.1016/j.energy.2018.04.058
Moradali MF, Rehm BH (2020) Bacterial biopolymers: from pathogenesis to advanced materials. Nat Rev Microbiol 18:195–210. https://doi.org/10.1038/s41579-019-0313-3
Vendruscolo F, Ninow JL (2014) Apple pomace as a substrate for fungal chitosan production in an airlift bioreactor. Biocatal Agric Biotechnol 3:338–342. https://doi.org/10.1016/j.bcab.2014.05.001
Namboodiri MT, Pakshirajan K (2019) Sustainable and green approach of chitosan production from Penicillium citrinum biomass using industrial wastewater as a cheap substrate. J Environ Manag 240:431–440. https://doi.org/10.1016/j.jenvman.2019.03.085
Domínguez Rivera Á, Martínez UrbinaLópez y López MÁVE (2019) Advances on research in the use of agro-industrial waste in biosurfactant production. World J Microbiol Biotechnol 35:1–18. https://doi.org/10.1007/s11274-019-2729-3
Jimoh AA, Lin J (2020) Biotechnological applications of Paenibacillus sp. D9 lipopeptide biosurfactant produced in low-cost substrates. Appl Biochem Biotechnol 191:921–941. https://doi.org/10.1007/s12010-020-03246-5
Panjiar N, Mattam AJ, Jose S, Gandham S, Velankar HR (2020) Valorization of xylose-rich hydrolysate from rice straw, an agroresidue, through biosurfactant production by the soil bacterium Serratia nematodiphila. Sci Total Environ 729:138933. https://doi.org/10.1016/j.scitotenv.2020.138933
Mangaraj S, Yadav A, Bal LM, Dash S, Mahanti NK (2019) Application of biodegradable polymers in food packaging industry: a comprehensive review. J Packag Technol Res 3:77–96. https://doi.org/10.1007/s41783-018-0049-y
Khattab MM, Dahman Y (2019) Production and recovery of poly-3-hydroxybutyrate bioplastics using agro-industrial residues of hemp hurd biomass. Bioproc Biosyst Eng 42:1115–1127. https://doi.org/10.1007/s00449-019-02109-6
Sirohi R, Pandey JP, Gaur VK, Gnansounou E, Sindhu R (2020) Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour Technol 311:123536. https://doi.org/10.1016/j.biortech.2020.123536
Al-Battashi HS, Annamalai N, Sivakumar N, Al-Bahry S, Tripathi BN, Nguyen QD, Gupta VK (2019) Lignocellulosic biomass (LCB): a potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev Environ Sci Bio/Technol 18:183–205. https://doi.org/10.1007/s11157-018-09488-4
Arumugam A, Anudakshaini T, Shruthi R, Jeyavishnu K, Sundarra Harini S, Sharad J (2020) Low-cost production of PHA using cashew apple (Anacardium occidentale L.) juice as potential substrate: optimization and characterization. Biomass Convers Biorefin 10:1167–1178. https://doi.org/10.1007/s13399-019-00502-5
Sasmitaloka KS, Sunarti TC, Rahayuningsih M (2017) Bioinsecticides production by Bacillus thuringiensis subsp. aizawai using agroindustrial by-product in Solid Fermentation. J Penelit Pascapanen Pertan 13:1–10. https://doi.org/10.21082/jpasca.v13n1.2016.1-10
Haridevan H, Evans DA, Ragauskas AJ, Martin DJ, Annamalai PK (2021) Valorisation of technical lignin in rigid polyurethane foam: a critical evaluation on trends, guidelines and future perspectives. Green Chem 23:8725–8753. https://doi.org/10.1039/D1GC02744A
Figueiredo P, Lintinen K, Hirvonen JT, Kostiainen MA, Santos HA (2018) Properties and chemical modifications of lignin: towards lignin-based nanomaterials for biomedical applications. Prog Mater Sci 93:233–269. https://doi.org/10.1016/j.pmatsci.2017.12.001
Wong SS, Shu R, Zhang J, Liu H, Yan N (2020) Downstream processing of lignin derived feedstock into end products. Chem Soc Rev 49:5510–5560. https://doi.org/10.1039/D0CS00134A
Mankar SV, Garcia Gonzalez MN, Warlin N, Valsange NG, Rehnberg N, Lundmark S, Jannasch P, Zhang B (2019) Synthesis, life cycle assessment, and polymerization of a vanillin-based spirocyclic diol toward polyesters with increased glass-transition temperature. ACS Sust Chem Eng 7:19090–19103. https://doi.org/10.1021/acssuschemeng.9b04930
Humbird C, Hsu D, D, Davis R, Tao L, Kinchin Aden A, Schoen P, Lukas J, Olthof B, Worley M (2011) Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol: dilute-acid pretreatment and enzymatic hydrolysis of corn stover. National Renewable Energy Lab.(NREL), Golden, CO (United States). https://doi.org/10.2172/1013269
Shen R, Tao L, Yang B (2019) Techno-economic analysis of jet-fuel production from biorefinery waste lignin. Biofuels Bioprod Biorefin 13:486–501. https://doi.org/10.1002/bbb.1952
Acknowledgements
The research leading to these results has received funding from the Norwegian Financial Mechanism 2014-2021 under the Project number 2020/37/K/ST8/03805. The authors are thankful to the University of Punjab, Pakistan for the scholastic support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmad, N., Aslam, S., Hussain, N. et al. Transforming Lignin Biomass to Value: Interplay Between Ligninolytic Enzymes and Lignocellulose Depolymerization. Bioenerg. Res. 16, 1246–1263 (2023). https://doi.org/10.1007/s12155-022-10541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-022-10541-y