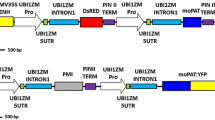
Abstract
Key message
An improved Agrobacterium -mediated transformation protocol is described for a recalcitrant commercial maize elite inbred with optimized media modifications and AGL1. These improvements can be applied to other commercial inbreds.
Abstract
This study describes a significantly improved Agrobacterium-mediated transformation protocol in a recalcitrant commercial maize elite inbred, PHR03, using optimal co-cultivation, resting and selection media. The use of green regenerative tissue medium components, high copper and 6-benzylaminopurine, in resting and selection media dramatically increased the transformation frequency. The use of glucose in resting medium further increased transformation frequency by improving the tissue induction rate, tissue survival and tissue proliferation from immature embryos. Consequently, an optimal combination of glucose, copper and cytokinin in the co-cultivation, resting and selection media resulted in significant improvement from 2.6 % up to tenfold at the T0 plant level using Agrobacterium strain LBA4404 in transformation of PHR03. Furthermore, we evaluated four different Agrobacterium strains, LBA4404, AGL1, EHA105, and GV3101 for transformation frequency and event quality. AGL1 had the highest transformation frequency with up to 57.1 % at the T0 plant level. However, AGL1 resulted in lower quality events (defined as single copy for transgenes without Agrobacterium T-DNA backbone) when compared to LBA4404 (30.1 vs 25.6 %). We propose that these improvements can be applied to other recalcitrant commercial maize inbreds.







Similar content being viewed by others
References
Beyer EM (1976) A potent inhibitor of ethylene action in plants. Plant Physiol 58:268–271
Cho M-J, Lemaux PG (2001) An efficient system for transformation and plant regeneration of sorghum using highly regenerative, green tissues. In Vitro Cell Dev Biol 37:38A
Cho M-J, Jiang W, Lemaux PG (1998) Transformation of recalcitrant barley cultivars through improvement of regenerability and decreased albinism. Plant Sci 138:229–244
Cho M-J, Buchanan BB, Lemaux PG (1999a) Development of transformation systems for monocotyledonous crop species and production of foreign proteins in transgenic barley and wheat seeds. In: Application of transformation technology in plant breeding. 30th Anniversary Korean Breeding Society, Suwon, Korea, November 19, pp 39–53
Cho M-J, Jiang W, Lemaux PG (1999b) High-frequency transformation of oat via microprojectile bombardment of seed-derived highly regenerative cultures. Plant Sci 148:9–17
Cho M-J, Ha CD, Lemaux PG (2000) Production of transgenic tall fescue and red fescue plants by particle bombardment of mature seed-derived highly regenerative tissues. Plant Cell Rep 19:1084–1089
Cho M-J, Choi HW, Lemaux PG (2001) Transformed T0 orchardgrass (Dactylis glomerata L.) plants produced from highly regenerative tissues derived from mature seeds. Plant Cell Rep 20:318–324
Cho M-J, Le VK, Okamoto D, Kim YB, Choi HW, Lemaux PG (2003) Generation of transgenic plants of creeping bentgrass (Agrostis palustris Huds.) plants from mature seed-derived highly regenerative tissues. In: Mujib A, Cho M-J, Predieri S, Banerjee S (eds) In vitro applications in crop improvement - recent progress. Science Publishers, Enfield, pp 67–77
Cho M-J, Yano H, Okamoto D, Kim H-K, Jung H-R, Newcomb K, Le VK, Yoo HS, Langham R, Buchanan BB, Lemaux PG (2004) Stable transformation of rice (Oryza sativa L.) via microprojectile bombardment of highly regenerative, green tissues derived from mature seed. Plant Cell Rep 22:483–489
Cho M-J, Klein TM, Zhao Z-Y (2013) Methods for tissue culture and transformation of sugarcane. US patent application 2013/0055472 A1
Chu CC, Wang CC, Sun CS, Hsu C, Yin KC, Chu CY (1975) Establishment of an efficient medium for anther culture in rice through comparative experiments on the nitrogen sources. Sci Sinica 18:659–668
D’Halluin K, Bonne E, Bossut M, De Beuckeleer M, Leemans J (1992) Transgenic maize plants by tissue electroporation. Plant Cell 4:1495–1505
Food and Agriculture Organization of the United Nations, Statistics Division 2012, http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor
Frame BR, Drayton PR, BagNall SV, Lewnau CJ, Bullock WP, Wilson HM, Dunwell JM, Thompson JA, Wang K (1994) Production of fertile transgenic maize plants by silicon carbide whisker-mediated transformation. Plant J 6:941–948
Frame BR, Shou H, Chikwamba RK, Zhang Z, Xiang C, Fonger TM, Pegg SEK, Li B, Nettleton DS, Pei D, Wang K (2002) Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol 129:13–22
