Abstract
By-products from the non-ferrous industry are an environmental problem; however, their economic value is high if utilized elsewhere. For example, by-products that contain alkaline compounds can potentially sequestrate CO2 through the mineral carbonation process. This review discusses the potential of these by-products for CO2 reduction through mineral carbonation. The main by-products that are discussed are red mud from the alumina/aluminum industry and metallurgical slag from the copper, zinc, lead, and ferronickel industries. This review summarizes the CO2 equivalent emissions generated by non-ferrous industries and various data about by-products from non-ferrous industries, such as their production quantities, mineralogy, and chemical composition. In terms of production quantities, by-products of non-ferrous industries are often more abundant than the main products (metals). In terms of mineralogy, by-products from the non-ferrous industry are silicate minerals. Nevertheless, non-ferrous industrial by-products have a relatively high content of alkaline compounds, which makes them potential feedstock for mineral carbonation. Theoretically, considering their maximum sequestration capacities (based on their oxide compositions and estimated masses), these by-products could be used in mineral carbonation to reduce CO2 emissions. In addition, this review attempts to identify the difficulties encountered during the use of by-products from non-ferrous industries for mineral carbonation. This review estimated that the total CO2 emissions from the non-ferrous industries could be reduced by up to 9–25%. This study will serve as an important reference, guiding future studies related to the mineral carbonation of by-products from non-ferrous industries.
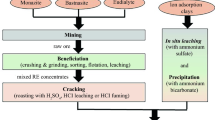
Graphical abstract











Similar content being viewed by others
Data availability
All the data needed for this research has been provided in the manuscript.
Notes
Modified with the addition of 0.6128 × %FeO based on a previous study (Srivastava et al. 2022a)
Abbreviations
- Alu:
-
Aluminum silicate hydrate (Al2Si70O143.H2O)
- An:
-
Andradite (Ca-Fe-Al-Si oxide)
- Ant:
-
Anatase (TiO2)
- Ber:
-
Berlinite (AlPO4)
- Bo:
-
Boehmite (AlO(OH))
- Byr:
-
Bayerite (Al(OH)3)
- Cal:
-
Calcite (CaCO3)
- Can:
-
Cancrinite (Na6Ca1.5Al6Si6O24(CO3)16)
- Ca1:
-
Katoite (Ca3Al2(SiO4)(OH)8)
- Ca2:
-
Reinhardbraunsite (Ca5(SiO4)2(OH)2)
- Ca3:
-
Calcium hydroxide (Ca(OH)2)
- COP26:
-
The 26th Congress of the Parties
- Crt:
-
Cristobalite (SiO2)
- Cor:
-
Cordierite (Mg2Al4Si5O18)
- Ch:
-
Chantalite (CaAl2(SiO4)(OH)4)
- Cham:
-
Chamosite ((Fe, Mg)5Al(AlSi3O10)(OH)8)
- Clin:
-
Clinoenstatite (MgSiO3)
- DC:
-
Direct carbonation
- Dpr:
-
Diaspore (AlO(OH))
- Dpd:
-
Diopside (CaMgSi2O6)
- En:
-
Enstatite (MgSiO3)
- Fl:
-
Fluorite (CaF2)
- Frs:
-
Forsterite (Mg2SiO4)
- Fr:
-
Franklinite (Zn, Fe, Mn)(Fe, Mn)2O4
- Fy:
-
Fayalite (Fe2SiO4)
- Gar:
-
Garnet (Ca3Al2(SiO4)3)
- Gis:
-
Gismondine (Ca2Al4Si4O16.9(H2O))
- Gi:
-
Gibbsite (Al(OH)3)
- Go:
-
Goethite (FeO(OH))
- GWP:
-
Global warming potential
- Hem:
-
Hematite (Fe2O3)
- Her:
-
Hercynite (MgAl2Fe1.8O4)
- IC:
-
Indirect carbonation
- Im:
-
Ilmenite (FeTiO3)
- Ka:
-
Kaolinite-1A (Al2Si2O5(OH)4)
- Kir:
-
Kirschsteinite (CaFeSiO4)
- LOI:
-
Loss on Ignition
- Mag:
-
Magnetite (Fe3O4)
- Mf:
-
Magnesioferrite (MgFe2O4)
- MF:
-
Magnesium iron silicate (Mg0.465Fe1.5353)Si2O6
- MFO:
-
Magnesium ferrous oxide (MgO)0.432(FeO)0.568
- Mul:
-
Mullite (3Al2O3.2SiO2)
- Mus:
-
Muscovite (KAl2(AlSi3O10)(OH)2)
- Nas:
-
Sodium aluminum silicate (Na5Al3Si3O15)
- Nep:
-
Nepheline ((Na,K)AlSiO4)
- Ol:
-
Olivine (Mg2SiO4)
- Prv:
-
Perovskite (CaTiO3)
- Py:
-
Pyroxene
- QSL:
-
Queneau-Schuhmann-Lurgi
- Qz:
-
Quartz (SiO2)
- Rtl:
-
Rutile (TiO2)
- Sdl:
-
Sodalite (Al3ClNa4Si3O12)
- Sl:
-
Silica (SiO2)
- So:
-
Sodium aluminum carbonate silicate (3NaAlSiO4.Na2CO3)
- Spn:
-
Spinels (MgAl2O4)
- S/L:
-
Solid/Liquid
- Trd:
-
Tridymite (SiO2)
- TSL:
-
Top submerged lance
- VO:
-
Vanadium pentoxide (V2O5)
- Wlm:
-
Willemite (Zn2SiO4)
- Wus:
-
Wustite (FeO)
- Z:
-
Zinc diiron(III) oxide (ZnFe2O4)
References
Abdul F, Pintowantoro S, Maulidani A (2020) Analysis the effect of charcoal mass variation to ni content, sinter strength and yield on sintering process of limonitic laterite nickel ore. In: Suwarno S, Djanali V, Mubarok F, Pramujati B (eds) In Key engineering materials, vol 867, Trans Tech Publications Ltd, pp 25–31. https://doi.org/10.4028/www.scientific.net/KEM.867.25
Abdulvaliyev RA, Akcil A, Gladyshev SV et al (2015) Gallium and vanadium extraction from red mud of Turkish alumina refinery plant: Hydrogarnet process. Hydrometallurgy 157:72–77. https://doi.org/10.1016/j.hydromet.2015.07.007
Akiyama J, Yamamoto Y (1987) Alkali-silica reactivity of ferronickel slag. Doboku Gakkai Ronbunshu 378:157–163
Albitar M, Mohamed Ali MS, Visintin P, Drechsler M (2015) Effect of granulated lead smelter slag on strength of fly ash-based geopolymer concrete. Constr Build Mater 83:128–135. https://doi.org/10.1016/j.conbuildmat.2015.03.009
Alex TC, Kalinkin AM, Nath SK et al (2013) Utilization of zinc slag through geopolymerization: Influence of milling atmosphere. Int J Miner Process 123:102–107. https://doi.org/10.1016/j.minpro.2013.06.001
Al-Jabri KS, Hisada M, Al-Oraimi SK, Al-Saidy AH (2009) Copper slag as sand replacement for high performance concrete. Cem Concr Compos 31:483–488. https://doi.org/10.1016/j.cemconcomp.2009.04.007
Almanzar F, Baker M, Elias N, Guzman E (2010) Mineral facilities of Europe, US Geological Survey. https://pubs.er.usgs.gov/publication/ofr20101257. Accessed 15 Nov 2022
Archambo M, Kawatra SK (2021) Red Mud: Fundamentals and new avenues for utilization. Miner Process Extr Metall Rev 42:427–450. https://doi.org/10.1080/08827508.2020.1781109
Archambo MS, Kawatra SK (2022) Extraction of rare earths from red mud iron nugget slags with oxalic acid precipitation. Miner Process Extr Metall Rev 43:656–663. https://doi.org/10.1080/08827508.2021.1927729
Authier-Martin M, Forte G, Ostap S, See J (2001) The mineralogy of bauxite for producing smelter-grade alumina. JOM 53:36–40. https://doi.org/10.1007/s11837-001-0011-1
Ayub SA, Tsegab H, Rahmani O, Pour AB (2020) Potential for co2 mineral carbonation in the paleogene segamat basalt of Malaysia. Minerals 10:1–14. https://doi.org/10.3390/min10121045
Azdarpour A, Asadullah M, Mohammadian E et al (2015) Mineral carbonation of red gypsum via pH-swing process: Effect of CO2 pressure on the efficiency and products characteristics. Chem Eng J 264:425–436. https://doi.org/10.1016/j.cej.2014.11.125
Baker M, Elias N, Guzmán E, Soto-Viruet Y (2010) Mineral facilities of Asia and the Pacific. US Department, of the Interior, US Geological Survey. https://pubs.er.usgs.gov/publication/ofr20101254. Accessed 15 Nov 2022
