Abstract
Breakthrough infections have been reported in fully vaccinated persons. Furthermore, rebound symptoms have been reported following the new FDA granted emergency use to combat SARS-CoV-2. Glycyrrhizin (GR) and boswellic acids (BAs) combination has been shown to have highly successful actions against COVID-19 in our recent clinical trial. However, the study is limited by the small sample size, and therefore, the aim of this article is to comprehensively evaluate recent evidence on the efficacy of GR and BAs in preventing the development of COVID-19 in patients with mild and moderate infections and in preventing post-COVID-19 cognitive impairment, which is the most important symptom after recovery from Covid-19 disease. We have reviewed and discussed information published since the outbreak of the COVID-19 pandemic until July 2022 on preclinical (in vivo, in vivo and bioinformatics) and clinical studies related to the antiviral, anti-inflammatory and immunomodulatory activity of Gr and BAs. Sixteen studies were performed to determine the efficacy of GR against SARS-CoV-2. Ten studies were used primarily for in vitro and in vivo assays and six used molecular docking studies. However, the antiviral activity of BAs against SARS-CoV-2 was determined in only five studies using molecular modeling and bioinformatics. All these studies confirmed that GR n and BAs have strong antiviral activity and can be used as a therapeutic agent for COVID-19 and as a protective agent against SARS-CoV-2. They may act by inhibiting the main protease SARS-CoV-2 (Mpro) responsible for replication and blocking spike protein-mediated cell entry. Only seven rigorously designed clinical trials regarding the usefulness of GR, BAs or their combinations in the treatment of COVID-19 have been published as of July 2022. Although there is no clinical study regarding the treatment of cognitive impairment after COVID-19 that has been published so far, several preclinical and clinical studies have demonstrated the potential effect of GR and BAs in the prevention and treatment of cognitive impairment by inhibiting the activity of several molecules that activate inflammatory signaling pathway. In conclusion, the findings of our study documented the beneficial use of GR and BAs to treat SARS-CoV-2 and its variants and prevent post-COVID cognitive impairment. However, it warrants further studies with a larger randomized sample size to ensure that the studies have sufficient evidence of benefits against COVID-19 and post-COVID-19 symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to WHO, the COVID-19 pandemic continues to threaten public health systems worldwide (WHO 2022). Breakthrough infections have been reported in fully vaccinated persons (Liu et al. 2022). Furthermore, it is becoming increasingly clear that many patients who become infected with SARS-CoV-2 subsequently develop a number of neuropsychiatric and cognitive complications defined as long COVID or post-COVID-19 condition. The National Institute for Health and Care Excellence (NICE) defined post-COVID-19 as on-going symptoms beyond 4–12 weeks after COVID-19 (WHO 2021). These symptoms may persist for a long time after recovery, which negatively affects the quality of life of patients. Fatigue and cognitive impairment, along with other enduring neuropsychiatric (e.g., depression) comprise the most common post-acute sequelae of SARS-CoV-2 (Renaud-Charest et al. 2021).
According to various studies, these post-COVID-19 symptoms are observed in about a third of patients who have suffered from COVID-19 of varying severity (Taquet et al. 2021; Nalbandian et al. 2021; Huang et al. 2021; Baker et al. 2021; Crivelli et al. 2022). There is increasing evidence that cognitive impairment is widely observed among the consequences of COVID-19 disease, even in patients who have developed mild disease symptoms (Tavares-Júnior et al. 2022; Crivelli et al 2022; Ceban et al. 2022; Hadad et al. 2022). The proportion of individuals with cognitive impairment after COVID-19 has been inconsistent, but a prospective follow-up study reported that 48–56% of patients may have cognitive impairment after severe COVID-19 infection (Miskowiak et al. 2022). It is not clear what is definitely the risk factor for developing cognitive impairment after recovery from COVID-19 but several studies have revealed that comorbidities, age, SARS-CoV-2-induced neuroinflammation, systemic inflammation, brain hypoxia, prolonged stay in intensive care unit, peripheral organ impairment and genetic predisposition may be the mechanism underlying cognitive impairment after COVID-19 (Haage and De Jager 2022; Crivelli et al. 2022).
Since the beginning of the pandemic, there have been tremendous efforts to find effective outpatient treatments for COVID-19. The development of treatments has been shown to be important for preventing severe infection especially in individuals who have a high risk of progressing to severe disease. Two groups for the treatment of COVID-19 are currently recommended for people with mild or moderate symptoms of COVID-19 (Queensland Health, 2022). These groups include antivirals that target vital steps in viral replication (antiviral therapy) such as nirmatrelvir plus ritonavir which is a peptidomimetic that inhibits the 3C-like protease, rendering it unable to process polyprotein precursors, thus preventing viral replication. It is taken orally in combination with low-dose ritonavir to inhibit CYP3A-mediated metabolism of nirmatrelvir. The other group is neutralizing antibodies targeting M spike protein (anti-spike monoclonal antibodies) such as Sotrovimab which is a recombinant human immunoglobulin monoclonal antibody targeting the spike protein receptor binding domain of SARS-CoV-2 (Bernal et al. 2022; Gottlieb et al. 2021; Ng TI et al. 2022; Hammond et al. 2022). On December 22, 2021, FDA granted emergency use authorization for nirmatrelvir plus ritonavir for non-hospitalized symptomatic patients with SARS-CoV-2 to prevent progression to severe disease (FDA Update, 2022). However, rebound symptoms after a period of improvement have been reported. Furthermore, several lab studies have recently shown that SARS-CoV-2 can mutate in ways that make it less susceptible to the most oral antivirals authorized to treat SARS-CoV-2 in the USA. SARS-CoV-2 accumulated three mutations at positions 50, 166 and 167 in the amino acid chain that reduced virus susceptibility to nirmatrelvir by 20-fold (Wang et al. 2022;Rubin 2022; Robert 2022; Alshanqeeti and Bhargava 2022; HAN Archive 2022).
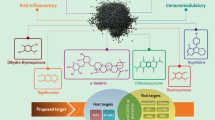
Nutraceuticals, including a variety of phytochemicals isolated from medicinal plants, dietary supplements, and functional foods, have long been used as an adjuvant treatment for many disease conditions, including viral infections. Several clinical studies are currently reporting the beneficial role of nutraceuticals in the treatment and/or prevention of COVID-19 (Subedi et al. 2021; Chavda et al. 2022). GR/licorice extract and BAs/Boswellia extract are nutraceuticals that have already been shown to possess anti-inflammatory, immunomodulatory, and antiviral activity against SARS-CoV-2 (Gomaa and Abdel- Wadood 2021; Gomaa et al. 2021). GR and BAs combination has been shown to have highly successful actions against COVID-19 in our recent clinical trial. We anticipated that the combination of the antiviral agent, GR, and the anti-inflammatory agent, immunomodulator, BAs, might have a more beneficial effect against COVID-19 (Gomaa et al. 2022). However, the study is limited by the small sample size; therefore, the aim of this article is to comprehensively evaluate recent evidence on the efficacy of GR and BAs in preventing the progression of COVID in patients with mild–moderate infection and in preventing post-COVID cognitive impairment, the widely observed among the consequences of COVID-19 disease.
