Abstract
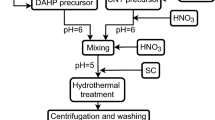
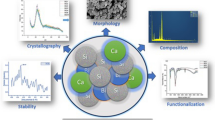
We synthetized hollow mesoporous bioactive glass nanospheres (HMBGS) and zinc-doped hollow mesoporous bioactive glass nanospheres (Zn-HMBGS) in which 5 mol% zinc was substituted for calcium using polyacrylic acid (PAA) as a template agent in sol-gel process. Then investigated the effect of zinc doping on morphology, size, structure, apatite-forming ability, biocompatibility and Bovine serum albumin (BSA) loading/release property of HMBGS. Results showed that Zn-HMBGS with an average particle size of 98.87 ± 7.08 nm were successfully fabricated and doping zinc did not significantly affect the spherical morphology, nano-scaled particle size and hollow mesoporous structure, but inhibited apatite-forming ability and improved biocompatibility of HMBGS. Additionally, the Zn-HMBGS possessed high specific surface area (120.014 m2g−1), large mesoporous size (5.26 ~ 6.68 nm) and hollow structure, exhibiting high loading capacity (202.17 ± 3.74 mg/g) and sustained release property for BSA. Due to their advantageous hollow mesoporous structure, good biocompatibility and efficient loading and stable release for BSA molecules, Zn-HMBGS show great potential as drug delivery systems for tissue regeneration.
Graphical Abstract

Highlights
-
Zinc-doped hollow mesoporous bioactive glass nanospheres (Zn-HMBGS) were prepared.
-
Zn-HMBGS exhibited high loading capacity and sustained release property for BSA.
-
Zn-HMBGS show great potential as drug delivery systems for tissue regeneration.













Similar content being viewed by others
References
Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, Tomsia AP (2011) Bioactive glass in tissue engineering. Acta Biomater 7:2355–2373. https://doi.org/10.1016/j.actbio.2011.03.016
Miguez PV, Hench LL, Boccaccini AR (2015) Bioactive glasses beyond bone and teeth: emerging applications in contact with soft tissues. Acta Biomater 13:1–15. https://doi.org/10.1016/j.actbio.2014.11.004
Hench LL, Splinter RJ, Allen WC, Greenlee TK (1971) Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res 5:117–141. https://doi.org/10.1002/jbm.820050611
Lossdörfer S, Schwartz Z, Lohmann C, Greenspan D, Ranly D, Boyan B (2004) Osteoblast response to bioactive glasses in vitro correlates with inorganic phosphate content. Biomaterials 25:2547–2555. https://doi.org/10.1016/j.biomaterials.2003.09.094
Valerio P, Pereira MM, Goes AM, Leite MF (2004) The effect of ionic products from bioactive glass dissolution on osteoblast proliferation and collagen production. Biomaterials 25:2941–2948. https://doi.org/10.1016/j.biomaterials.2003.09.086
Li B, Luo WQ, Wang YY, Wu HY, Zhang CC (2016) Bioactive SiO2-CaO-P2O5 hollow nanospheres for drug delivery. J Non-Cryst Solids 447:98–103. https://doi.org/10.1016/j.jnoncrysol.2016.05.041
Das MP, Pandey G, Neppolian B, Das J (2021) Design of poly-L-glutamic acid embedded mesoporous bioactive glass nanospheres for pH-stimulated chemotherapeutic drug delivery and antibacterial susceptibility. Colloid Surf B 202:111700. https://doi.org/10.1016/j.colsurfb.2021.111700
Li X, Liang QM, Zhang W, Li YL, Ye JD, Zhao FJ, Chen XF, Wang SR (2017) Bio-inspired bioactive glasses for efficient microRNA and drug delivery. J Mater Chem B 5:6376–6384. https://doi.org/10.1039/C7TB01021D
Hu Q, Jiang WH, Li YL, Chen XF, Liu JM, Chen T, Miao GH (2018) The effects of morphology on physicochemical properties, bioactivity and biocompatibility of micro-/nano-bioactive glasses. Adv Powder Technol 28:1812–1819. https://doi.org/10.1016/j.apt.2018.04.017
Firuzeh M, Labbaf S, Sabouri Z (2021) A facile synthesis of mono-dispersed, spherical and mesoporous bioactive glass nanoparticles for biomedical applications. J Non-Cryst Solids 554:120598. https://doi.org/10.1016/j.jnoncrysol.2020.120598
Hu Q, Li YL, Miao GH, Zhao NR, Chen XF (2014) Size control and biological properties of monodispersed mesoporous bioactive glass sub-micron spheres. Rsc Adv 4:22678–22687. https://doi.org/10.1039/C4RA01276C
Vichery C, Nedelec JM (2016) Bioactive glass nanoparticles: from synthesis to materials design for biomedical applications. Materials 9:288. https://doi.org/10.3390/ma9040288