Balakrishnan M, Batra VS, Hargreaves JSJ et al (2009) Hydrogen production from methane in the presence of red mud –making mud magnetic. Green Chem 11:42–47. https://doi.org/10.1039/b815834g
Bałdyga J, Henczka M, Sokolnicka K (2010) Utilization of carbon dioxide by chemically accelerated mineral carbonation. Mater Lett 64:702–704. https://doi.org/10.1016/j.matlet.2009.12.043
Bale CW, Bélisle E, Chartrand P et al (2016) FactSage thermochemical software and databases, 2010–2016. Calphad 54:35–53. https://doi.org/10.1016/j.calphad.2016.05.002
Bao J, Li K, Ning P et al (2021) Study on the role of copper converter slag in simultaneously removing SO2 and NOx using KMnO4/copper converter slag slurry. J Environ Sci (china) 108:33–43. https://doi.org/10.1016/j.jes.2021.02.004
Barna R, Moszkowicz P, Gervais C (2004) Leaching assessment of road materials containing primary lead and zinc slags. Waste Manag 24:945–955. https://doi.org/10.1016/j.wasman.2004.07.014
Bartzas G, Komnitsas K (2015) Life cycle assessment of ferronickel production in Greece. Resour Conserv Recycl 105:113–122. https://doi.org/10.1016/j.resconrec.2015.10.016
Bodor M, Santos RM, van Gerven T, Vlad M (2013) Recent developments and perspectives on the treatment of industrial wastes by mineral carbonation - A review. Cent Eur J Eng 3:566–584. https://doi.org/10.2478/s13531-013-0115-8
Bonenfant D, Kharoune L, Sauvé S et al (2008) CO2 sequestration by aqueous red mud carbonation at ambient pressure and temperature. Ind Eng Chem Res 47:7617–7622. https://doi.org/10.1021/ie7017228
Chen Z, Cang Z, Yang F et al (2021) Carbonation of steelmaking slag presents an opportunity for carbon neutral: a review. Journal of CO2 Utilization 54:101738. https://doi.org/10.1016/j.jcou.2021.101738
Cheng S, Du T, Long Y et al (2020) Value added utilization of ferronickel slags as raw materials of 4A zeolite for CO2 reduction. Adsorption 26:1113–1126
Ciacci L, Eckelman MJ, Passarini F et al (2014) Historical evolution of greenhouse gas emissions from aluminum production at a country level. J Clean Prod 84:540–549. https://doi.org/10.1016/j.jclepro.2014.03.062
Cooling DJ, Hay PS, Guilfoyle L (2002) Carbonation of bauxite residue. In: Proceedings of the 6th international alumina quality workshop. vol 2, pp 185–190
Coppola A, Scala F, Azadi M (2022) Direct Dry Carbonation of Mining and Industrial Wastes in a Fluidized Bed for Offsetting Carbon Emissions. Processes 10. https://doi.org/10.3390/pr10030582
Costa G, Baciocchi R, Polettini A et al (2007) Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues. Environ Monit Assess 135:55–75
Ćwik A, Casanova I, Rausis K et al (2018) Carbonation of high-calcium fly ashes and its potential for carbon dioxide removal in coal fired power plants. J Clean Prod 202:1026–1034. https://doi.org/10.1016/j.jclepro.2018.08.234
Daval D, Sissmann O, Menguy N et al (2011) Influence of amorphous silica layer formation on the dissolution rate of olivine at 90 °C and elevated pCO2. Chem Geol 284(1–2):193–209
Davidson AJ, Binks SP, Gediga J (2016) Lead industry life cycle studies: environmental impact and life cycle assessment of lead battery and architectural sheet production. Int J Life Cycle Assess 21:1624–1636. https://doi.org/10.1007/s11367-015-1021-5
de Andrade Lima LRP, Bernardez LA (2011) Characterization of the lead smelter slag in Santo Amaro, Bahia, Brazil. J Hazard Mater 189:692–699. https://doi.org/10.1016/j.jhazmat.2011.02.091
Dodoo-Arhin D, Nuamah RA, Agyei-Tuffour B et al (2017) Awaso bauxite red mud-cement based composites: Characterisation for pavement applications. Case Stud Construct Mater 7:45–55. https://doi.org/10.1016/j.cscm.2017.05.003
Du Z, Lin B (2018) Analysis of carbon emissions reduction of China’s metallurgical industry. J Clean Prod 176:1177–1184. https://doi.org/10.1016/j.jclepro.2017.11.178
El-Naas MH, el Gamal M, Hameedi S, Mohamed AMO (2015) CO2 sequestration using accelerated gas-solid carbonation of pre-treated EAF steel-making bag house dust. J Environ Manag 156:218–224. https://doi.org/10.1016/j.jenvman.2015.03.040
Eloneva S, Said A, Fogelholm C-J, Zevenhoven R (2012) Preliminary assessment of a method utilizing carbon dioxide and steelmaking slags to produce precipitated calcium carbonate. Appl Energy 90:329–334. https://doi.org/10.1016/j.apenergy.2011.05.045
Eros J, Candelario-Quintana L (2006) Mineral facilities of Africa and the Middle East-US Geological Survey Open-File Report. http://pubs.usgs.gov/of/2006/1135/index.html. Accessed 15 Nov 2022
Ettler V, Johan Z (2014) 12years of leaching of contaminants from Pb smelter slags: Geochemical/mineralogical controls and slag recycling potential. Appl Geochem 40:97–103. https://doi.org/10.1016/j.apgeochem.2013.11.001
Ettler V, Komárková M, Jehlička J et al (2004) Leaching of lead metallurgical slag in citric solutions - Implications for disposal and weathering in soil environments. Chemosphere 57:567–577. https://doi.org/10.1016/j.chemosphere.2004.07.022
Fagerlund J, Experience N, Inês R, Ron Z (2012) CO2 Fixation using magnesium silicate minerals part 1: process description and performance. Energy 41(1):184–191. https://doi.org/10.1016/j.energy.2011.08.032
Fan Y, Shibata E, Iizuka A, Nakamura T (2014) Crystallization behaviors of copper smelter slag studied using time-temperature-transformation diagram. Mater Trans 55(6):958–963
Fernández-Caliani JC, Ríos G, Martínez J, Jiménez F (2012) Occurrence and speciation of copper in slags obtained during the pyrometallurgical processing of chalcopyrite concentrates at the Huelva smelter (Spain). J Min MetallSect B 48:161–171. https://doi.org/10.2298/JMMB111111027F
Ferreira R, Pinto F (2020) INSG insight comment on the effect of the COVID-19 pandemic on the global nickel market. https://insg.org/wp-content/uploads/2020/10/INSG_Insight_32-Comment-on-the-effect-of-the-Covid-19-pandemic-on-the-global-nickel-market.pdf. Accessed 6 Oct 2022
Gabasiane TS, Danha G, Mamvura TA et al (2021) Environmental and socioeconomic impact of copper slag—a review. Crystals 11(12):1504. https://doi.org/10.3390/cryst11121504
Gao W, Wang C, Yin F et al (2012) Situation and technology progress of lead smelting in china. Adv Mat Res 581–582:904–911
Gao F, Huang Z, Li H, et al (2021) Recovery of magnesium from ferronickel slag to prepare hydrated magnesium sulfate by hydrometallurgy method. J Clean Prod 303. https://doi.org/10.1016/j.jclepro.2021.127049
Gao Y, Guo T, Li Z, et al (2022) Mechanism of retarder on hydration process and mechanical properties of red mud-based geopolymer cementitious materials. Constr Build Mater 356. https://doi.org/10.1016/j.conbuildmat.2022.129306
Gorai B, Jana RK, Premchand (2003) Characteristics and utilisation of copper slag - A review. Resour Conserv Recycl 39:299–313. https://doi.org/10.1016/S0921-3449(02)00171-4
Goronovski A, Vind J, Vassiliadou V et al (2019) Radiological assessment of the Bayer process. Miner Eng 137:250–258. https://doi.org/10.1016/j.mineng.2019.04.016