Methods
We have reviewed information published through July 2022. Searches were conducted to find relevant articles and studies relating to the use of GR/licorice extract, and BAs/Boswellia extract in COVID-19. These studies demonstrated the effects, use and safety of GR/licorice extract and BAs/Boswellia extract in preventing/treating COVID-19 and post-COVID cognitive impairment, in both clinical and preclinical studies (in vitro, in vivo and bioinformatics). The studies were collected by searching on online electronic databases (academic libraries such as PubMed, Scopus, Medline, Embase, Web of Science and the Egyptian Knowledge Bank). Articles were excluded if they were not available in English, in an indexed journal or in the international clinical trial registration platform regarding clinical studies (RCT). The opinion papers, retrieval of rapid reviews, guidelines documents, scope reviews, and panel reviews were not considered for our review. All articles were then approved and reviewed by two people (AG, YA) for relevance and included in this review.
Results and discussion
Antiviral activity of glycyrrhizin and boswellic acids against SARS-CoV-2 and mechanism of action
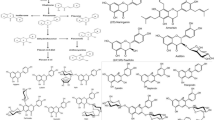
Determining the in vitro antiviral activity of potentially effective candidates is a necessary step for clinical studies, as extrapolation from in vitro to in vivo assumes that in vivo cellular drug accumulation is similar to that seen in in vitro experiments (Bocci et al. 2020). Since the 1979s, many studies reported that glycyrrhizin showed antiviral effect directly by inhibiting the replication of various DNA and RNA viruses including SARS-CoV and disrupting viral uptake into the host cells at low concentrations without cytotoxicity or indirectly by activating the immune function (Gomaa and Abdel- Wadood 2021; Huan et al. 2021; Ng et al. 2021). After the WHO officially declared a pandemic on March 11, 2020, several studies were conducted to determine the effectiveness of GR in treating COVID-19 using an in vitro assay and in silico structure-based virtual screening/docking on both human host targets and viral proteins. Since the beginning of this pandemic, 16 studies have been carried out to identify the efficacy of GR against SARS-CoV-2. Ten studies primarily used in vitro and in vivo assays. Six used molecular docking studies. All these studies confirmed that GR can be used as a therapeutic agent for COVID-19 and as a preventive agent against SARS-CoV-2 (Table 1).
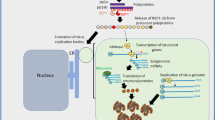
The antiviral activity of glycyrrhizin and its derivatives has provided scientific evidence guiding clinical applications. All studies classified the mechanism of actions underlying the direct antiviral effect into two mechanisms: (1) inhibition of virus proteins (spike (S) proteins) mediating cell binding (ACE2) and blocking virus entry. (2) Inhibition of 3C-like protease or main protease (Mpro) and inhibits virus replication and aggregation in the host. Compared with other biologically active substances, in molecular docking with a biological test in vitro, it was found that GR, is the most effective and safe molecule to combat the coronavirus in general and specifically affect SARS-CoV-2. It was most effective in blocking the interaction between the domain-binding receptor (DBR) (more specifically, nsp7) of the spike protein of SARS-CoV-2 and ACE2 (Yu et al. 2021; Li et al.; Jezova et al. 2021; Yi et al. 2021).
In Vero E6 cells, GR fought SARS-CoV-2 by active inhibition of the main protease (Mpro) of SARS-CoV-2 and inhibited SARS-CoV-2 replication (van de Sand et al. 2021; Gowda et al. 2021) In another study using computational and experimental investigations in vitro, licorice and GR treatment when used concurrently, post-infection, and pre-treatment have demonstrated potent anti-SARS-CoV-2 ability through two mechanisms of action as SARS-CoV-2 inhibitors for main protease (Mpro) responsible for replication and prevent cell binding mediated by spike protein. The results of this study suggested that GR can be used as a protective agent against COVID-19 (Tolah et al. 2021). In vivo study on a mouse model of COVID-19 combined with in vitro experiments; GR nanoparticles exert antiviral and anti-inflammatory effects, preventing organ damage and death by prominently suppressing the replication of COVID-19 (Zhao Z et al. 2021).
Several studies have reviewed the antiviral activity of BAs/Boswellia extract in vitro, in vivo and theoretically (Gomaa et al. 2021; Jamshidi et al. 2022). The antiviral activity of BAs against SARS-CoV-2 was determined using molecular modeling and bioinformatics. No in vitro or in vivo studies have been published determining the efficacy of boswellic acids on SARS-CoV-2. To date, only four theoretical studies have been published using bioinformatics methods (Table 1). Recently, the activity of BAs, GR and several natural compounds was determined by studying molecular docking to measure the binding of these compounds to the SARS-CoV-2 E protein. BAs and GR were found to have strong antiviral activities against enveloped viruses because they are inhibitors of the SARS-CoV-2 E protein (Fatima et al. 2022). Data from other bioinformatics studies suggested that BAs possess antiviral properties because BAs target SARS-CoV-2 viruses at an atomic scale on three functional proteins, which are responsible for human cell adhesion (spikes of the virus) or viral RNA replication (main proteins of SARS-CoV-2, Mpro) (Caliebe et al. 2021; Kadhim et al. 2021; Roy and Menon 2022). However, experimental in vitro and in vivo studies are required to document the antiviral activities of BA/Boswellia extract against SARS-CoV-2 as demonstrated with GR. Meanwhile, the mechanism of action of GR and BA against SARS-CoV-2 is similar and can be improved using them together as with HCV combination therapy (Fig. 1).
Effect of glycyrrhizin and boswellic acids on inflammatory pathways triggered by SARS-CoV-2 in vitro and in vivo animal models
It has been established that all complications of COVID-19 infection result from the hyperinflammation associated with SARS-CoV-2 infection. The SARS-CoV-2 N protein enhances NLRP3 inflammasome activation to induce excessive inflammation (Pan et al. 2021). This finding suggests that drugs with anti-inflammatory properties and NLRP3 inhibition may play an important role in the treatment of COVID-19. The anti-inflammatory activity of BAs and GR has been documented in in vitro, in vivo, and clinical studies. In these studies, experimental inflammation was induced by an inflammatory stimulus other than SARS-CoV-2. Recently, the immunomodulatory and anti-inflammatory properties of BAs and GR were reviewed by many investigators (Gomaa et al. 2021; Gomaa and Abdel-wadood 2021; Zheng et al. 2021; Renda et al. 2022). From the outbreak of the COVID-19 pandemic until July 2022, only six studies on the treatment of COVID-19 inflammation (in vivo, in vitro and bioinformatics) have been published. These studies confirm the anti-inflammatory activity of glycyrrhizin against inflammation caused by SARS-CoV-2 (Table 2).
