Abstract
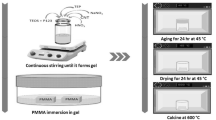
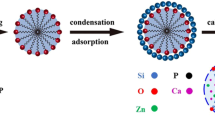
This paper reports a facile method for fabricating monodispersed mesoporous bioactive glass sub-micron spheres (MBGS) using dodecylamine (DDA) as a catalyst and template agent in sol–gel process. The effects of synthesis conditions including the amount of DDA, temperature of hydrolysis and the volume ratio of alcohol to water (AW ratio) on the resulting particle size, morphology, monodispersity and pore size distribution of MBGS are investigated and discussed. The results indicate that the particle size, morphology, monodispersity and pore size distribution of MBGS depend on the amount of DDA, the temperature of hydrolysis and the AW ratio. Meanwhile, using DDA as the structure directing agent and hydrolysis catalyst under optimal synthesis conditions (e.g. 4 g DDA, hydrolysis temperature at 40 °C and AW ratio at 4) is in favor of obtaining MBGS with mesoporous surface structure, large specific surface area (362.073 m2 g−1), relatively homogeneous particle size (~560 nm) as well as good apatite-forming activity. The unique structure and properties may turn MBGS into a good candidate as a drug delivery carrier or an injectable biomaterial for bone tissue regeneration.









Similar content being viewed by others
References
Hench LL, Polak JM (2002) Third-generation biomedical materials. Science 295(5557):1014
Hench LL, Splinter RJ, Allen W, Greenlee T (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5(6):117–141
Lossdörfer S, Schwartz Z, Lohmann C, Greenspan D, Ranly D, Boyan B (2004) Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials 25(13):2547–2555
Valerio P, Pereira MM, Goes AM, Leite MF (2004) The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 25(15):2941–2948
Hench LL (2006) The story of Bioglass®. J Mater Sci Mater Med 17(11):967–978
Bo L et al (2010) Fabrication, structure and biological properties of organic acid-derived sol–gel bioactive glasses. Biomed Mater 5(5):054103
Lei B, Chen XF, Wang YJ, Zhao NR, Du C, Fang LM (2010) Surface nanoscale patterning of bioactive glass to support cellular growth and differentiation. J Biomed Mater Res A 94A(4):1091–1099
Hum J, Boccaccini A (2012) Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: a review. J Mater Sci Mater Med 23(10):2317–2333
Bellucci D, Cannillo V, Sola A (2010) Shell scaffolds: a new approach towards high strength bioceramic scaffolds for bone regeneration. Mater Lett 64(2):203–206
Rahaman MN, Day DE, Sonny Bal B, Fu Q, Jung SB, Bonewald LF, Tomsia AP (2011) Bioactive glass in tissue engineering. Acta Biomater 7(6):2355–2373
Chen XF, Lei B, Wang YJ, Zhao N (2009) Morphological control and in vitro bioactivity of nanoscale bioactive glasses. J Non-Cryst Solids 355(13):791–796
Meseguer-Olmo L, Bernabeu-Esclapez A, Ros-Martinez E, Sanchez-Salcedo S, Padilla S, Martin A, Vallet-Regí M, Clavel-Sainz M, Lopez-Prats F, Meseguer-Ortiz C (2008) In vitro behaviour of adult mesenchymal stem cells seeded on a bioactive glass ceramic in the SiO2–CaO–P2O5 system. Acta Biomater 4(4):1104–1113
Jones JR, Tsigkou O, Coates EE, Stevens MM, Polak JM, Hench LL (2007) Extracellular matrix formation and mineralization on a phosphate-free porous bioactive glass scaffold using primary human osteoblast (HOB) cells. Biomaterials 28(9):1653–1663
Valerio P, Guimaraes M, Pereira M, Leite M, Goes A (2005) Primary osteoblast cell response to sol–gel derived bioactive glass foams. J Mater Sci Mater Med 16(9):851–856
Lei B, Chen X, Han X, Li Z (2011) Unique physical–chemical, apatite-forming properties and human marrow mesenchymal stem cells (HMSCs) response of sol–gel bioactive glass microspheres. J Mater Chem 21(34):12725–12734
Lei B, Chen XF, Koh YH (2011) Effects of acidic catalysts on the microstructure and biological property of sol–gel bioactive glass microspheres. J Sol-Gel Sci Technol 58(3):656–663
Lei B, Chen X, Wang Y, Zhao N (2009) Synthesis and in vitro bioactivity of novel mesoporous hollow bioactive glass microspheres. Mater Lett 63(20):1719–1721