Gu Q, Wang T, Wu W, et al (2021) Influence of pretreatments on accelerated dry carbonation of MSWI fly ash under medium temperatures. Chem Eng J 414. https://doi.org/10.1016/j.cej.2021.128756
Gürkan EH, Tibet Y, Çoruh S (2021) Application of full factorial design method for optimization of heavy metal release from lead smelting slag. Sustainability (Switzerland) 13. https://doi.org/10.3390/su13094890
Han YS, Ji S, Lee PK, Oh C (2017) Bauxite residue neutralization with simultaneous mineral carbonation using atmospheric CO2. J Hazard Mater 326:87–93. https://doi.org/10.1016/j.jhazmat.2016.12.020
Hartman M, Svoboda K, Pohořelý M, Šyc M (2013) Thermal Decomposition of Sodium Hydrogen Carbonate and Textural Features of Its Calcines. Ind Eng Chem Res 52:10619–10626. https://doi.org/10.1021/ie400896c
Hawker W, Vaughan J, Hayes PC, Jak E (2014) A synergisitc hydro-and pyro-metallurgical process for low-energy copper production Corrosion of Titanium View project Processing of high silica bauxite view project. In: Taylor A (ed) Proceedings of ALTA. pp 365–384
Hayes PC, Schlesinger ME, Steil H-U, Siegmund A (2010) Lead smelter survey. Lead Zinc 343–414
He L, Yu D, Lv W et al (2013) A Novel Method for CO 2 Sequestration via Indirect Carbonation of Coal Fly Ash. Ind Eng Chem Res 52:15138–15145. https://doi.org/10.1021/ie4023644
He Z, Hu X, Chou KC (2022) Synergetic modification of industrial basic oxygen furnace slag and copper slag for efficient iron recovery. Process Saf Environ Prot 165:487–495. https://doi.org/10.1016/j.psep.2022.07.044
Ho HJ, Iizuka A, Shibata E (2019) Carbon Capture and Utilization Technology without Carbon Dioxide Purification and Pressurization: A Review on Its Necessity and Available Technologies. Ind Eng Chem Res 58:8941–8954. https://doi.org/10.1021/acs.iecr.9b01213
Ho HJ, Iizuka A, Shibata E et al (2020) CO2utilization via direct aqueous carbonation of synthesized concrete fines under atmospheric pressure. ACS Omega 5:15877–15890. https://doi.org/10.1021/acsomega.0c00985
Ho H-J, Iizuka A, Kubo H (2022a) Direct aqueous carbonation of dephosphorization slag under mild conditions for CO2 sequestration and utilization: Exploration of new dephosphorization slag utilization. Environ Technol Innov 28:102905. https://doi.org/10.1016/j.eti.2022.102905
Ho HJ, Iizuka A, Shibata E (2021) Chemical recycling and use of various types of concrete waste: a review. J Clean Prod 284:124785. https://doi.org/10.1016/j.jclepro.2020.124785
Ho HJ, Iizuka A, Shibata E, et al (2021b) Utilization of CO2 in direct aqueous carbonation of concrete fines generated from aggregate recycling: Influences of the solid–liquid ratio and CO2 concentration. J Clean Prod 312. https://doi.org/10.1016/j.jclepro.2021.127832
Ho HJ, Iizuka A, Lee CH, Chen WS (2022) Mineral carbonation using alkaline waste and byproducts to reduce CO2 emissions in Taiwan. Environ Chem Lett 21(2):865–884. https://doi.org/10.1007/s10311-022-01518-6
Ho HJ, Iizuka A, Shibata E, Ojumu T (2022c) Circular indirect carbonation of coal fly ash for carbon dioxide capture and utilization. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.108269
Hoang J, Reuter MA, Matusewicz R et al (2009) Top submerged lance direct zinc smelting. Miner Eng 22:742–751. https://doi.org/10.1016/j.mineng.2008.12.014
Hu H, Deng Q, Li C et al (2014) The recovery of Zn and Pb and the manufacture of lightweight bricks from zinc smelting slag and clay. J Hazard Mater 271:220–227. https://doi.org/10.1016/j.jhazmat.2014.01.035
Hu J, Liu W, Wang L et al (2017) Indirect mineral carbonation of blast furnace slag with (NH4)2SO4 as a recyclable extractant. J Energy Chem 26:927–935. https://doi.org/10.1016/j.jechem.2017.06.009
Hua Y, Heal KV, Friesl-Hanl W (2017) The use of red mud as an immobiliser for metal/metalloid-contaminated soil: A review. J Hazard Mater 325:17–30
Huijgen WJJ, Comans RNJ (2006) Carbonation of steel slag for CO2 sequestration: Leaching of products and reaction mechanisms. Environ Sci Technol 40:2790–2796. https://doi.org/10.1021/es052534b
Huijgen WJJ, Witkamp GJ, Comans RNJ (2005) Mineral CO2 sequestration by steel slag carbonation. Environ Sci Technol 39:9676–9682. https://doi.org/10.1021/es050795f
Huijgen WJJ, Witkamp GJ, Comans RNJ (2006) Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem Eng Sci 61:4242–4251. https://doi.org/10.1016/j.ces.2006.01.048
Huntzinger DN, Gierke JS, Kawatra SK et al (2009) Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ Sci Technol 43:1986–1992. https://doi.org/10.1021/es802910z
Ibrahim MH, El-Naas MH, Benamor A et al (2019) Carbon mineralization by reaction with steel-making waste: a review. Processes 7(2):115. https://doi.org/10.3390/pr7020115
International Aluminium (2022a) Green house gas emissions-primary aluminium. https://international-aluminium.org/statistics/greenhouse-gas-emissions-intensity-primary-aluminium/. Accessed 24 March 2023
International Aluminium (2022b) Alumina production. https://international-aluminium.org/statistics/alumina-production/. Accessed 26 Oct 2022b
International Copper Study Group (2022) The World Copper Factbook 2022-ICSG 2022. https://icsg.org/copper-factbook/. Accessed 16 Nov 2022
International Energy Agency (2022) Total copper demand by sector and scenario, 2020–2040. https://www.iea.org/data-and-statistics/charts/total-copper-demand-by-sector-and-scenario-2020-2040. Accessed 26 Oct 2022
International Lead and Zinc Study Group (2022) Lead and Zinc Statistics. https://ilzsg.org/static/statistics.aspx?from=1. Accessed 15 Nov 2022
International Nickel Study Group (2022) Press release INSG April 2022 web meetings. https://insg.org/index.php/pressreleasesmain/. Accessed 16 Nov 2022
Ito H, Hayashi H, Sasaki H (2002) Rapid separation of oil particles from low-concentrated O/W emulsions in the presence of anionic surfactants using fibrous slag. J Colloid Interface Sci 252:214–221. https://doi.org/10.1006/jcis.2002.8445
Jones R, Hayman D, Denton G (1998) Recovery of cobalt, nickel, and copper from slags, using DC-arc furnace technology. Pyrometallurgy Division 2125:1–17
Kaikake A (2007) Recent ferronickel smelting operation at Hyuga Smelting Co. J MMIJ 123:686–688
Kapusta JPT (2004) JOM world nonferrous smelters survey, part I: Copper. JOM 56:21–27. https://doi.org/10.1007/s11837-004-0086-6
Kashefi K, Pardakhti A, Shafiepour M, Hemmati A (2020) Process optimization for integrated mineralization of carbon dioxide and metal recovery of red mud. J Environ Chem Eng 8:103638. https://doi.org/10.1016/j.jece.2019.103638
Katayama T (1992) Petrographic study on the potential alkali-reactivity of ferro-nickel slags for concrete aggregates. In: Proc. 9th intern. conf. alkali-aggregate reaction in concrete. London, pp 497–507