Interestingly, one of these studies provides a theoretical and practical basis for using glycyrrhizin as a promising drug to treat the COVID-19 cytokine storm that is a major complication of COVID-19 disease (Li H et al. 2022). Furthermore, in mice sepsis ARDS model of acute respiratory distress syndrome which is the most important complications of COVID-19, GR prevents the development of acute respiratory distress syndrome (Gu et al. 2022). GR exerts this effect through multiple mechanism and pathways (Li H et al. 2022). One of these mechanism depend on prevention of SARS-CoV-2 (S1 and Orf3a protein) induced HMGB1 release which induce high release of proinflammatory cytokines IL-1β, IL-8, and IL-6 (Gu et al. 2022; Gowda et al. 2021). Another suggestion involves inhibition of NLRP3 inflammasome inflammatory pathway that is activated by SARS-CoV-2 infection and causes excessive inflammation (Zhao et al. (2021a, b); Wang et al. 2022). Recently, Gu et al (2022) suggested that GR inhibits HMGB1/TLR9 pathways and neutrophils extracellular traps formation. In addition, Zheng et al. (2021) suggested that GA inhibit the inflammation by inhibiting the key targeting to COVID-19 that activates the response to reactive oxygen species.
Several studies in vivo and in vitro confirmed the immunomodulatory and anti-inflammatory activity of BAs/Boswellia extract (Khajehdeh et al. 2022); however, no study demonstrated the anti-inflammatory or immunomodulatory activity of BA in animal model of COVID-19 yet. Recently, Aldahlawi et al. (2020) and Zimmermann-Klemd et al. (2020) demonstrated that Boswellia sacra essential oil, Boswellia extract and BAs exert immunomodulatory effects on T cells and dendritic cells where it deviates the differentiation of monocytes into immature DCs. More recently, according to in vitro LPS-induced inflammation on H9C2 cells, an in vivo zebrafish larval model and molecular docking study, Boswellia extract and BAs inhibited inflammation and cytotoxicity and showed strong redox activity. The mechanism of action may be by increasing anti-inflammatory activity by decreasing specific inflammatory gene expression (iNOS, TNF-α, IL-1, and COX-2) (Siddhu et al. 2022; Taherzadeh et al. 2022). Taken together, the results of the above studies show evidence that GA and BA suppress SARS-CoV-2-induced inflammation through inhibition of the NLRP3 inflammasome pathway and HMGB1 release. Moreover, its immunomodulatory activity contributes to preventing the development of cytokine storms.
Efficacy of glycyrrhizin/licorice extract and boswellic acids/Boswellia extract in the treatment of COVID-19 in clinical trials
GR/licorice was used in clinical trials to treat liver disease, viral infections (HCV, HBV), gastrointestinal disorders, oral diseases, and various skin disorders (Kwon et al. 2020; Huan et al. 2021; Leite et al. 2022) while BAs/Boswellia extract was used to treat osteoarthritis, ischemic stroke, cognitive impairment post-traumatic brain injury, MS, bronchial asthma and brain tumors (Baram et al. 2019; Abdel-Tawab et al. 2021; Varma et al. 2021). The pharmacological bases for the use of GR/licorice and BAs/Boswellia extract in clinical trials have been attributed to their anti-inflammatory, antioxidant and immunomodulatory properties. Since the outbreak of COVID-19, there have been few clinical studies on GR/licorice or BAs/Boswellia extract as a potential treatment for COVID-19, and there are many studies on Chinese prescriptions with licorice as a major ingredient (Sun et al. 2021). Only seven clinical trials with a rigorous design regarding the usefulness of GR, BAs and their combinations have been published through July 2022. These studies provide evidence of the benefit of using GR and BAs in COVID-19 management (Table 3).
Importantly, our study showed that the combinations of GR and BAs were highly successful against COVID-19 (Gomaa et al. 2022). In this study, patients with moderate SARS-CoV-2 received a GR capsule (licorice extract 300 mg contains 60 mg of GR) twice daily 1 h before the BAs capsule (Boswellia extract 300 mg contains 200 mg of BAs). BAs capsules were taken after meals. The treatment was continued for 14 days even if symptoms of infection disappeared before the end of the treatment course. The results of our trial showed that there was significant decrease in percentage of mortality in group that received GR + BAs compared to placebo group with significantly shorter recovery time, in the intervention group. Clinical status on the ordinal score scale showed a significant decrease in the score of the GR + BA group. Clinical deterioration occurred in 0 patients in the intervention group and in 5 patients in the placebo group who required admission to the ICU for mechanical ventilation. There was a significant decrease in CRP and an increase in the percentage of lymphocytes in the intervention group compared with the placebo group.
Interestingly, in our preliminary, uncontrolled trial (20 patients), no patients had long-COVID-19 or post-COVID-19 symptoms as cognitive impairment, but five patients experienced fatigue and one had tachycardia after treatment had ended. These patients continued to receive half the dose of treatment of licorice extract and Boswellia serrata gum with vitamin C and zinc for a further 14 days or until these symptoms disappeared. All symptoms disappeared a month after starting treatment (unpublished data). However, hospitalized patients attending our randomized clinical trial were not followed up after discharge from hospital.
In another randomized, double-blind, placebo-controlled clinical trial conducted in a rigorous design, evidence of the efficacy of BAs in improving clinical symptoms and inflammatory markers in patients with moderate COVID-19 was shown. BAs formulated as syrup have been used three times daily for 14 days. The average length of hospital stay or recovery time was significantly shorter in the BAs group than in the placebo group. Furthermore, clinical manifestations were significantly improved in the BA group more than in the placebo group. This study showed a significant decrease in inflammatory markers as the percentage of neutrophils, neutrophil-to-lymphocyte ratio (NLR), CRP, TNFα and IL-6 levels in the BAs group compared with the placebo (Barzin Tond et al. 2022).
Two other trials suggested that topical use of GR as a nasal or oropharyngeal spray or mucous patch was effective in eradicating SARS-CoV-2, which colonized the nasopharyngeal area and accelerated laboratory and clinical recovery of patients infected with SARS-CoV-2 in the early stages of the disease. Therefore, it has the potential to prevent the progress of the disease and be a prophylactic agent against COVID-19. It may reduce the spread of the virus and, therefore, may play an important role in controlling the outbreak of SARS-CoV-2 (Elsersy et al. 2022; Pourahmad et al. 2022). Furthermore, in a randomized, double-blind, placebo-controlled trial, 2 weeks of inhalation of frankincense (Boswellia carterii) with another blend of essential oil extracts improved energy levels in healthy COVID-19 survivors suffering from a lack of energy for more than 5 months after recovery (long COVID-19). In addition, the results of this study showed that this combination could significantly relieve fatigue among women experiencing fatigue after recovery from COVID-19 (Hawkins et al. 2022).
In retrospective, single-center, observational study, diammonium glycyrrhizinate (DG) combined with vitamin C significantly attenuated the prognoses of COVID-19 disease in 207 hospitalized patients. New onset of complications such as ARDS, acute liver injury and acute myocardial injury was significantly fewer in group of patients treated by diammonium glycyrrhizinate with vitamin C compared with the non-DG group (Tan et al. 2022). This study is further support for the use of glycyrrhizin/licorice extract in the treatment of COVID-19. However, one of the weaknesses of this study is the lack of registration of this study in an international clinical trial site. It is clear from previous results that GR/licorice extract may be more effective than BAs/Boswellia extract in fighting COVID-19 while the combination of GR and BAs was the best in combating COVID-19.
