Abstract
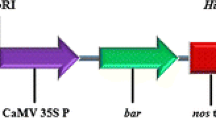
We have developed an improved Agrobacterium-mediated transformation and rapid regeneration system for four cultivars (‘CO(Ra)-14’, ‘PR-202’, ‘Try-1’ and ‘Paiyur-2’) of finger millet using optimized transformation and direct plant regeneration conditions. The shoot apical meristems (SAMs) were used as explants in this study. Agrobacterium strain EHA105 carrying binary vector pCAMBIA1301 was used to optimize the transformation conditions. Concentration of hygromycin, the optical density of the culture, infection time, age of the explants, co-cultivation period, the concentrations of acetosyringone and antibiotics were optimized to improve the transformation frequency. The highest frequency of mean transient gus expression (85.1%) was achieved in cultivar ‘CO(Ra)-14’. The entire transformation procedure, from initiating SAMs to planting putative transgenic plantlets in the greenhouse, was completed within 45 days with the highest stable transformation frequency of 11.8% for ‘CO(Ra)-14’. PCR, gus staining and Southern blot analyses were performed in T0 and T1 generations to confirm the gene integration. Six events from T0 had a single copy of the transgene and showed a normal Mendelian pattern of segregation. To our knowledge, this is the first report on the high frequency transformation of finger millet by Agrobacterium and subsequent recovery of transgenic plants via direct plant regeneration without a callus phase, in short duration (45 days). The proposed protocol could be supportive in breaking through the bottleneck in transformation and regeneration of finger millet cultivars.






Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- AS:
-
Acetosyringone
- BAP:
-
6-Benzylaminopurine
- CEH:
-
Casein enzymichydrolysate
- CTAB:
-
N-Cetyl-N,N,N-trimethylammonium bromide
- gusA :
-
β-Glucuronidase
- hpt :
-
Hygromycin phosphotransferase
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog
- nos :
-
Nopaline synthase
- npt :
-
Neomycin phosphotransferase
- RH:
-
Relative humidity
- SAMs:
-
Shoot apical meristems
- X-gluc:
-
5-Bromo-4-chloro-3-indolyl-β-d-glucuronide
- YEP:
-
Yeast-peptone-NaCl.
References
Arockiasamy S, Ignacimuthu S (2007) Regeneration of transgenic plants from two indica rice (Oryza sativa L.) cultivars using shoot apex explants. Plant Cell Rep 26:1745–1753
Arvinth S, Arun S, Selvakesavan RK, Srikanth J, Mukunthan N, Kumar PA, Premachandran MN, Subramonian N (2010) Genetic transformation and pyramiding of aprotinin-expressing sugarcane with cry1Ab for shoot borer (Chilo infuscatellus) resistance. Plant Cell Rep 29:383–395
Babu AG, Geetha KN, Manjunatha V, Shankar AG (2012) An efficient high throughput plant regeneration and transformation protocol for production of transgenics tolerant to salt in finger millet. Int J Forestry Crop Improv 3:16–20
Barton MK (2010) Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamo. Developmental Biol 341:95–113
Baskaran P, Dasgupta I (2012) Gene delivery using microinjection of Agrobacterium to embryonic shoot apical meristem of elite indica rice cultivars. J Plant Biochem Biotechnol 21:268–274
Bayer GY, Yemets AI, Blume YB (2014) Obtaining the transgenic lines of finger millet Eleusine coracana (L.) Gaertn. with dinitroaniline resistance. Cytology Genetics 48:139–144
Bilang R, Zhang S, Leduc N, Iglesias VA, Gisel A, Simmonds J, Potrykus I, Sautter C (1993) Transient gene expression in vegetative shoot apical meristems of wheat after ballistic microtargetting. Plant J 4:735–744