Katsiotis NS, Tsakiridis PE, Velissariou D et al (2015) Utilization of Ferronickel Slag as Additive in Portland Cement: A Hydration Leaching Study. Waste Biomass Valorization 6:177–189. https://doi.org/10.1007/s12649-015-9346-7
Keller V, Stopic S, Xakalashe B et al (2020) Effectiveness of fly ash and red mud as strategies for sustainable acid mine drainage management. Minerals 10:1–21. https://doi.org/10.3390/min10080707
Kemache N, Pasquier LC, Cecchi E et al (2017) Aqueous mineral carbonation for CO2 sequestration: From laboratory to pilot scale. Fuel Process Technol 166:209–216. https://doi.org/10.1016/j.fuproc.2017.06.005
Keskinkilic E (2019) Nickel laterite smelting processes and some examples of recent possible modifications to the conventional route. Metals 9(9):974. https://doi.org/10.3390/met9090974
Khairul MA, Zanganeh J, Moghtaderi B (2019) The composition, recycling and utilisation of Bayer red mud. Resour Conserv Recycl 141:483–498
Klauber C, Gräfe M, Power G (2011) Bauxite residue issues: II. options for residue utilization. Hydrometallurgy 108:11–32. https://doi.org/10.1016/j.hydromet.2011.02.007
Kojima T, Nagamine A, Ueno N, Uemiya S (1997) Absorption and fixation of carbon dioxide by rock weathering. Energy Convers Manag 38:S461–S466
Krevor SCM, Lackner KS (2011) Enhancing serpentine dissolution kinetics for mineral carbon dioxide sequestration. Int J Greenhouse Gas Control 5:1073–1080. https://doi.org/10.1016/j.ijggc.2011.01.006
Kumar N (2021) Copper slag characteristics and applications: A review. Asian J Multidimensional Res 10:381–386
Kuri JC, Nuruzzaman M, Sarker PK (2022) Sodium sulphate resistance of geopolymer mortar produced using ground ferronickel slag with fly ash. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.09.258
Kuri JC, Majhi S, Sarker PK, Mukherjee A (2021) Microstructural and non-destructive investigation of the effect of high temperature exposure on ground ferronickel slag blended fly ash geopolymer mortars. J Build Eng 43. https://doi.org/10.1016/j.jobe.2021.103099
Kusin FM, Hasan SNMS, Molahid VLM et al (2022) Carbon dioxide sequestration of iron ore mining waste under low-reaction condition of a direct mineral carbonation process. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-23677-3
Kwon S, Fan M, DaCosta HFM, Russell AG (2011) Factors affecting the direct mineralization of CO2 with olivine. J Environ Sci 23:1233–1239. https://doi.org/10.1016/S1001-0742(10)60555-4
Lackner KS, Wendt CH, Butt DP et al (1995) Carbon dioxide disposal in carbonate minerals. Energy 20:1153–1170
Larachi F, Daldoul I, Beaudoin G (2010) Fixation of CO2 by chrysotile in low-pressure dry and moist carbonation: Ex-situ and in-situ characterizations. Geochim Cosmochim Acta 74:3051–3075. https://doi.org/10.1016/j.gca.2010.03.007
Li J, Jacobs AD, Hitch M (2019) Direct aqueous carbonation on olivine at a CO2 partial pressure of 6.5 MPa. Energy 173:902–910. https://doi.org/10.1016/j.energy.2019.02.125
Li Y, Liu YM (2021) Progress and trend of bulk utilization technology of metallurgical solid wastes in China. Gongcheng Kexue Xuebao/Chinese J Eng 43:1713–1724
Li M, Mi Z, Coffman DM, Wei YM (2018) Assessing the policy impacts on non-ferrous metals industry’s CO2 reduction: Evidence from China. J Clean Prod 192:252–261. https://doi.org/10.1016/j.jclepro.2018.05.015
Li Z, Ma G, Zhang X et al (2021) Characteristics and chemical speciation of waste copper slag. Environ Sci Pollut Res 28:20012–20022. https://doi.org/10.1007/s11356-020-11830-9
Li J, Sun Z, Wang L, et al (2022a) Properties and mechanism of high-magnesium nickel slag-fly ash based geopolymer activated by phosphoric acid. Constr Build Mater 345. https://doi.org/10.1016/j.conbuildmat.2022.128256
Li L, Ling TC, Pan SY (2022b) Environmental benefit assessment of steel slag utilization and carbonation: a systematic review. Sci Total Environ 806:150280. https://doi.org/10.1016/j.scitotenv.2021.150280
Li M, Gao F, Sun B, et al (2022c) Zero carbon-emission technology route construction and multifactor analysis of aluminum production in China. J Clean Prod 370. https://doi.org/10.1016/j.jclepro.2022.133535
Li Y, Fang J, Cheng L, et al (2022d) Mechanical performance, hydration characteristics and microstructures of high volume blast furnace ferronickel slag cement mortar by wet grinding activation. Constr Build Mater 320. https://doi.org/10.1016/j.conbuildmat.2021.126148
Li W, Wang T, Zhu X (2022e) Clean dealkalization technology from aluminum industry hazardous tailings—red mud by displacement with Mg-based agent. Environ Sci Pollut Res 29:55957–55970. https://doi.org/10.1007/s11356-022-19754-2
Lim M, Han GC, Ahn JW, You KS (2010) Environmental remediation and conversion of carbon dioxide (CO2) into useful green products by accelerated carbonation technology. Int J Environ Res Public Health 7:203–228
Lin B, Wang X (2015) Carbon emissions from energy intensive industry in China: Evidence from the iron & steel industry. Renew Sustain Energy Rev 47:746–754
Liu J, Guo R (2020) Hydration properties of alkali-activated quick cooled copper slag and slow cooled copper slag. J Therm Anal Calorim 139:3383–3394. https://doi.org/10.1007/s10973-019-08708-5
Liu Y, Lin C, Wu Y (2007) Characterization of red mud derived from a combined Bayer Process and bauxite calcination method. J Hazard Mater 146:255–261. https://doi.org/10.1016/j.jhazmat.2006.12.015
Liu W, Su S, Xu K et al (2018) CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition. J Clean Prod 178:98–107. https://doi.org/10.1016/j.jclepro.2017.12.281
Liu W, Song L, Xu C (Charles) et al (2019) Combined synthesis of Li4SiO4 sorbent with high CO2 uptake in the indirect carbonation of blast furnace slag process. Chem Eng J 370:71–80. https://doi.org/10.1016/j.cej.2019.03.186
Liu W, Teng L, Rohani S et al (2021) CO2 mineral carbonation using industrial solid wastes: a review of recent developments. Chem Eng J 416:129093. https://doi.org/10.1016/j.cej.2021.129093
Lu T, Tikana L, Herrmann C, et al (2022) Environmental hotspot analysis of primary copper production in China and its future improvement potentials. J Clean Prod 370. https://doi.org/10.1016/j.jclepro.2022.133458
Luo Y, He D (2021) Research status and future challenge for CO2 sequestration by mineral carbonation strategy using iron and steel slag. Environ Sci Pollut Res 28:49383–49409
Ma S, Zhang Z, Liu X, et al (2022) Reuse of red mud in magnesium potassium phosphate cement: Reaction mechanism and performance optimization. J Build Eng 61. https://doi.org/10.1016/j.jobe.2022.105290
Madhavan N, Brooks G, Rhamdhani MA, Bordignon A (2022) Contribution of CO2 Emissions from Basic Oxygen Steelmaking Process. Metals (Basel) 12. https://doi.org/10.3390/met12050797
Mankhand T (2012) Recovery of valuable materials from aluminum dross. J Sustain Planet 3:86–94
Matsuo Y, Takebe H, Ohta Y, Morinaga K (1989) A Study on Cooling Condition and Structure of Ferro Nickel Slag. Shigen-to-Sozai 105:1067–1071