Potential effect of glycyrrhizin/licorice extract and boswellic acids/Boswellia extracts in prevention of post-COVID-19 cognitive impairment
A recent study indicated that hospitalization and the severity of COVID-19 can increase the risk of post-COVID-19 AD (Li C et al. 2022). However, another study showed that cognitive impairment was not predicted by age, pre-existing conditions, or severity of COVID-19 disease (Hadad et al. 2022). Whatever the potential mechanisms underlying the cognitive impairment after recovery from COVID-19, there is not yet a study that tests a specific drug to prevent or treat this symptom. Meanwhile, GR/licorice extract and BAs/Boswellia extract have been shown to treat and prevent the development of cognitive impairment resulting from many causes in experimental and clinical studies (Ravanfar et al. 2019; Rajabian et al. 2020; Gomaa et al. 2021; Haghaei et al. 2021; Siddiqui et al. 2021, Gong et al. 2022). Our first study on the effect of Boswellia serrata extract on cognitive impairment showed that Boswellia serrata extract was effective in the prevention and treatment of cognitive impairment in animal models by inhibiting oxidative stress and proinflammatory cytokines (Gomaa et al. 2019).
Based on evidences, it is concluded that inflammation and oxidative stress plays a crucial role in cognitive impairment pathophysiology. Systemic inflammation may play an important role in promoting neurodegeneration, and cognitive decline. Inflammation is characterized by increased blood levels of proinflammatory cytokines. Furthermore, increased levels of oxidative stress in the brain over cellular antioxidant defenses can damage cellular structures and increase accumulation of beta-amyloid and neurofibrillary tangles. Increased oxidative stress and systemic inflammation may occur as a result of events such as infection, chronic disease, physical and psychological stress, and cellular aging (Walker et al. 2019; Buccellato et al. 2021).
Interestingly, it has been observed that HMGB1 (High Mobility Group Box-1) is elevated largely in serum of COVID-19 patients. HMGB1 is a proinflammatory cytokine with a strong ability to stimulate the inflammatory response and its elevated level have been associated with disease severity and the development of a cytokine storm (Al‑kuraishy et al. 2022). Moreover, HMGB1 has been suggested to be elevated in neuroinflammation, cognitive impairment, postoperative cognitive dysfunction and cognitive impairment in the late stage of TBI (traumatic brain injury) (Paudel et al. 2020; Lin et al. 2021; Tan et al. 2021). GR has been confirmed to have antagonistic properties to HMGB1 and NLRP3 inflammasome which are activated by TLR2, the main contributor to neuroinflammation and cognitive dysfunction (Lin et al. 2021; Tan et al. 2021).
In addition, GR has been shown to have a blocking effect against lipopolysaccharide-induced neuroinflammation and cognitive impairment in C57 mice by suppressing activation of the TLR4 signaling pathway that inhibits production of proinflammatory cytokines in the brain of C57 mice (Cho et al. 2018; Liu et al. 2019). Furthermore, in aged mice, GR improves learning and memory in aged mice by modulating T/B cell proliferation through the inhibition of several genes related to macrophages and neutrophils (Jiang et al. 2020). There are few clinical studies that have shown the effectiveness of GR in managing cognitive dysfunction. In a randomized, placebo-controlled clinical study, elderly people with mild cognitive impairment (MCI) used licorice extract for 12 weeks with or without training, and it can slow or halt the progression of MCI by significantly reducing inflammatory markers (IL-1β and TNF-α) but their decrease was more pronounced in group used licorice with training (Kohanpour et al. 2017).
Several in vivo studies demonstrated the benefit of BAs/Boswellia extract in cognitive impairment and plasticity impairments induced in the animal model by LPS-induced excessive inflammation. It works by inhibiting inflammation and oxidative stress. It may modulate the activity of multiple molecular targets that influence the signaling pathways that contribute to the pathogenesis of cognitive impairment (Borooni et al. 2020; Marefati et al. 2020, 2021, 2022). Mohammed et al. (2022) observed that BA modulated the expression of parameters related to the Wnt/-catenin pathway and decreased the expression of TNF-α IL-1β, attenuated lipid peroxidation, and raised total brain antioxidants. Another study indicated that a possible mechanism for the beneficial effects of BAs may include inhibition of the 5-LOX/COX pathway in arachidonic acid metabolism, activation of Nrf2 (has anti-inflammatory activity), through binding to ARE (antioxidant response element) and inhibition of NF-kB (Siddiqui et al. 2021). Furthermore, BAs eliminate memory impairment by enhancing the activity of PPARγ and its downstream regulators, matrix metalloproteinase 2 genes in the hippocampus (Gunasekaran et al. 2021). In a double-blind, randomized, placebo-controlled clinical trial, the effect of BAs on cognitive impairment after traumatic brain injury (TBI) was investigated. The results of this study after 3 months of follow-up showed that BA was safe, well tolerated, and had a positive effect on the cognitive function of patients with TBI (Meshkat, 2022). In another clinical trial, Boswellia papyrifera significantly improved visuospatial memory, but verbal memory was not changed (Sedighi et al. 2014).
Aforementioned data provide evidence that systemic inflammation associated with COVID-19 infection is a prominent cause of neurodegeneration, and cognitive impairment. Interestingly, not only preclinical studies but also studies conducted in clinical settings have well established the potential effect of GR/licorice and BAs/Boswellia extract in the prevention and treatment of cognitive impairment by inhibiting the activity of multiple molecules that activate the signaling pathways of inflammation.
Limitations
This review has some limitations. The most obvious limitation is the small and few clinical trials. The absence of preclinical in vitro and in vivo studies testing the antiviral activity of BA is another limitation. Third, the lack of studies comparing the activity of GR alone or GR + BA with the newly approved anti-COVID-19. Fourth: there is no follow-up for patients after their discharge from the hospital. Fifth, the potential interaction between GR or BAs and standard protocol drugs has not been studied. Finally, all clinical studies were performed only on adult patients and the data may not be applicable to pediatric groups.
Conclusions
Several studies demonstrated the antiviral, anti-inflammatory and immunomodulatory activity of GR and BAs. All these studies confirmed that GR or GR + BAs have strong antiviral activity and can be used as a therapeutic agent for COVID-19 and as a protective agent against SARS-CoV-2. Our recent clinical trial showed that the combination of GR and BAs was highly successful against COVID-19. There are several clinical and preclinical studies indicating the benefit of GR and BAs in preventing cognitive impairment due to systemic inflammation, such as in COVID-19. However, more randomized controlled trials with effective, large populations are needed to show a definitive conclusion about therapeutic efficacy of GR and BAs in treatment of COVID-19 and prevention of post-COVID-19 symptoms. Based on the safety, clinical trials, and preclinical evidence presented in this review together with the failure of newly approved antiviral agents, GR or GR with BAs, should be disseminated globally and systematically in the treatment of SARS-CoV-2 and its variants and prevention of post-COVID cognitive impairment. However, more randomized controlled trials with effective, large populations are needed to show a definitive conclusion about therapeutic efficacy of GR and BAs in treatment of COVID-19 and prevention of post-COVID-19 symptoms.
Availability of data and materials
Data on relevant human studies in the current review. https://drive.google.com/file/d/17YZvAhCFoBMV6vdnjLFzfNN52vtpCKri/view?usp=sharing.