Ceasar SA, Ignacimuthu S (2008) Efficient somatic embryogenesis and plant regeneration from shoot apex explants of different Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol Plant 44:427–435
Ceasar SA, Ignacimuthu S (2009) Genetic engineering of millets: current status and future prospects. Biotechnol Lett 31:779–788
Ceasar SA, Ignacimuthu S (2010) Effects of cytokinins, carbohydrates and amino acids on induction and maturation of somatic embryos in kodo millet (Paspalum scorbiculatum Linn.). Plant Cell Tiss Organ Cult 102:153–162
Ceasar SA, Ignacimuthu S (2011) Agrobacterium-mediated transformation of finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants. Plant Cell Rep 30:1759–1770
Ceasar SA, Ignacimuthu S (2015) Finger millet [Eleusine coracana (L.) Gaertn.]. In: Wang K (ed) Agrobacterium Protocols, vol 1. Part of the Methods in Molecular Biology book series (MIMB, vol 1223). Springer, New York, pp 135–142
Chandrashekar A (2010) Finger millet: Eleusine coracana Chap. 6. Adv Food Nutri Res 59:215–262
Cho MJ, Choi HW, Okamoto D, Zhang S, Lemaux PG (2003) Expression of green fluorescent protein and its inheritance in transgenic oat plants generated from shoot meristematic cultures. Plant Cell Rep 21:467–474
Cho M, Wu E, Kwan J, Yu M, Banh J, Linn W, Anand A, Li Z, TeRonde S, Register JC III, Jones TJ, Zhao Z (2014) Agrobacterium-mediated high-frequency transformation of an elite commercial maize (Zea mays L.) inbred line. Plant Cell Rep 33:1767–1777
Devi P, Sticklen M (2002) Culturing shoot-tip clumps of pearl millet [Pennisetum glaucum (L.) R. Br.] and optimal microprojectile bombardment parameters for transient expression. Euphytica 125:45–50
Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB (2014) Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol 51:1021–1040
Dey M, Bakshi S, Galiba G, Sahoo L, Panda SK (2012) Development of a genotype independent and transformation amenable regeneration system from shoot apex in rice (Oryza sativa spp. Indica) using TDZ. 3. Biotech 2:233–240
Do PT, Lee H, Mookkan M, Folk WR, Zhang ZJ (2016) Rapid and efficient Agrobacterium-mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar gene as a selectable marker. Plant Cell Rep 35:2065–2076
Dosad S, Chawla HS (2016) In vitro plant regeneration and transformation studies in millets: current status and future prospects. Indian J Plant Physiol 21:239–254
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Gupta P, Raghuvanshi S, Tyagi AK (2001) Assessment of the efficiency of various gene promoters via biolistics in leaf and regenerating seed callus of millets, Eleusine coracana and Echinochloa crusgalli. Plant Biotechnol 18:275–282
Hema R, Vemanna RS, Sreeramulu S, Reddy CP, Senthil-Kumar M, Udayakumar M (2014) Stable expression of mtlD gene imparts multiple stress tolerance in finger millet. PLoS ONE 9(6):e99110
Ignacimuthu S, Ceasar SA (2012) Development of transgenic finger millet (Eleusine coracana (L.) Gaertn.) resistant to leaf blast disease. J Biosci 37:1–13
Jagga-Chugh SJ, Kachhwaha S, Sharma M, Kothari-Chajer A, Kothari SL (2012) Optimization of factors influencing microprojectile bombardment-mediated genetic transformation of seed-derived callus and regeneration of transgenic plants in Eleusine coracana (L.) Gaertn. Plant Cell Tiss Organ Cult 109:401–441
Jha P, Shashi, Rustagi A, Agnihotri PK, Kulkarni VM, Bhat V (2011) Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell Tiss Organ Cult 107:501–512
Karthikeyan A, Pandian SK, Ramesh M (2011a) Transgenic indica rice cv. ADT 43 expressing a D1-pyrroline-5-carboxylate synthetase (P5CS) gene from Vigna aconitifolia demonstrates salt tolerance. Plant Cell Tiss Organ Cult 107:383–395