Hong Z, Luz GM, Hampel PJ, Jin M, Liu A, Chen X, Mano JF (2010) Mono-dispersed bioactive glass nanospheres: preparation and effects on biomechanics of mammalian cells. J Biomed Mater Res A 95(3):747–754
Labbaf S, Tsigkou O, Müller KH, Stevens MM, Porter AE, Jones JR (2011) Spherical bioactive glass particles and their interaction with human mesenchymal stem cells in vitro. Biomaterials 32(4):1010–1018
Hong Z, Reis RL, Mano JF (2008) Preparation and in vitro characterization of scaffolds of poly (l-lactic acid) containing bioactive glass ceramic nanoparticles. Acta Biomater 4(5):1297–1306
Tanev PT, Pinnavaia TJ (1995) A neutral templating route to mesoporous molecular sieves. Science 267(5199):865–867
Yu J, Zhao L, Cheng B (2006) Preparation of monodispersed microporous SiO2 microspheres with high specific surface area using dodecylamine as a hydrolysis catalyst. J Solid State Chem 179(1):226–232
Shi X, Wang Y, Wei K, Ren L, Lai C (2008) Self-assembly of nanohydroxyapatite in mesoporous silica. J Mater Sci Mater Med 19(8):2933–2940
Hu Q, Chen X, Zhao N, Li Y (2013) Facile synthesis and in vitro bioactivity of monodispersed mesoporous bioactive glass sub-micron spheres. Mater Lett 106(1):452–455
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73(1):373–380
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27(15):2907–2915
Zhao H, Xin Y, Wang H, Zhang Z, Liu S (2011) A comparison of the formation of SiO2 particles under the catalysis of dodecylamine and ammonia solutions. J Inorg Organomet Polym Mater 21(4):925–928
Di Renzo F, Testa F, Chen J, Cambon H, Galarneau A, Plee D, Fajula F (1999) Textural control of micelle-templated mesoporous silicates: the effects of co-surfactants and alkalinity. Microporous Mesoporous Mater 28(3):437–446
Branda F, Silvestri B, Luciani G, Costantini A (2007) The effect of mixing alkoxides on the Stöber particles size. Colloids Surf A 299(1–3):252–255
Wang C–C, Ying JY (1999) Sol-gel synthesis and hydrothermal processing of anatase and rutile titania nanocrystals. Chem Mater 11(11):3113–3120
Liu S, Cool P, Collart O, Van Der Voort P, Vansant EF, Lebedev OI, Van Tendeloo G, Jiang M (2003) The influence of the alcohol concentration on the structural ordering of mesoporous silica: cosurfactant versus cosolvent. J Phys Chem B 107(38):10405–10411
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26(1):62–69
Macquarrie DJ, Jackson DB, Tailland S, Utting KA (2001) Organically modified hexagonal mesoporous silicas (HMS)—remarkable effect of preparation solvent on physical and chemical properties. J Mater Chem 11(7):1843–1849
Coleman N, Hench L (2000) A gel-derived mesoporous silica reference material for surface analysis by gas sorption 1. Textural features. Ceram Int 26(2):171–178
Ryoo R, Park IS, Jun S, Lee CW, Kruk M, Jaroniec M (2001) Synthesis of ordered and disordered silicas with uniform pores on the border between micropore and mesopore regions using short double-chain surfactants. J Am Chem Soc 123(8):1650–1657
Yun H, Kim S, Lee S, Song I (2010) Synthesis of high surface area mesoporous bioactive glass nanospheres. Mater Lett 64(16):1850–1853
Acknowledgments
This work was supported by the Key Project of the National Natural Science Foundation of China (Grant No. 50830101), National Natural Science Foundation of China (Grant No. 51072055, Grant No. 51172073, Grant No. 51202069), the National 973 project of China (2011CB606204), Research Fund for the Doctoral Program of Higher Education of China (20110172110002) and the Fundamental Research Funds for the Central University (2012ZP0001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Q., Chen, X., Zhao, N. et al. Fabrication and characterization of dodecylamine derived monodispersed mesoporous bioactive glass sub-micron spheres. J Sol-Gel Sci Technol 69, 9–16 (2014). https://doi.org/10.1007/s10971-013-3167-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-013-3167-6