Meinshausen M, Lewis J, McGlade C et al (2022) Realization of Paris Agreement pledges may limit warming just below 2 °C. Nature 604:304–309. https://doi.org/10.1038/s41586-022-04553-z
Memary R, Giurco D, Mudd G, Mason L (2012) Life cycle assessment: A time-series analysis of copper. J Clean Prod 33:97–108. https://doi.org/10.1016/j.jclepro.2012.04.025
Meshram A, Singh KK (2018) Recovery of valuable products from hazardous aluminum dross: A review. Resour Conserv Recycl 130:95–108. https://doi.org/10.1016/j.resconrec.2017.11.026
Meyer NA, Vögeli JU, Becker M et al (2014) Mineral carbonation of PGM mine tailings for CO2 storage in South Africa: A case study. Miner Eng 59:45–51
Miao XW, Bai ZT, Lu GH et al (2020) Review of comprehensive utilization of typical ferroalloy slags. Gongcheng Kexue Xuebao/Chinese J Eng 42:663–679
Mishra B, Gostu S (2017) Materials sustainability for environment: Red-mud treatment. Front Chem Sci Eng 11:483–496
Mistry M, Gediga J, Boonzaier S (2016) Life cycle assessment of nickel products. Int J Life Cycle Assess 21:1559–1572. https://doi.org/10.1007/s11367-016-1085-x
Morrison C, Hooper R, Lardner K (2003) The use of ferro-silicate slag from ISF zinc production as a sand replacement in concrete. Cem Concr Res 33:2085–2089. https://doi.org/10.1016/S0008-8846(03)00234-5
Mosavinezhad SHG, Nabavi SE (2012) Effect of 30% Ground Granulated Blast Furnace, Lead and Zinc Slags as sand replacements on the strength of concrete. KSCE J Civ Eng 16:989–993. https://doi.org/10.1007/s12205-012-1240-2
Mukiza E, Zhang LL, Liu X, Zhang N (2019) Utilization of red mud in road base and subgrade materials: A review. Resour Conserv Recycl 141:187–199
Murai K, Yamamuro Y (1988) Utilizations of Ferro-Nickel Slag. Resour Process 35:42–48
Murari K, Siddique R, Jain KK (2015) Use of waste copper slag, a sustainable material. J Mater Cycles Waste Manag 17:13–26
Myers CA, Nakagaki T, Akutsu K (2019) Quantification of the CO2 mineralization potential of ironmaking and steelmaking slags under direct gas-solid reactions in flue gas. Int J Greenhouse Gas Control 87:100–111. https://doi.org/10.1016/j.ijggc.2019.05.021
Nikulshina V, Gálvez ME, Steinfeld A (2007) Kinetic analysis of the carbonation reactions for the capture of CO2 from air via the Ca(OH)2-CaCO3-CaO solar thermochemical cycle. Chem Eng J 129:75–83
Nilsson AE, Aragonés MM, Torralvo FA et al (2017) A review of the carbon footprint of Cu and Zn production from primary and secondary sources. Minerals 7(9):168. https://doi.org/10.3390/min7090168
Norgate T, Jahanshahi S (2011) Assessing the energy and greenhouse gas footprints of nickel laterite processing. Miner Eng 24:698–707. https://doi.org/10.1016/j.mineng.2010.10.002
Norgate TE, Jahanshahi S, Rankin WJ (2007) Assessing the environmental impact of metal production processes. J Clean Prod 15:838–848. https://doi.org/10.1016/j.jclepro.2006.06.018
Nuruzzaman M, Kuri JC, Sarker PK (2022) Strength, permeability and microstructure of self-compacting concrete with the dual use of ferronickel slag as fine aggregate and supplementary binder. Constr Build Mater 318. https://doi.org/10.1016/j.conbuildmat.2021.125927
O’Connor WK, Dahlin DC, Rush GE et al (2002) Carbon dioxide sequestration by direct mineral carbonation: process mineralogy of feed and products. Min Metall Explor 19:95–101. https://doi.org/10.1007/BF03403262
Olajire AA (2013) A review of mineral carbonation technology in sequestration of CO2. J Pet Sci Eng 109:364–392
Orth A, Anastasijevic N, Eichberger H (2007) Low CO2 emission technologies for iron and steelmaking as well as titania slag production. Miner Eng 20:854–861. https://doi.org/10.1016/j.mineng.2007.02.007
Ostap S (1986) Control of silica in the bayer process used for alumina production. Can Metall Q 25:101–106. https://doi.org/10.1179/cmq.1986.25.2.101
Pan SY, Chang EE, Chiang PC (2012) CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual Res 12:770–791. https://doi.org/10.4209/aaqr.2012.06.0149
Pan D, Li L, Tian X et al (2019) A review on lead slag generation, characteristics, and utilization. Resour Conserv Recycl 146:140–155
Pardo N, Moya JA (2013) Prospective scenarios on energy efficiency and CO2 emissions in the European Iron & Steel industry. Energy 54:113–128. https://doi.org/10.1016/j.energy.2013.03.015
Pasquier LC, Mercier G, Blais JF et al (2014) Parameters optimization for direct flue gas CO2 capture and sequestration by aqueous mineral carbonation using activated serpentinite based mining residue. Applied Geochemistry 50:66–73. https://doi.org/10.1016/j.apgeochem.2014.08.008
Pei D, Li Y, Hua S, et al (2021) In situ XRD study on function mechanism of pyroxene and anorthite in Si-Ca ceramics from ferronickel slag. Mater Lett 305. https://doi.org/10.1016/j.matlet.2021.130839
Pei J, Pan X, Qi Y, et al (2022) Preparation of ultra-lightweight ceramsite from red mud and immobilization of hazardous elements. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.108157
Penpolcharoen M (2005) Utilization of secondary lead slag as construction material. Cem Concr Res 35:1050–1055. https://doi.org/10.1016/j.cemconres.2004.11.001
Perederiy I, Papangelakis VG (2017) Why amorphous FeO-SiO2 slags do not acid-leach at high temperatures. J Hazard Mater 321:737–744
Pérez-Moreno SM, Gázquez MJ, Bolívar JP (2015) CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments. Chem Eng J 262:737–746. https://doi.org/10.1016/j.cej.2014.10.023
Phiri TC, Singh P, Nikoloski AN (2021) The potential for copper slag waste as a resource for a circular economy: a review – part II. Miner Eng 172:107150. https://doi.org/10.1016/j.mineng.2021.107150
Piatak NM, Parsons MB, Seal RR (2015) Characteristics and environmental aspects of slag: A review. Appl Geochem 57:236–266
Pintowantoro S, Abdul F (2019) Selective reduction of laterite nickel ore. Mater Trans 60:2245–2254. https://doi.org/10.2320/matertrans.MT-M2019101
Pintowantoro S, Panggabean PC, Setiyorini Y, Abdul F (2022) Smelting and Selective Reduction of Limonitic Laterite Ore in Mini Blast Furnace. J Inst Eng (India) Series D. https://doi.org/10.1007/s40033-022-00348-8
Potysz A, van Hullebusch ED, Kierczak J (2018) Perspectives regarding the use of metallurgical slags as secondary metal resources – A review of bioleaching approaches. J Environ Manag 219:138–152
Prasad PS, Ramana GV (2016) Imperial smelting furnace (zinc) slag as a structural fill in reinforced soil structures. Geotext Geomembr 44:406–428. https://doi.org/10.1016/j.geotexmem.2016.01.009
Prasetyo AB, Khaerul A, Mayangsari W et al (2021) Magnesium Extraction of Ferronickel Slag Processed by Alkali Fusion and Hydrochloric Acid Leaching. J Min MetallSect B 57:225–233. https://doi.org/10.2298/JMMB200224018P
Qi C, Ye L, Ma X et al (2017) Life cycle assessment of the hydrometallurgical zinc production chain in China. J Clean Prod 156:451–458. https://doi.org/10.1016/j.jclepro.2017.04.084