Abbreviations
- ARDS:
-
Acute respiratory distress Syndrome
- B. serrata :
-
Boswellia serrate
- Bas:
-
Boswellic acids
- KBA:
-
11-Keto-β-boswellic acid
- AKBA:
-
3-O-acetyl-11-keto-β- boswellic acid
- ACE2:
-
Angiotensin-converting enzyme 2
- Bax:
-
Bcl-2-associated X protein
- COX-2:
-
Cyclooxygenase-2
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- GR:
-
Glycyrrhizin
- HMGB1:
-
High-mobility group box 1 protein
- iNOS:
-
Inducible nitric oxide synthase
- IL-1β:
-
Interleukin one beta
- IL-6:
-
Interleukin-6
- JAK:
-
Janus kinase
- JNK:
-
C-Jun N-terminal kinase
- 5-LOX:
-
5-Lipoxygenase
- LTB4:
-
Leukotriene B4
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
- NLRP3:
-
Nod-like receptor protein3
- NF-κB:
-
Nuclear factor kappa B
- SARS-CoV:
-
Severe acute respiratory syndrome coronavirus
- TLR:
-
Toll-Like Receptor 2
- TNF-α:
-
Tumor necrosis factor alpha
References
Abdel-Tawab M (2021) Considerations to be taken when carrying out medicinal plant research—what we learn from an insight into the IC50 values, bioavailability and clinical efficacy of exemplary anti-inflammatory herbal components. Pharmaceuticals 14:437. https://doi.org/10.3390/ph14050437
Ahmad S, Waheed Y, Abro A, Abbasi SW, Ismail S (2021) Molecular screening of glycyrrhizin-based inhibitors against ACE2 host receptor of SARS-CoV-2. J Mol Model 27(7):206. https://doi.org/10.1007/s00894-021-04816-y
Aldahlawi AM, Alzahrani AT, Elshal MF (2020) Evaluation of immunomodulatory effects of Boswellia sacra essential oil on T-cells and dendritic cells. BMC Complement Med Ther 20:352. https://doi.org/10.1186/s12906-020-03146-5
Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE (2022) High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology 30(3):811–820. https://doi.org/10.1007/s10787-022-00988-y
Alshanqeeti S, Bhargava A (2022) COVID-19 rebound after paxlovid treatment: a case series and review of literature. Cureus 14(6):e26239. https://doi.org/10.7759/cureus.26239
Baker HA, Safavynia SA, Evered LA (2021) The “third wave”: impending cognitive and functional decline in COVID-19 survivors. Br J Anaesth 1:44–47. https://doi.org/10.1016/j.bja.2020.09.045
Baram SM, Karima S, Shateri S et al (2019) Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double- blind, placebo-controlled, pilot trial. Inflammopharmacology 27:1101–1112. https://doi.org/10.1007/s10787-019-00627
Barzin Tond S, Balenci L, Khajavirad N, Salehi M, Tafakhori A, Shahmohammadi MR et al (2022) Inflawell® improves neutrophil-to-lymphocyte ratio and shortens hospitalization in patients with moderate COVID-19, in a randomized double-blind placebo-controlled clinical trial. Inflammopharmacology 30(2):465–475. https://doi.org/10.1007/s10787-022-00928-w
Bernal AJ, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A et al (2022) Molnupiravir for oral treatment of Covid-19 in Nonhospitalized patients. N Engl J Med 386(6):509–520. https://doi.org/10.1056/NEJMoa2116044
Bocci G, Bradfute S, Ye C, Garcia M, Parvathareddy J, Reichard W et al (2020) Virtual and in vitro antiviral screening revive therapeutic drugs for COVID-19. ACS Pharmacol Transl Sci 3(6):1278–1292. https://doi.org/10.1021/acsptsci.0c00131
Borooni S, Nourbakhsh F, Tajbakhsh E, Behshood P (2020) The effects of aqueous extract of boswellia serrata on memory impairment induced by lipopolysaccharide. IJML 7(4):300–311
Buccellato FR, D’Anca M, Fenoglio C, Scarpini E, Galimberti D (2021) Role of oxidative damage in Alzheimer’s disease and neurodegeneration: from pathogenic mechanisms to biomarker discovery. Antioxidants (Basel) 10(9):1353. https://doi.org/10.3390/antiox10091353
Caliebe RH, Scior T, Ammon HPT (2021) Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: bioinformatic studies. Arch Pharm (weinheim) 354(11):e2100160. https://doi.org/10.1002/ardp.202100160
Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM et al (2022) Fatigue and cognitive impairment in Post-COVID-19 Syndrome: a systematic review and meta-analysis. Brain Behav Immun 101:93–135. https://doi.org/10.1016/j.bbi.2021.12.020
Chavda VP, Patel AB, Vihol D, Vaghasiya D, Ahmed K, Trivedi K, Dave D (2022) Herbal remedies, nutraceuticals, and dietary supplements for covid-19 management: an update. Clin Complem Med Pharmacol 2(1):100021. https://doi.org/10.1016/j.ccmp.2022.100021
Chen R, Huang Y, Quan J, Liu J, Wang H, Billiar TR, Zeh Lotze MT., HJ, Kang R, Tang D, (2020) HMGB1 as a potential biomarker and therapeutic target for severe COVID-19. Heliyon 6(12):e05672. https://doi.org/10.1016/j.heliyon.2020.e05672
Cho MJ, Kim JH, Park CH, Lee AY, Shin YS, Lee JH, Park CG, Cho EJ (2018) Comparison of the effect of three licorice varieties on cognitive improvement via an amelioration of neuroinflammation in lipopolysaccharide-induced mice. Nutr Res Pract 12(3):191–198. https://doi.org/10.4162/nrp.2018.12.3.191
Coronavirus (COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19, December 22, 2021, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19
Crivelli L, Palmer K, Calandri I, Guekht A, Beghi E, Carroll W et al (2022) Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement 18(5):1047–1066. https://doi.org/10.1002/alz.12644
Elsersy HE, Zahran MAH, Elbakry A-E, Abd-Elwahab M, Ahmed MM, Elgandy MS, Mohammed M, Elewa NM (2022) Combined nasal, oropharyngeal povidone iodine plus glycyrrhizic acid sprays, accelerate clinical and laboratory recovery and reduces household transmission of SARS-CoV-2: a randomized placebo-controlled clinical trial. Front Med 9:863917. https://doi.org/10.3389/fmed.2022.863917
Fatima SW, Alam S, Khare SK (2022) Molecular and structural insights of β-boswellic acid and glycyrrhizic acid as potent SARS-CoV-2 Envelope protein inhibitors. Phytomed Plus. 2(2):100241. https://doi.org/10.1016/j.phyplu.2022.100241
Fomenko VV, Rudometova NB, Yarovaya OI, Rogachev AD, Fando AA et al (2022) Synthesis and in vitro study of antiviral activity of glycyrrhizin nicotinate derivatives against HIV-1 pseudoviruses and SARS-CoV-2 viruses. Molecules 27:295. https://doi.org/10.3390/molecules27010295
Gomaa AA, Abdel-Wadood YA (2021) The potential of glycyrrhizin and licorice extract in combating COVID-19 and associated conditions. Phytomed plus 1(3):100043. https://doi.org/10.1016/j.phyplu.2021.100043