Frame BR, McMurray JM, Fonger TM, Main ML, Taylor KW, Torney FJ, Paz MM, Wang K (2006) Improved Agrobacterium-mediated transformation of three maize inbred lines using MS salts. Plant Cell Rep 25:1024–1034
Fromm ME, Morrish F, Armstrong C, Williams R, Thomas J, Klein TM (1990) Inheritance and expression of chimeric genes in the progeny of transgenic maize plants. Biotechnology 8:833–839
Golovkin MV, Abraham M, Morocz S, Bottka S, Feher A, Dudits D (1993) Production of transgenic maize plants by direct DNA uptake into embryogenic protoplasts. Plant Sci 90:41–52
Gordon-Kamm WJ, Spencer TM, Mangano ML, Adams TR, Daines RJ, Start WG, O’Brien JV, Chambers SA, Adams WR Jr, Willetts NG, Rice TB, Mackey CJ, Krueger RW, Kausch AP, Lemaux PG (1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2:603–618
Ha CD, Lemaux PG, Cho M-J (2001) Stable transformation of a recalcitrant Kentucky bluegrass (Poa pratensis L.) cultivar using mature seed-derived highly regenerative tissues. In Vitro Cell Dev Biol Plant 37:6–11
Huang X, Wei Z (2005) Successful Agrobacterium-mediated genetic transformation of maize elite inbred lines. Plant Cell Tissue Organ Cult 83:187–200
Ishida Y, Satto H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14:745–750
Ishida Y, Hiei Y, Komari T (2007) Agrobacterium-mediated transformation of maize. Nat Protoc 2(7):1614–1621
Kim H-K, Lemaux PG, Buchanan BB, Cho M-J (1999) Reduction of genotype limitation in wheat (Triticum aestivum L.) transformation. In Vitro Cell Dev Biol 35:43
Lemaux PG, Cho M-J, Zhang S, Bregitzer P (1999) Transgenic Cereals: Hordeum vulgare (barley). In: Vasil IK (ed) Molecular improvement of cereal crops. Kluwer, Dordrecht, The Netherlands, pp 255–316
Li Y-C, Ren J, Cho M-J, Zhou S, Kim Y-B, Guo H, Wong JH, Niu H, Kim H-K, Morigasaki S, Lemaux PG, Frick OL, Yin J, Buchanan BB (2009) The level of expression of thioredoxin is linked to fundamental properties and applications of wheat seeds. Mol Plant 2:430–441
Lowe KS, Cahoon RE, Scelonge CJ, Yumin T, Gordon-Kamm WJ, Bruce WB, Newman LJ (2007) Wuschel (wus) gene homologs. US patent 7256322 B2
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Negrotto D, Jolley M, Beer S, Wench AR, Hansen G (2000) The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation. Plant Cell Rep 19:798–803
Oestreich DC (1995) Inbred corn line PHR03. US patent 5436390
Ombori O, Muoma JMO, Machuka J (2013) Agrobacterium-mediated genetic transformation of selected tropical inbred and hybrid maize (Zea mays L.) lines. Plant Cell Tissue Organ Cult 113:11–23
Petolino JF, Hopkins NL, Kosegi BD, Skokut M (2000) Whisker-mediated transformation of embryogenic callus of maize. Plant Cell Rep 19:781–786
Songstad DD, Duncan DR, Widholm JM (1988) Effect of l-aminocyclopropane-l-carboxylic acid, silver nitrate, and norbornadiene on plant regeneration from maize callus cultures. Plant Cell Rep 7:262–265
Vega JM, Yu W, Kennon AR, Chen X, Zhang ZJ (2008) Improvement of Agrobacterium-mediated transformation in Hi-II maize (Zea mays) using standard binary vectors. Plant Cell Rep 27:297–305
Wan Y, Widholm JM, Lemaux PG (1995) Type-I callus as a bombardment target for generating fertile transgenic maize (Zea mays L.). Planta 196:7–14
Wu LM, Wei YM, Zheng YL (2006) Effect of silver nitrate on the tissue culture of immature wheat embryos. Plant Physiol 53:530–534
Wu E, Lenderts B, Glassman K, Berezowska-Kaniewska M, Christensen H, Asmus T, Zhen S, Chu Y, Cho M-J, Zhao Z-Y (2014) Optimized Agrobacterium-mediated sorghum transformation protocol and molecular data of transgenic sorghum plants. In Vitro Cell Dev Biol Plant 50:9–18
Zhao Z-Y, Gu W, Cai T, Tagliani LA, Hondred DA, Bond D, Krell S, Rudert ML, Bruce WB, Pierce DA (1998) Molecular analysis of T0 plants transformed by Agrobacterium and comparison of Agrobacterium-mediated transformation with bombardment transformation in maize. Maize Genet Coop Newslett 72:34–37
Zhao Z-Y, Cai T, Tagliani L, Miller M, Wang N, Pang H, Rudert M, Schroeder S, Hondred D, Seltzer J, Pierce D (2000) Agrobacterium-mediated sorghum transformation. Plant Mol Biol 44:789–798
Zhao Z-Y, Gu W, Cai T, Tagliani L, Hondred D, Bond D, Schroeder S, Rudert M, Pierce D (2001) High throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol Breed 8:323–333
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prakash Lakshmanan.
Rights and permissions
About this article
Cite this article
Cho, MJ., Wu, E., Kwan, J. et al. Agrobacterium-mediated high-frequency transformation of an elite commercial maize (Zea mays L.) inbred line. Plant Cell Rep 33, 1767–1777 (2014). https://doi.org/10.1007/s00299-014-1656-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1656-x