Ragipani R, Bhattacharya S, Suresh AK (2021) A review on steel slag valorisation: Via mineral carbonation. React Chem Eng 6:1152–1178
Rao M, Li G, Jiang T et al (2013) Carbothermic reduction of nickeliferous laterite ores for nickel pig iron production in China: A review. JOM 65:1573–1583. https://doi.org/10.1007/s11837-013-0760-7
Rashid MI, Benhelal E, Anderberg L et al (2022) Aqueous carbonation of peridotites for carbon utilisation: a critical review. Environ Sci Pollut Res 29(50):75161–75183. https://doi.org/10.1007/s11356-022-23116-3
Reddy KR, Gopakumar A, Chetri JK (2019) Critical review of applications of iron and steel slags for carbon sequestration and environmental remediation. Rev Environ Sci Biotechnol 18:127–152
Renforth P (2019) The negative emission potential of alkaline materials. Nat Commun 10. https://doi.org/10.1038/s41467-019-09475-5
Reynes JF, Mercier G, Blais JF, Pasquier LC (2021) Feasibility of a mineral carbonation technique using iron-silicate mining waste by direct flue gas co2 capture and cation complexation using 2,2′-bipyridine. Minerals 11. https://doi.org/10.3390/min11040343
Ritchie Hannah, Roser Max, Rosado Pablo (2020) CO2 and Greenhouse Gas Emissions. https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions. Accessed 5 Oct 2022
Rubinos DA, Spagnoli G (2019) Assessment of red mud as sorptive landfill liner for the retention of arsenic (V). J Environ Manag 232:271–285. https://doi.org/10.1016/j.jenvman.2018.09.041
Rushendra Revathy TD, Palanivelu K, Ramachandran A (2016) Direct mineral carbonation of steelmaking slag for CO2 sequestration at room temperature. Environ Sci Pollut Res 23:7349–7359. https://doi.org/10.1007/s11356-015-5893-5
Rushendra Revathy TD, Ramachandran A, Palanivelu K (2021) Sequestration of CO2 by red mud with flue gas using response surface methodology. Carbon Manag 12:139–151. https://doi.org/10.1080/17583004.2021.1893127
Saha AK, Khan MNN, Sarker PK (2018) Value added utilization of by-product electric furnace ferronickel slag as construction materials: A review. Resour Conserv Recycl 134:10–24
Samal S, Ray AK, Bandopadhyay A (2013) Proposal for resources, utilization and processes of red mud in India — A review. Int J Miner Process 118:43–55. https://doi.org/10.1016/j.minpro.2012.11.001
Santos RM, van Audenaerde A, Chiang YW et al (2015) Nickel extraction from olivine: Effect of carbonation pre-treatment. Metals (basel) 5:1620–1644. https://doi.org/10.3390/met5031620
Sanwani E, Jeremy E, Chaerun SK et al (2022) Use of Mixotrophic Bacteria as Flocculating Agents to Separate Iron from Red Mud (Alumina Refinery Residue). J Sustain Metall 8:443–457. https://doi.org/10.1007/s40831-021-00479-4
Sas Z, Sha W, Soutsos M et al (2019) Radiological characterisation of alkali-activated construction materials containing red mud, fly ash and ground granulated blast-furnace slag. Sci Total Environ 659:1496–1504. https://doi.org/10.1016/j.scitotenv.2019.01.006
Sasaki S, Sato T, Kawasaki K, Takayanagi K (2013) 佐 々 木 正 司 1 佐 藤 努 2 川 崎 康 一 3 高 柳 和 弘 4 フェロニッケルスラグの土壌改良材への応用に関する研究 *-冷却速度やホタテ貝殻 の混合割合と溶解挙動の結晶化学的関係-application of ferro nickel slag to soil improvement agent-relationship of mixing ratio of scallop shell and cooling rate with dissolution behavior of the ferro nickel slag-29. J MMIJ 129(1):29–35. https://doi.org/10.2473/journalofmmij.129.29
Schlögl S, Diendorfer P, Baldermann A, Vollprecht D (2022) Use of industrial residues for heavy metals immobilization in contaminated site remediation: a brief review. Int J Environ Sci Technol 20(2):2313–2326. https://doi.org/10.1007/s13762-022-04184-x
Seignez N, Gauthier A, Bulteel D et al (2007) Effect of Pb-rich and Fe-rich entities during alteration of a partially vitrified metallurgical waste. J Hazard Mater 149:418–431. https://doi.org/10.1016/j.jhazmat.2007.04.007
Selamat SN, Nor NHM, Rashid MHA, et al (2017) Review of CO2 reduction technologies using mineral carbonation of iron and steel making slag in Malaysia. In: Journal of Physics: Conference Series. IOP Publishing, vol 914, no 1, p 012012. https://doi.org/10.1088/1742-6596/914/1/012012
Sglavo VM, Campostrini R, Maurina S, et al (2000) Bauxitèred mud’ in the ceramic industry. Part 1: thermal behaviour. J Eur Ceram Soc 20(3):235–244. https://doi.org/10.1016/S0955-2219(99)00088-6
Shang W, Peng Z, Xu F et al (2021) Preparation of enstatite-spinel based glass-ceramics by co-utilization of ferronickel slag and coal fly ash. Ceram Int 47:29400–29409. https://doi.org/10.1016/j.ceramint.2021.07.108
Shaojun Y, Yunhan X (2008) Steam catalysis in CaO carbonation under low steam partial pressure. Ind Eng Chem Res 47:4043–4048. https://doi.org/10.1021/ie8000265
Sharifi Y, Afshoon I, Nematollahzade M et al (2020b) Effect of copper slag on the resistance characteristics of SCC exposed to the acidic environment. Asian J Civil Eng 21:597–609. https://doi.org/10.1007/s42107-019-00218-x
Sharifi Y, Afshoon I, Asad-Abadi S, Aslani F (2020a) Environmental protection by using waste copper slag as a coarse aggregate in self-compacting concrete. J Environ Manag 271. https://doi.org/10.1016/j.jenvman.2020.111013
Sheikh E, Mousavi SR, Afshoon I (2022) Producing green Roller Compacted Concrete (RCC) using fine copper slag aggregates. J Clean Prod 368. https://doi.org/10.1016/j.jclepro.2022.133005
Shi C, Meyer C, Behnood A (2008) Utilization of copper slag in cement and concrete. Resour Conserv Recycl 52:1115–1120
Shin S, Kim M-J (2022) Utilization of residual by-products remaining after indirect carbonation: Zeolite synthesis and conversion mechanism. J Environ Chem Eng 10:108599. https://doi.org/10.1016/j.jece.2022.108599
Singh J, Singh SP (2019) Geopolymerization of solid waste of non-ferrous metallurgy – A review. J Environ Manag 251. https://doi.org/10.1016/j.jenvman.2019.109571
Snæbjörnsdóttir SÓ, Sigfússon B, Marieni C (2020) Carbon dioxide storage through mineral carbonation. Nat Rev Earth Environ 1:90–102
Song H-Y, Seo J-B, Kang S-K et al (2014) CO 2 Fixation by Magnesium Hydroxide from Ferro-Nickel Slag. Clean Technol 20:42–50. https://doi.org/10.7464/ksct.2014.20.1.042
Song S, Sun W, Wang L, et al (2019) Recovery of cobalt and zinc from the leaching solution of zinc smelting slag. J Environ Chem Eng 7. https://doi.org/10.1016/j.jece.2018.11.022
Song Y, Wu W, Xu L, et al (2020) A critical review on generation, characteristics, and utilization of zinc slag. In: Siegmund A, Alam S, Grogan J, et al. (eds) PbZn 2020: 9th international symposium on lead and zinc Processing. The Minerals, Metals & Materials Series. Springer, Cham, pp 275–281. https://doi.org/10.1007/978-3-030-37070-1_23
Song Y, Dong M, Wang Z, et al (2022) Effects of red mud on workability and mechanical properties of autoclaved aerated concrete (AAC). J Build Eng 61. https://doi.org/10.1016/j.jobe.2022.105238