Lei B, Chen XF, Han X, Zhou JA (2012) Versatile fabrication of nanoscale sol-gel bioactive glass particles for efficient bone tissue regeneration. J Mater Chem B 22:16906–16913. https://doi.org/10.1039/C2JM31384G
Lin KP, Gan Y, Zhu PD, Li SS, Lin C, Yu SL, Zhao S, Shi JH, Li RM, Yuan JF (2021) Hollow mesoporous polydopamine nanospheres: synthesis, biocompatibility and drug delivery. Nanotechnology 32:28560. https://doi.org/10.1088/1361-6528/abf4a9
Zhao DC, Yang NL, Xu LK, Du J, Yang Y, Wang D (2022) Hollow structures as drug carriers: recognition, response, and release. Nano Res 15:739–757. https://doi.org/10.1007/s12274-021-3595-5
Bao Y, Shi CH, Wang T, Li XL, Ma JZ (2016) Recent progress in hollow silica: template synthesis, morphologies and applications. Micropor Mesopor Mat 227:121–136. https://doi.org/10.1016/j.micromeso.2016.02.040
Zhao FJ, Lei B, Li X, Mo YF, Wang RX, Chen DF, Chen XF (2018) Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 178:36–47. https://doi.org/10.1016/j.biomaterials.2018.06.004
Westhauser F, Decker S, Nawaz Q, Rehder F, Saur M, Wilesmann S, Moghaddam A, Kunisch E, Boccaccini AR (2021) Impact of Zinc- or Copper-Doped Mesoporous Bioactive Glass Nanoparticles on the Osteogenic Differentiation and Matrix Formation of Mesenchymal Stromal Cells. Materials 14:1457–1467. https://doi.org/10.3390/ma14081864
Bahati D, Meriame B, Mabrouk KE (2023) Synthesis, characterization, and in vitro apatite formation of strontium-doped sol-gel-derived bioactive glass nanoparticles for bone regeneration applications. Ceram Int 49:23020–23034. https://doi.org/10.1016/j.ceramint.2023.04.128
Wen SZ, Yu BR, Liu L, Zhu QX (2023) Thermophysical properties and flexural strength of strontium-fluorine Co-doped hydroxyapatite. J Ceram 44:305–311. https://doi.org/10.13957/j.cnki.tcxb.2023.02.011
Wang SY, Li R, Xia DD, Zhao X, Zhu Y, Gu RL, Yoon JM, Liu YS (2021) The impact of Zn-doped synthetic polymer materials on bone regeneration: a systematic review. Stem Cell Res Ther 12:123. https://doi.org/10.1186/s13287-021-02195-y
Naseri S, Lepry WC, Nazhat SN (2017) Bioactive glasses in wound healing: hope or hype? J Mater Chem B 5:6167–6174. https://doi.org/10.1039/C7TB01221G
Miao GH, Li ZM, Meng YC, Wu JW, Li YL, Hu Q, Chen XF, Yang XC, Chen XM (2019) Preparation, characterization, in vitro bioactivity and protein loading/release property of mesoporous bioactive glass microspheres with different compositions. Adv Powder Technol 30:1848–1857. https://doi.org/10.1016/j.apt.2019.06.002
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27:2907–2915. https://doi.org/10.1016/j.biomaterials.2006.01.017
Coleman NJ, Hench LL (2000) A gel-derived mesoporous silica reference material for surface analysis by gas sorption2. Durability and stability. Ceram Int 26:179–186. https://doi.org/10.1016/S0272-8842(99)00045-0
Taleb MFA, Alkahtani A, Mohamed SK (2015) Radiation synthesis and characterization of sodium alginate/chitosan/hydroxyapatite nanocomposite hydrogels: a drug delivery system for liver cancer. Polym Bull 72:725–742. https://doi.org/10.1007/s00289-015-1301-z
Anirudhan TS, Christa J (2018) Binusreejayan, pH and magnetic field sensitive folic acid conjugated protein-polyelectrolyte complex for the controlled and targeted delivery of 5-fluorouracil. J Ind Eng Chem 57:199–207. https://doi.org/10.1016/j.jiec.2017.08.024
Zhang W, Zhao FJ, Huang DQ, Fu XL, Li X, Chen XF (2016) Strontium-Substituted Submicrometer Bioactive Glasses Modulate Macrophage Responses for Improved Bone Regeneration. ACS Appl Mater Interfaces 8:30747–30758. https://doi.org/10.1021/acsami.6b10378
Li ZM, Xie KK, Yang S, Yu T, Xiao Y, Zhou YH (2021) Multifunctional Ca-Zn-Si-based micro-nano spheres with anti-infective, anti-inflammatory, and dentin regenerative properties for pulp capping application. J Mater Chem B 9:8289–8299. https://doi.org/10.1039/D1TB01517F
Hu Q, Li YL, Zhao NR, Ning CY, Chen XF (2014) Facile synthesis of hollow mesoporous bioactive glass sub-micron spheres with a tunable cavity size. Mater Lett 134:130–133. https://doi.org/10.1016/j.matlet.2014.07.041
Tsigkou O, Labbaf S, Steven MM, Porter AE, Jones JR (2014) Monodispersed bioactive glass submicron particles and their effect on bone marrow and adipose tissue-derived stem cells. Adv Healthc Mater 3:115–125. https://doi.org/10.1002/adhm.201300126