Gomaa AA, Farghaly HA, Abdel-Wadood YA, Gomaa GA (2021a) Potential therapeutic effects of boswellic acids/Boswellia serrata extract in the prevention and therapy of type 2 diabetes and Alzheimer’s disease. Naunyn Schmiedebergs Arch Pharmacol 394(11):2167–2185. https://doi.org/10.1007/s00210-021-02154-7
Gomaa AA, Makboul RM, Al-Mokhtar MA, Nicola MA (2019) Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Biomed Pharmacother 109:281–292. https://doi.org/10.1016/j.biopha.2018.10.056
Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA, Hammam DS (2022) Advancing combination treatment with glycyrrhizin and boswellic acids for hospitalized patients with moderate COVID-19 infection: a randomized clinical trial. Inflammopharmacology. 30(2):477–486. https://doi.org/10.1007/s10787-022-00939-7
Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA (2021) Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology 29(4):1033–1048. https://doi.org/10.1007/s10787-021-00841-8
Gong Y, Jiang X, Yang S, Huang Y, Hong J, Ma Y, Fang X, Fang Y, Wu J (2022) The Biological Activity of 3-O-Acetyl-11-keto-β-Boswellic Acid in Nervous System Diseases. Neuromolecular Med 18:1–11. https://doi.org/10.1007/s12017-022-08707-0
Gottlieb RL, Vaca CE, Paredes J, Mera J, Webb BJ, Perez G et al (2021) Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 386(4):305–315. https://doi.org/10.1056/NEJMoa2116846
.Gowda P, Patrick S, Joshi SD, Kumawat RK, Sen E (2021) Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 142:155496. https://doi.org/10.1016/j.cyto.2021.155496
Gu J, Ran X, Deng J, Zhang A, Peng G, Du J, Wen D, Jiang B, Xia F (2022) Glycyrrhizin alleviates sepsis-induced acute respiratory distress syndrome via suppressing of HMGB1/TLR9 pathways and neutrophils extracellular traps formation. Int Immunopharmacol 108:108730. https://doi.org/10.1016/j.intimp.2022.108730
Gunasekaran V, Avarachan J, Augustine A, Khayum A (2021) 3-O-Acetyl-11-keto-β-boswellic acid ameliorates acquired, consolidated and recognitive memory deficits through the regulation of hippocampal PPAR γ, MMP9 and MMP2 genes in dementia model. Heliyon 7(12):e08523. https://doi.org/10.1016/j.heliyon.2021.e08523
Haage V, De Jager PL (2022) Neuroimmune contributions to Alzheimer’s disease: a focus on human data. Mol Psychiatry 6:1–18. https://doi.org/10.1038/s41380-022-01637-0
Hadad R, Khoury J, Stanger C, Fisher T, Schneer S, Ben-Hayun R, Possin K et al (2022) Cognitive dysfunction following COVID-19 infection. J Neurovirol 26:1–8. https://doi.org/10.1007/s13365-022-01079-y
Haghaei H, Soltani S (2021) Aref hosseini S, Rashidi M, Karima S Boswellic Acids as Promising leads in drug development against Alzheimer’s Disease. Pharm Sci 27(1):14–31. https://doi.org/10.34172/PS.2020.25
Hammond J, Leister-Tebbe H, Gardener A et al (2022) Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2118542
HAN Archive - 00467.(2022). COVID-19 Rebound After Paxlovid Treatment. (2022). https://emergency.cdc.gov/han/2022/han00467.asp Accessed May 31, 2022.
Hawkins J, Hires C, Keenan L, Dunne E (2022) Aromatherapy blend of thyme, orange, clove bud, and frankincense boosts energy levels in post-COVID-19 female patients: a randomized, double-blinded, placebo controlled clinical trial. Complement Ther Med. 67:102823. https://doi.org/10.1016/j.ctim.2022.102823
Huan C, Xu Y, Zhang W, Guo T, Pan H, Gao S (2021) Research progress on the antiviral activity of glycyrrhizin and its derivatives in liquorice. Front Pharmacol 12:680674. https://doi.org/10.3389/fphar.2021.680674
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397:220–232
Jamshidi Z, Hashemi M, Yazdian-Robati R, Etemad L, Salmasi Z, Kesharwani P (2022) Effects of Boswellia species on viral infections with particular attention to SARS-CoV-2. Inflammopharmacology 26:1–13. https://doi.org/10.1007/s10787-022-01037-4
Jezova D, Karailiev P, Karailievova L, Puhova A, Murck H (2021) Food enrichment with glycyrrhiza glabra extract suppresses ACE2 mRNA and protein expression in rats—possible implications for COVID-19. Nutrients 13:2321. https://doi.org/10.3390/nu13072321
Jiang R, Gao J, Shen J, Zhu X, Wang H, Feng S, Huang C, Shen H, Liu H (2020) Glycyrrhizic acid improves cognitive levels of aging mice by regulating t/b cell proliferation. Front Aging Neurosci 12:570116. https://doi.org/10.3389/fnagi.2020.570116
Kadhim MM, Washeel A, Mrebee Zarzoor A, Kadhum WR (2021) Inhibition of SARS-CoV-2 reproduction using Boswellia carterii: a theoretical study. J Mol Liq 337:116440. https://doi.org/10.1016/j.molliq.2021.116440
Khajehdehi M, Khalaj-Kondori M, Baradaran B (2022) Molecular evidences on anti-inflammatory, anticancer, and memory-boosting effects of frankincense. Phytother Res 36(3):1194–1215. https://doi.org/10.1002/ptr.7399
Kohanpour MA, Peeri M, Azarbayjani MA (2017) The Effects of Glycyrrhiza glabra L. extract use with aerobic training on inflammatory factors and cognitive state in elderly with mild cognitive impairment. J Herbmed Pharmacol 6(4):178–184
Kwon YJ, Son DH, Chung TH, Lee YJ (2020) A review of the pharmacological efficacy and safety of licorice root from corroborative clinical trial findings. J Med Food 23(1):12–20. https://doi.org/10.1089/jmf.2019.4459
Leite CDS, Bonafé GA, Carvalho Santos J, Martinez CAR, Ortega MM, Ribeiro ML (2022) The anti-inflammatory properties of licorice (Glycyrrhiza glabra)-derived compounds in intestinal disorders. Int J Mol Sci 23(8):4121. https://doi.org/10.3390/ijms23084121
Li C, Liu J, Lin J, Shang H (2022) COVID-19 and risk of neurodegenerative disorders: a Mendelian randomization study. Transl Psychiatry 12(1):283. https://doi.org/10.1038/s41398-022-02052-3
Li H, You J, Yang X, Wei Y, Zheng L, Zhao Y, Huang Y, Jin Z, Yi C (2022) Glycyrrhetinic acid: A potential drug for the treatment of COVID-19 cytokine storm. Phytomedicine 102:154153. https://doi.org/10.1016/j.phymed.2022b.154153
Li X, Qiu Q, Li M, Lin H, Cao S, Wang Q, Chen Z et al (2021) Chemical composition and pharmacological mechanism of ephedra-glycyrrhiza drug pair against coronavirus disease 2019 (COVID-19). Aging (Albany NY) 13(4):4811–4830. https://doi.org/10.18632/aging.202622