Srivastava S, Snellings R, Nielsen P, Cool P (2022) Accelerated carbonation of ferrous and non-ferrous slags to produce clinker-free carbonate-bonded blocks: Synergetic reduction in environmental leaching. Constr Build Mater 348:128630. https://doi.org/10.1016/j.conbuildmat.2022.128630
Srivastava S, Snellings R, Nielsen P, Cool P (2022a) Insights into CO2-mineralization using non-ferrous metallurgy slags: CO2(g)-induced dissolution behavior of copper and lead slags. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.107338
Statista (2022) The largest zinc smelters worldwide in 2018, based on production output. https://www.statista.com/statistics/240622/leadig-zinc-smelters-worldwide/. Accessed 15 Nov 2022
Steinour H (1959) Some effects of carbon dioxide on mortars and concrete-discussion. J Am Concr Inst 30:905–907
Štulović M, Radovanović D, Kamberović Ž, Korać M, Anđić Z (2019) Assessment of leaching characteristics of solidified products containing secondary alkaline lead slag. Int J Environ Res Public Health 16(11):2005
Sun Y, Yao MS, Zhang JP, Yang G (2011) Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution. Chem Eng J 173:437–445. https://doi.org/10.1016/j.cej.2011.08.002
Sun W, Li X, Liu R et al (2021) Recovery of Valuable Metals from Nickel Smelting Slag Based on Reduction and Sulfurization Modification. Minerals 11:1022. https://doi.org/10.3390/min11091022
Sushil S, Batra VS (2008) Catalytic applications of red mud, an aluminium industry waste: A review. Appl Catal B 81:64–77
Szépvölgyi J (2011) A chemical engineer’s view of the red mud disaster. Nachrichten aus der Chemie 59(5):V–VII
Taneez M, Hurel C (2019) A review on the potential uses of red mud as amendment for pollution control in environmental media. Environ Sci Pollut Res 26:22106–22125
Tang H, Peng Z, Shang W, et al (2022) Preparation of refractory materials from electric furnace ferronickel slag and blast furnace ferronickel slag: A comparison. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.107929
Tao M, Zhang X, Wang S, et al (2019) Life cycle assessment on lead–zinc ore mining and beneficiation in China. J Clean Prod 237. https://doi.org/10.1016/j.jclepro.2019.117833
Teir Sebastian and Picaset (2008) Fixation of carbon dioxide by producing carbonates from minerals and steelmaking slags. Helsinki Univ Technol. Teknillinen korkeakoulu. https://aaltodoc.aalto.fi/handle/123456789/4489
Tian S, Jiang J, Chen X et al (2013) Direct gas-solid carbonation kinetics of steel slag and the contribution to insitu sequestration of flue gas CO2 in steel-making plants. Chemsuschem 6:2348–2355. https://doi.org/10.1002/cssc.201300436
Tian H, Guo Z, Pan J, et al (2021) Comprehensive review on metallurgical recycling and cleaning of copper slag. Resour Conserv Recycl 168. https://doi.org/10.1016/j.jhazmat.2020.124420
Tong Z, Ma G, Zhou D et al (2019) The Indirect Mineral Carbonation of Electric Arc Furnace Slag Under Microwave Irradiation. Sci Rep 9:7676. https://doi.org/10.1038/s41598-019-44162-x
Tripathi B, Chaudhary S (2016) Performance based evaluation of ISF slag as a substitute of natural sand in concrete. J Clean Prod 112:672–683. https://doi.org/10.1016/j.jclepro.2015.07.120
Tripathi B, Misra A, Chaudhary S (2013) Strength and Abrasion Characteristics of ISF Slag Concretehttps://doi.org/10.1061/(ASCE)MT.1943-5533
Turri L, Gérardin K, Muhr H et al (2019) CO2 sequestration by carbonation of olivine: a new process for optimal separation of the solids produced. Green Process Synth 8:480–487. https://doi.org/10.1515/gps-2019-0016
Uibu M, Kuusik R, Andreas L, Kirsimäe K (2011) The CO2-binding by Ca-Mg-silicates in direct aqueous carbonation of oil shale ash and steel slag. In: Energy Procedia. Elsevier Ltd, pp 925–932. https://doi.org/10.1016/j.egypro.2011.01.138
Ukwattage NL, Ranjith PG, Li X (2017) Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement (lond) 97:15–22. https://doi.org/10.1016/j.measurement.2016.10.057
United Nations Framework Convention on Climate Change (2021) Glasgow Climate Pact. https://unfccc.int/documents/310475. Accessed 15 Nov 2022
United States Geological Survey (2022) Mineral year book-Copper. httpswww.usgs.govcentersnational-minerals-information-centercopper-statistics-and-information. Accessed 26 Oct 2022
van Genderen E, Wildnauer M, Santero N, Sidi N (2016) A global life cycle assessment for primary zinc production. Int J Life Cycle Assess 21:1580–1593. https://doi.org/10.1007/s11367-016-1131-8
Veetil SP, Pasquier LC, Blais JF et al (2015) Direct gas–solid carbonation of serpentinite residues in the absence and presence of water vapor: a feasibility study for carbon dioxide sequestration. Environ Sci Pollut Res 22:13486–13495. https://doi.org/10.1007/s11356-015-4580-x
Wang S, Ang HM, Tadé MO (2008) Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 72:1621–1635
Wang J, Zhong M, Wu P et al (2021a) A Review of the Application of Steel Slag in CO2 Fixation. ChemBioEng Rev 8:189–199
Wang F, Dreisinger DB, Jarvis M, Hitchins T (2018) The technology of CO2 sequestration by mineral carbonation: current status and future prospects. Canadian Metallurgical Quarterly 57:46–58
Wang F, Dreisinger D (2023) An integrated process of CO2 mineralization and selective nickel and cobalt recovery from olivine and laterites. Chem Eng J 451. https://doi.org/10.1016/j.cej.2022.139002
Wang M, Liu X (2021) Applications of red mud as an environmental remediation material: A review. J Hazard Mater 408
Wang L, Sun N, Tang H, Sun W (2019) A review on comprehensive utilization of red mud and prospect analysis. Minerals 9
Wang R, Shi Q, Li Y, et al (2021b) A critical review on the use of copper slag (CS) as a substitute constituent in concrete. Constr Build Mater 292. https://doi.org/10.1016/j.jclepro.2020.125136
Wang S, Jin H, Deng Y, Xiao Y (2021c) Comprehensive utilization status of red mud in China: A critical review. J Clean Prod 289
Wang Q, Ma H, Liu M, et al (2022) A new method of full resource utilization of copper slag. Hydrometallurgy 212. https://doi.org/10.1016/j.hydromet.2022.105899
Warner A, Diaz C, Dalvi A et al (2006) JOM World Nonferrous Smelter Survey, Part III: Nickel: Laterite. JOM 58:11–20
Watanabe T, Ono S, Arai H, Matsumori T (1987) Direct reduction of garnierite ore for production of ferro-nickel with a rotary Kiln at Nippon Yakin Kogyo Co. Ltd., Oheyama works. Int J Miner Process 19(1–4):173–187. https://doi.org/10.1016/0301-7516(87)90039-1
Wei D, Jun-Hui X, Yang P et al (2022) Extraction of Scandium and Iron from Red Mud. Miner Process Extr Metall Rev 43:61–68. https://doi.org/10.1080/08827508.2020.1833195
Wei W, Samuelsson PB, Tilliander A, et al (2020) Energy consumption and greenhouse gas emissions of nickel products. Energies (Basel) 13. https://doi.org/10.3390/en13215664
Wei-Hua G, Chun-Hua Y, Xiao-Fang C, Ya-Lin W (2013) Modeling and optimization problems and challenges arising in nonferrous metallurgical processes. Acta Automatica Sinica 39:197–207