Chasapis CT, Spiliopoulou CA, Loutsidou AC, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86:521–534. https://doi.org/10.1007/s00204-011-0775-1
Moghanian A, Firoozi S, Tahriri M (2017) Synthesis and in vitro studies of sol-gel derived lithium substituted 58S bioactive glass. Ceram Int 43:12835–12843. https://doi.org/10.1016/j.ceramint.2017.06.174
Li YL, Liang QM, Lin C, Li X, Chen XF, Hu Q (2017) Facile synthesis and characterization of novel rapid-setting spherical sub-micron bioactive glasses cements and their biocompatibility in vitro. Mat Sci Eng C-Mater 75:646–652. https://doi.org/10.1016/j.msec.2017.02.095
Nescakova Z, Zheng K, Liverani L, Nawaz Q, Galuskova D, Kankova H, Michalek M, Galusek D, Boccaccini AR (2019) Multifunctional zinc ion doped sol-gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact Mater 4:312–321. https://doi.org/10.1016/j.bioactmat.2019.10.002
Kanzaki N, Onuma K, Treboux G, Tsutsumi S, Ito A (2000) Inhibitory effect of magnesium and zinc on crystallization kinetics of hydroxyapatite (0001) face. J Phys Chem B 104:4189–4194. https://doi.org/10.1021/jp9939726
Zheng K, Lu M, Rutkowski B, Dai XY, Yang YY, Taccardi N, Stachewicz U, Czyrska-Filemonowicz A, Hüser N, Boccaccini AR (2016) ZnO quantum dots modified bioactive glass nanoparticles with pH-sensitive release of Zn ions, fluorescence, antibacterial and osteogenic properties. J Mater Chem B 4:7936–7949. https://doi.org/10.1039/C6TB02053D
Du RL, Chang J, Ni SY, Zhai WY (2006) Characterization and in vitro bioactivity of zinc-containing bioactive glass and glass-ceramics. J Biomater Appl 20:341–360. https://doi.org/10.1177/0885328206054535
Boonsongrit Y, Abe H, Sato K, Naito M, Yoshimura M, Ichikawa H, Fukumori Y (2008) Controlled release of bovine serum albumin from hydroxyapatite microspheres for protein delivery system. Mat Sci Eng B 148:162–165. https://doi.org/10.1016/j.mseb.2007.09.006
El-Fiqi A (2022) Sol-gel synthesis, properties and protein loading/delivery capacity of hollow bioactive glass nanospheres with large hollow cavity and mesoporous shell. Front Mater Sci 16:22060. https://doi.org/10.1007/s11706-022-0608-6
Hu Q, Jiang WH, Chen XF, Li YY, Liang QM (2017) The effects of Sr concentration on physicochemical properties, bioactivity and biocompatibility of sub-micron bioactive glasses spheres. Adv Powder Technol 28:2713–2722. https://doi.org/10.1016/j.apt.2017.07.024
Ding XB, Zheng J, Ju FY, Wang L, Kong JH, Feng JY, Liu T (2021) Facile fabrication of hollow mesoporous bioactive glass spheres: From structural behaviour to in vitro biology evaluation. Ceram Int 47:34836–34844. https://doi.org/10.1016/j.ceramint.2021.09.024
Li CD, Wang C, Boccaccini AR, Zhen K (2023) Sol-gel processing and characterization of binary P2O5-CaO and ternary P2O5-CaO-Li2O mesoporous phosphate bioactive glasses. J Non-Cryst Solids 17:100159. https://doi.org/10.1016/j.nocx.2023.100159
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52362038, 52362041, 52072162, 51962014), the key Project of Jiangxi Natural Science Foundation (2020ACBL214008), Jiangxi Provincial Natural Science Foundation (20224BAB204020, 20232BAB204012, 20224BAB214024), the Science Foundation of Jiangxi Provincial Department of Education (GJJ2201006), Jingdezhen science and technology project (20212GYZD009-07) and National College Student Innovation and Entrepreneurship Training Program Support Project (NO. 202310408027).
Author information
Authors and Affiliations
Contributions
Qing Hu: Project administration, Conceptualization, Writing-review, editing, Supervision. Huidong Tang, Hezhen Wu, Yanqiao Xu, Wentao Li and Xianjian Wang: Methodology, Formal analysis, Writing-original draft preparation. Guo Feng, Feng Jiang: Formal analysis, Writing-Review & Editing. Jian Liang, Jianmin Liu: Conceptualization, Technical supports.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Q., Tang, H., Wu, H. et al. Facile synthesis, characterization, biocompatibility and protein loading/release property of zinc-doped hollow mesoporous bioactive glass nanospheres. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06374-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06374-0