Li J, Xu D, Wang L, Zhang M, Zhang G, Li E, He S (2021) Glycyrrhizic acid inhibits SARS-CoV-2 infection by blocking spike protein-mediated cell attachment. Molecules 26:6090. https://doi.org/10.3390/molecules26206090
Lin F, Shan W, Zheng Y, Pan L, Zuo Z (2021) Toll-like receptor 2 activation and up-regulation by high mobility group box-1 contribute to post-operative neuroinflammation and cognitive dysfunction in mice. J Neurochem. 158(2):328–341. https://doi.org/10.1111/jnc.15368
Liu C, Lee J, Ta C, Soroush A, Rogers JR, Kim JH, Natarajan K, Zucker J, Perl Y, Weng C (2022) Risk factors associated with SARS-CoV-2 breakthrough infections in fully mRNA-vaccinated individuals: retrospective analysis. JMIR Public Health Surveill 8(5):e35311. https://doi.org/10.2196/35311
Liu W, Huang S, Li Y, Zhang K, Zheng X (2019) Suppressive effect of glycyrrhizic acid against lipopolysaccharide-induced neuroinflammation and cognitive impairment in C57 mice via toll-like receptor 4 signaling pathway. Food Nutr Res. https://doi.org/10.29219/fnr.v63.1516
Maddah M, Bahramsoltani R, Yekta N, Rahimi R, Aliabadi R, Pourfath M (2021) Proposing high-affinity inhibitors from Glycyrrhiza glabra L. against SARS-CoV-2 infection: virtual screening and computational analysis New. J Chem 45:15977–15995
Marefati N, Beheshti F, Etemadizadeh P, Hosseini M, Anaeigoudari A (2022) Gum resin extract of Boswellia serrata attenuates lipopolysaccharide-induced inflammation and oxidative damage in hepatic and renal tissues of rats. Asian Pac J Trop Biomed [Serial Online] 12:20–5
Marefati N, Beheshti F, Memarpour S, Bayat R, Naser SM, Sadeghnia HR, Ghazavi H, Hosseini M (2020) The effects of acetyl-11-keto-β-boswellic acid on brain cytokines and memory impairment induced by lipopolysaccharide in rats. Cytokine. 2020(131):155107. https://doi.org/10.1016/j.cyto.2020.155107
Marefati N, Beheshti F, Memarpour S, Rezaei M, Hosseini M (2021) The effects of pre-treatment with olibanum and its constituent, boswellic acid on synaptic plasticity impairments induced by lipopolysaccharide in rats. Avicenna J Phytomed 11(1):68–78
Meshkat S, Mahmoodi Baram S, Rajaei S, Mohammadian F, Kouhestani E et al (2022) Boswellia serrata extract shows cognitive benefits in a double-blind, randomized, placebo-controlled pilot clinical trial in individuals who suffered traumatic brain injury. Brain Inj 36(4):553–559. https://doi.org/10.1080/02699052.2022.2059816
Miskowiak KW, Fugledalen L, Jespersen AE, Sattler SM, Podlekareva D, Rungby J, Porsberg CM, Johnsen S (2022) Trajectory of cognitive impairments over 1 year after COVID-19 hospitalisation: Pattern, severity, and functional implications. Eur Neuropsychopharmacol 59:82–92. https://doi.org/10.1016/j.euroneuro.2022.04.004
Mohamed EA, Ahmed HI, Zaky HS et al (2022) Boswellic acids ameliorate neurodegeneration induced by AlCl3: the implication of Wnt/β-catenin pathway. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-20611-5
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS et al (2021) Post-acute COVID-19 syndrome. Nat Med 27:601–615
Ng SL, Khaw KY, Ong YS, Goh HP, Kifli N, The SP, Ming LC, Kotra V, Goh BH (2021) Licorice: a potential herb in overcoming SARS-CoV-2 infections. J Evid Based Integr Med 26:2515690X21996662. https://doi.org/10.1177/2515690X21996662
Ng TI, Correia I, Seagal J et al (2022) Antiviral drug discovery for the treatment of COVID-19 infections. Viruses 14:961. https://doi.org/10.3390/v14050961
Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, Xiao F, et al.(2021). SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun 12(1):4664. https://doi.org/10.1038/s41467-021-25015-6. Erratum in: Nat Commun. 2021 12(1):5306
Paudel YN, Angelopoulou E, Semple B, Piperi C, Othman I, Shaikh MF (2020) Potential neuroprotective effect of the hmgb1 inhibitor glycyrrhizin in neurological disorders, ACS Chem. Neurosci 11:485–500. https://doi.org/10.1021/acschemneuro.9b00640
Petrov P, Mihaylov A, Shopova M, Boncheva M, Marquez D (2021) Efficacy and safety of viusid and asbrip in hospitalized patients with mild and moderate COVID-19: a randomized controlled trial. Adv Infect Dis 11:171–184. https://doi.org/10.4236/aid.2021.112017
Pourahmad M, Shahzamani K, Nikokar F, Saniei M, Saniei M, Soltani R (2022) The effects of licorice mucoadhesive patches on the results of nasopharyngeal swab real-time polymerase chain reaction test of SARS-CoV-2; a randomized triple-blind placebo-controlled clinical trial. Immunopathol Persa. https://doi.org/10.34172/ipp.2022.31400
Queensland Health. COVID-19 Treatment Guidelines for mild-moderate disease (Adults) v1.4 Published 28/06/2022 Page 2 of 37. Published by the State of Queensland (Queensland Health)
Rajabian A, Sadeghnia H, Fanoudi S, Hosseini A (2020) Genus Boswellia as a new candidate for neurodegenerative disorders. Iran J Basic Med Sci 23(3):277–286. https://doi.org/10.22038/IJBMS.2020.35288.8419
Ravanfar P, Namazi G, Borhani-Haghighi A, Zafarmand S (2018) Neurologic effects of licorice: a review. Phcog Rev 12:115–119
Rehman MF, Akhter S, Batool AI, Selamoglu Z, Sevindik M, Eman R, Mustaqeem M, Akram MS et al (2021) Effectiveness of natural antioxidants against SARS-CoV-2? Insights from the In-Silico World. Antibiotics 10:1011. https://doi.org/10.3390/antibiotics10081011
Renaud-Charest O, Lui LMW, Eskander S, Ceban F, Ho R, Di Vincenzo JD et al (2021) Onset and frequency of depression in post-COVID-19 syndrome: a systematic review. J Psychiatr Res. 2021(144):129–137. https://doi.org/10.1016/j.jpsychires.2021.09.054
Renda G, Gökkaya İ, Şöhretoğlu D (2022) Immunomodulatory properties of triterpenes. Phytochem Rev 21(2):537–563. https://doi.org/10.1007/s11101-021-09785-x
Robert FSE (2022) Bad news for Paxlovid? Coronavirus can find multiple ways to evade COVID-19 drug. Science 377(6602):29. https://doi.org/10.1126/science.add7226
Roy A, Menon T (2022) Evaluation of bioactive compounds from Boswellia serrata against SARS-CoV-2. Vegetos 35(2):404–414. https://doi.org/10.1007/s42535-021-00318-7
Rubin R (2022) From positive to negative to positive again-the mystery of why COVID-19 rebounds in some patients who take paxlovid. JAMA 327(24):2380–2382. https://doi.org/10.1001/jama.2022.9925