Wu X, Gu F, Su C et al (2022) Preparing high-strength ceramsite from ferronickel slag and municipal solid waste incineration fly ash. Ceram Int. https://doi.org/10.1016/j.ceramint.2022.07.357
Xiao YD, Jin HX, Wang ML, Guo YL (2022) Collaborative Utilization Status of Red Mud and Phosphogypsum: A Review. J Sustain Metall. https://doi.org/10.1007/s40831-022-00569-x
Yadav S, Mehra A (2021) A review on ex situ mineral carbonationhttps://doi.org/10.1007/s11356-020-12049-4/Published
Yamamoto Y, Akiyama A (1988) Popout of concrete incorporating ferro-nickel slags. Doboku Gakkai Ronbunshu 390:171–178
Yan H, Zhang J, Zhao Y, Zheng C (2013) CO2 Sequestration from flue gas by direct aqueous mineral carbonation of wollastonite. Sci China Technol Sci 56:2219–2227. https://doi.org/10.1007/s11431-013-5318-y
Yan H, Zhang J, Zhao Y et al (2015) CO2 Sequestration by direct aqueous mineral carbonation under low-medium pressure conditions. Journal of Chemical Engineering of Japan 48:937–946. https://doi.org/10.1252/jcej.14we381
Yang Y, Guo YQ, Zhu WS, Huang JB (2019) Environmental impact assessment of China’s primary aluminum based on life cycle assessment. Trans Nonferrous Met Soc China (English Edition) 29:1784–1792. https://doi.org/10.1016/S1003-6326(19)65086-7
Yang L, Peng Z, Huang Y et al (2021) Co-utilization of ferronickel slag and fly ash cenosphere for production of superior thermal insulation materials. Ceram Int 47:10019–10026. https://doi.org/10.1016/j.ceramint.2020.12.148
Yanjia W, Chandler W (2010) The Chinese nonferrous metals industry—energy use and CO2 emissions. Energy Policy 38:6475–6484. https://doi.org/10.1016/j.enpol.2009.03.054
Yao L, Liu D, Ke Y, et al (2020) Synthesis and hydration characteristic of geopolymer based on lead smelting slag. Int J Environ Res Public Health 17. https://doi.org/10.3390/ijerph17082762
Yaswanth KK, Revathy J, Gajalakshmi P (2022) Influence of copper slag on Mechanical, durability and microstructural properties of GGBS and RHA blended strain hardening geopolymer composites. Constr Build Mater 342. https://doi.org/10.1016/j.conbuildmat.2022.128042
Yin NH, Sivry Y, Guyot F et al (2016) Evaluation on chemical stability of lead blast furnace (LBF) and imperial smelting furnace (ISF) slags. J Environ Manag 180:310–323. https://doi.org/10.1016/j.jenvman.2016.05.052
You N, Li B, Cao R, et al (2019) The influence of steel slag and ferronickel slag on the properties of alkali-activated slag mortar. Constr Build Mater 227. https://doi.org/10.1016/j.conbuildmat.2019.07.340
Zeng H, Lyu F, Sun W et al (2020) Progress on the industrial applications of red mud with a focus on China. Minerals 10:1–28
Zhang S, Rezaie HR, Sarpoolaky H, Lee WE (2000) Alumina dissolution into silicate slag. J Am Ceram Soc 83:897–903
Zhang J, Zhang R, Geerlings H, Bi J (2010) A novel indirect wollastonite carbonation route for co2 sequestration. Chem Eng Technol 33:1177–1183. https://doi.org/10.1002/ceat.201000024
Zhang R, Zheng S, Ma S, Zhang Y (2011) Recovery of alumina and alkali in Bayer red mud by the formation of andradite-grossular hydrogarnet in hydrothermal process. J Hazard Mater 189:827–835. https://doi.org/10.1016/j.jhazmat.2011.03.004
Zhang J, Zhang R, Geerlings H, Bi J (2012) Mg-silicate carbonation based on an HCl- and NH 3-Recyclable Process: Effect of Carbonation Temperature. Chem Eng Technol 35:525–531. https://doi.org/10.1002/ceat.201100425
Zhang W, Li H, Chen B et al (2015) CO2 emission and mitigation potential estimations of China’s primary aluminum industry. J Clean Prod 103:863–872. https://doi.org/10.1016/j.jclepro.2014.07.066
Zhang J, Tian X, Chen W, et al (2022a) Measuring environmental impacts from primary and secondary copper production under the upgraded technologies in key Chinese enterprises. Environ Impact Assess Rev 96. https://doi.org/10.1016/j.eiar.2022.106855
Zhang Y, Zhang Y, Zhu H, et al (2022b) Life cycle assessment of pollutants and emission reduction strategies based on the energy structure of the nonferrous metal industry in China. Energy 261. https://doi.org/10.1016/j.energy.2022.125148
Zhang Z, Wang Q, Huang Z (2022c) Value-added utilization of copper slag to enhance the performance of magnesium potassium phosphate cement. Resour Conserv Recycl 180. https://doi.org/10.1016/j.resconrec.2022.106212
Zhao Q, Liu C, Mei X et al (2022a) Research progress of steel slag-based carbon sequestration. Fundam Res. https://doi.org/10.1016/j.fmre.2022.09.023
Zhao Z, Liu W, Jiang Y, et al (2022b) Solidification of heavy metals in lead smelting slag and development of cementitious materials. J Clean Prod 359. https://doi.org/10.1016/j.jclepro.2022.132134
Zhou Y, Wang L, Xiao T et al (2020) Legacy of multiple heavy metal (loid) s contamination and ecological risks in farmland soils from a historical artisanal zinc smelting area. Sci Total Environ 720:137541
Zhu S, Gao C, Song K, et al (2022) An assessment of environmental impacts and economic benefits of multiple aluminum production methods. J Clean Prod 370. https://doi.org/10.1016/j.jclepro.2022.133523
Zolotova ES, Ivanova NS, Ryabinin VF et al (2021) Element mobility from the copper smelting slag recycling waste into forest soils of the taiga in Middle Urals. Environ Sci Pollut Res 28:1141–1150. https://doi.org/10.1007/s11356-020-10577-7
Zulhan Z, Agustina N (2021) A novel utilization of ferronickel slag as a source of magnesium metal and ferroalloy production. J Clean Prod 292. https://doi.org/10.1016/j.jclepro.2020.125307
Zuo Z, Feng Y, Dong X et al (2022) Energy absorption characteristics and kinetics of carbonaceous solid waste gasification with copper slag as heat carrier. Int J Hydrogen Energy 47:20076–20086. https://doi.org/10.1016/j.ijhydene.2022.04.116
Acknowledgements
We thank Gabrielle David, PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Fakhreza Abdul contributed to conceptualization, methodology, formal analysis, investigation, data curation, and visualization. Atsushi Iizuka contributed to conceptualization, methodology, investigation, supervision, and writing—review and editing. Hsing-Jung Ho contributed to conceptualization, investigation, and writing—review and editing. Ken Adachi contributed to supervision and writing—review and editing. Etsuro Shibata contributed to conceptualization, investigation, supervision, and writing—review and editing. The first draft of the manuscript was written by Fakhreza Abdul, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ioannis A. Katsoyiannis
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdul, F., Iizuka, A., Ho, HJ. et al. Potential of major by-products from non-ferrous metal industries for CO2 emission reduction by mineral carbonation: a review. Environ Sci Pollut Res 30, 78041–78074 (2023). https://doi.org/10.1007/s11356-023-27898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27898-y