Karthikeyan A, Pandian SK, Ramesh M (2011b) Agrobacterium-mediated transformation of leaf base derived callus tissues of popular indica rice (Oryza sativa L. sub sp. indica cv. ADT 43). Plant Sci 181:258–268
Karthikeyan A, Shilpha J, Pandian SK, Ramesh M (2012) Agrobacterium-mediated transformation of indica rice cv. ADT 43. Plant Cell Tiss Organ Cult 109:153–165
Latha AM, Rao KV, Reddy VD (2005) Production of transgenic plants resistant to leaf blast disease in finger millet (Eleusine coracana (L.) Gaertn.). Plant Sci 169:657–667
Latha MA, Rao KV, Reddy TP, Reddy VD (2006) Development of transgenic pearl millet (Pennisetum glaucum (L.) R. Br.) plants resistant to downy mildew. Plant Cell Rep 25:927–935
Ling HQ, Kriseleit D, Ganal MW (1998) Effect of ticarcillin potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Rep 17:843–847
Liu G, Godwin ID (2012) Highly efficient sorghum transformation. Plant Cell Rep 31:999–1007
Mayavan S, Subramanyam K, Jaganath B, Sathish D, Manickavasagam M, Ganapathi A (2015) Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep 34:1835–1848
Mbithi-Mwikya S, Ooghe W, Camp JV, Ngundi D, Huyghebaert A (2000) Amino acid profiles after sprouting, autoclaving, and lactic acid fermentation of finger millet (Eleusine coracana) and kidney beans (Phaseolus vulgaris L.). J Agric Food Chem 48:3081 – 3085
Mousa WK, Schwan A, Davidson J, Strange P, Liu H, Zhou T, Auzanneau F, Raizada MN (2015) An endophytic fungus isolated from finger millet (Eleusine coracana) produces anti-fungal natural products. Front Microbiol 6:1157
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Patel M, Dewey RE, Qu R (2013) Enhancing Agrobacterium tumefaciens-mediated transformation efficiency of perennial ryegrass and rice using heat and high maltose treatments during bacterial infection. Plant Cell Tiss Organ Cult 114:19–29
Perales M, Reddy GV (2012) Stem cell maintenance in shoot apical meristems. Curr Opin Plant Biol 15:10–16
Rahman H, Jagadeeshselvam N, Valarmathi R, Sachin B, Sasikala R, Senthil N, Sudhakar D, Robin S, Muthurajan R (2014) Transcriptome analysis of salinity responsiveness in contrasting genotypes of finger millet (Eleusine coracana L.) through RNA-sequencing. Plant Mol Biol 85:485–503
Satish L, Ceasar SA, Shilpha J, Rency AS, Rathinapriya P, Ramesh M (2015) Direct plant regeneration from in vitro-derived shoot apical meristems of finger millet (Eleusine coracana (L.) Gaertn.). In Vitro Cell Dev Biol Plant 52:192–200
Satish L, Rathinapriya P, Ceasar SA, Rency AS, Pandian S, Rameshkumar R, Subramanian A, Ramesh M (2016a) Effects of cefotaxime, amino acids and carbon source on somatic embryogenesis and plant regeneration in four Indian genotypes of foxtail millet (Setaria italica L.). In Vitro Cell Dev Biol Plant 52:140–153
Satish L, Rathinapriya P, Rency AS, Ceasar SA, Pandian S, Rameshkumar R, Ramesh M (2016b) Somatic embryogenesis and regeneration using Gracilaria edulis and Padina boergesenii seaweed liquid extracts and genetic fidelity in finger millet (Eleusine coracana). J Appl Phycol 28:2083–2098 b)
Satish L, Rency SA, Rathinapriya P, Ceasar SA, Pandian S, Rameshkumar R, Rao TB, Balachandran SM, Ramesh M (2016c) Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn.). Plant Cell Tiss Organ Cult 124:15–31 c)
Sharma M, Kothari-Chajer A, Jagga-Chugh S, Kothari SL (2011) Factors influencing Agrobacterium tumefaciens-mediated genetic transformation of Eleusine coracana (L.) Gaertn. Plant Cell Tiss Organ Cult 105:93–104
Sood S, Kumar A, Babu BK, Gaur VS, Pandey D, Kant L, Pattnayak A (2016) Gene discovery and advances in finger millet [Eleusine coracana (L.) Gaertn.] genomics—An important nutri-cereal of future. Front Plant Sci 7:1634