Sedighi B, Pardakhty A, Kamali H, Shafiee K, Hasani BN (2014) Effect of Boswellia papyrifera on cognitive impairment in multiple sclerosis. Iran J Neurol 13(3):149–53
Siddhu NSS, Guru A, Satish Kumar RC et al (2022) Pro-inflammatory cytokine molecules from Boswellia serrate suppresses lipopolysaccharides induced inflammation demonstrated in an in-vivo zebrafish larval model. Mol Biol Rep 49:7425–7435. https://doi.org/10.1007/s11033-022-07544-5
Siddiqui A, Shah Z, Jahan RN, Othman I, Kumari Y (2021) Mechanistic role of boswellic acids in Alzheimer’s disease: emphasis on anti-inflammatory properties. Biomed Pharmacother 144:112250. https://doi.org/10.1016/j.biopha.2021.112250
Sinha SK, Prasad SK, Islam MA, Gurav SS, Patil RB, AlFaris NA et al (2021) Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J Biomol Struct Dyn 39(13):4686–4700. https://doi.org/10.1080/07391102.2020.1779132
Subedi L, Tchen S, Gaire BP, Hu B, Hu K (2021) Adjunctive nutraceutical therapies for COVID-19. Int J Mol Sci 22(4):1963. https://doi.org/10.3390/ijms22041963
Sun Z, He G, Huang N, Thilakavathy K, Lim JCW, Kumar SS, Xiong C (2021) Glycyrrhizic acid: a natural plant ingredient as a drug candidate to treat COVID-19. Front Pharmacol 12:707205. https://doi.org/10.3389/fphar.2021.707205
Taherzadeh D, Baradaran Rahimi V, Amiri H, Ehtiati S, Yahyazadeh R, Hashemy SI, Askari VR (2022) Acetyl-11-Keto-β-boswellic acid (AKBA) prevents lipopolysaccharide-induced inflammation and cytotoxicity on H9C2 cells. Evid Based Complement Alternat Med 2022:2620710. https://doi.org/10.1155/2022/2620710
Tan R, Xiang X, Chen W, Yang Z, Hu W, Qu H, Liu J (2022) Efficacy of diammonium glycyrrhizinate combined with vitamin C for treating hospitalized COVID-19 patients: a retrospective, observational study. QJM. https://doi.org/10.1093/qjmed/hcab184
Tan SW, Zhao Y, Li P, Ning YL, Huang ZZ, Yang N, Liu D, Zhou YG (2021) HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J Neuroinflammation 18(1):241. https://doi.org/10.1186/s12974-021-02274-0
Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ (2021) 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 8:416–427
Tavares-Júnior JWL, de Souza ACC, Borges JWP, Oliveira DN, Siqueira-Neto JI, Sobreira-Neto MA, Braga-Neto P (2022) COVID-19 associated cognitive impairment: a systematic review. Cortex 152:77–97. https://doi.org/10.1016/j.cortex.2022.04.006
Tolah AM, Altayeb LM, Alandijany TA, Dwivedi VD, El-Kafrawy SA, Azhar EI (2021) Computational and In vitro experimental investigations reveal anti-viral activity of licorice and glycyrrhizin against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals 14:1216. https://doi.org/10.3390/ph14121216
Tsai MS, Shih WT, Yang YH, Lin YS, Chang GH, Hsu CM et al (2022) Potential inhibitor for blocking binding between ACE2 and SARS-CoV-2 spike protein with mutations. Biomed Pharmacother 149:112802. https://doi.org/10.1016/j.biopha.2022.112802
van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E et al (2021) Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 13:609. https://doi.org/10.3390/v13040609
Varma K, Haponiuk J, Gopi S (2021) Antiinflammatory activity of Boswellia, Editor(s): Sreeraj Gopi. Augustine Amalraj, Ajaikumar Kunnumakkara, Sabu Thomas, Inflammation and Natural Products, Academic Press 2021:147–159. https://doi.org/10.1016/B978-0-12-819218-4.00010-9
Walker KA, Ficek BN, Westbrook R (2019) Understanding the Role of systemic inflammation in Alzheimer’s disease. ACS Chem Neurosci 10(8):3340–3342. https://doi.org/10.1021/acschemneuro.9b00333
Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. (2022a) COVID-19 rebound after paxlovid and molnupiravir during January-June 2022a. medRxiv [Preprint].:2022a.06.21.22276724
Wang Z, Xu G, Li Z, Xiao X, Tang J, Bai Z (2022) NLRP3 inflammasome pharmacological inhibitors in glycyrrhiza for NLRP3-driven diseases treatment: extinguishing the fire of inflammation. J Inflamm Res 15:409–422. https://doi.org/10.2147/JIR.S344071
WHO. (2022). Weekly Epidemiological Update on COVID-19 - 13 July 2022, https://www.who.int › Publications › m › item
WHO. (2021). A clinical case definition of post COVID-19 condition by a Delphi consensus. Oct 6, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_ definition-2021.1. Accessed Dec 12, 2021.
Yi Y, Li J, Lai X, Zhang M, Kuang Y, Bao YO, Yu R, Hong W, Muturi E, Xue H, Wei H, Li T et al (2021) Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J Adv Res 36:201–210. https://doi.org/10.1016/j.jare.2021.11.012
Yu S, Zhu Y, Xu J, Yao G, Zhang P, Wang M, Zhao Y, Lin G, Chen H, Chen L, Zhang J (2021) Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS CoV-2. Phytomedicine 85:153364. https://doi.org/10.1016/j.phymed.2020.153364
Zhao N, Di B, Xu LL (2021a) The NLRP3 inflammasome and COVID-19: activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev 61:2–15. https://doi.org/10.1016/j.cytogfr.2021.06.002
Zhao Z, Xiao Y, Xu L, Liu Y, Jiang G, Wang W, Li B et al (2021b) Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl Mater Interfaces 13(18):20995–21006. https://doi.org/10.1021/acsami.1c02755
Zheng W, Huang X, Lai Y, Liu X, Jiang Y, Zhan S (2021) Glycyrrhizic acid for COVID-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front Pharmacol 12:631206. https://doi.org/10.3389/fphar.2021.631206
Zimmermann-Klemd AM, Reinhardt JK, Nilsu T, Morath A, Falanga CM et al (2020) Boswellia carteri extract and 3-O-acetyl-alpha-boswellic acid suppress T cell function. Fitoterapia 146:104694. https://doi.org/10.1016/j.fitote.2020.104694
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was not funded by any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AA. Gomaa: resources, writing—original draft, writing—review and editing, supervision. YA Abdel-Wadood: resources, review and editing. MA Gomaa: resources, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomaa, A.A., Abdel-Wadood, Y.A. & Gomaa, M.A. Glycyrrhizin and boswellic acids, the golden nutraceuticals: multitargeting for treatment of mild–moderate COVID-19 and prevention of post-COVID cognitive impairment. Inflammopharmacol 30, 1977–1992 (2022). https://doi.org/10.1007/s10787-022-01062-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01062-3