Sticklen MB, Oraby HF (2005) Shoot apical meristem: a sustainable explant for genetic transformation of cereal crops. In Vitro Cell Dev Biol Plant 41:187–200
Tang H, Ren Z, Karzal G (2000) An evaluation of antibiotics for the elimination of Agrobacterium tumefaciens from walnut somatic embryos and for the effect on the proliferation of somatic embryos and regeneration of transgenic plants. Plant Cell Rep 19:881–887
Tran TN, Sanan-Mishra N (2015) Effect of antibiotics on callus regeneration during transformation of IR 64 rice. Biotechnol Rep 7:143–149
Travella S, Ross SM, Harden J, Everett C, Snape JW, Harwood WA (2005) A comparison of transgenic barley lines produced by particle bombardment and Agrobacterium-mediated techniques. Plant Cell Rep 23:780–789
Visarada KBRS, Kishore NS (2015) Advances in genetic transformation. In: Madhusudhana R, Rajendrakumar P, Patil JV (Eds.) Sorghum Molecular Breeding. Springer, New York, pp 199–215
Wilmink A, Dons JJM (1993) Selective agents and marker genes for use in transformation of monocotyledonous plants. Plant Mol Biol Rep 11:165–185
Yookongkaew N, Srivatanakul M, Narangajavana J (2007) Development of genotype-independent regeneration system for transformation of rice (Oryza sativa ssp. indica). J Plant Res 120:237–245
Zhang S, Cho M-J, Kopret T, Yun R, Bregitzer P, Lemaux PG (1999) Genetic transformation of commercial cultivars of oat (Avena sativa L.) and barley (Hordeum vulgare L.) using in vitro shoot meristematic cultures derived from germinated seedlings. Plant Cell Rep 18:959–966
Zhong H, Sun B, Warkentin D, Zhang S, Wu R, Wu T, Sticklen MB (1996) The competence of maize shoot meristems for integrative transformation and inherited expression of transgenes. Plant Physiol 110:1097–1107
Acknowledgements
The author L. Satish sincerely thanks the University Grants Commission, New Delhi, India for financial support in the form of UGC BSR JRF and SRF (UGC order no. F.4-1/2006 (BSR)/7-326/2011/BSR) for the Ph.D. program. We thank Department of Small Millets, Millet Research Station, Tamil Nadu Agricultural University, Coimbatore for providing the seed material used in the present study. We thank Dr. R. Sathishkumar, Associate Professor for providing transgenic greenhouse facility at Plant Genetic Engineering Lab, Department of Biotechnology, Bharathiar University, Coimbatore, India. Also the authors gratefully acknowledge the Bioinformatics Infrastructure Facility of Alagappa University (funded by the Department of Biotechnology, Government of India: Grant No. BT/BI/25/001/2006) for providing the computational facility. We sincerely thank Dr. D. J. Pilbeam, Visiting Research Fellow, School of Biology, University of Leeds, UK for checking the language of the article.
Author information
Authors and Affiliations
Contributions
LS and MR conceived and supervised the study; LS conducted the experiments; LS and SAC analyzed data and wrote the manuscript; SAC and MR revised and proofread the manuscript. All authors agreed on the final appearance of the manuscript after careful review.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Additional information
Communicated by Danny Geelen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Satish, L., Ceasar, S.A. & Ramesh, M. Improved Agrobacterium-mediated transformation and direct plant regeneration in four cultivars of finger millet (Eleusine coracana (L.) Gaertn.). Plant Cell Tiss Organ Cult 131, 547–565 (2017). https://doi.org/10.1007/s11240-017-1305-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1305-5