Abstract
Aims
COVID-19 is a significant global threat to public health. Despite the availability of vaccines and anti-viral drugs, there is an urgent need for alternative treatments to help prevent and/or manage COVID-19 symptoms and the underlying dysregulated immune response. We hypothesized that administration of Inflawell® syrup, a Boswellia extract formulation enriched for boswellic acids (BAs), can reduce the excessive or persistent inflammation and thereby prevent disease progression. BAs are medicinally activated triterpenoids found in the resins of Boswellia spp., and possess an immense therapeutic potential due to their anti-inflammatory and immunoregulatory activities. We investigated the effect of Inflawell® syrup, on moderate COVID-19 patients along with the current standard of care treatment.
Methods
A randomized placebo-controlled double-blind clinical trial was conducted, following definitive confirmation of COVID-19. Forty-seven hospitalized patients with moderate COVID-19 were enrolled and received either the Inflawell® syrup or placebo. Clinical symptoms and markers of inflammation were evaluated at baseline and completion of the trial.
Results
Our clinical trial revealed an increase in the percentage of oxygen saturation level in patients that received the BAs compared to placebo (P < 0.0001). In addition, the average duration of hospitalization was significantly shorter in the BAs group compared with the placebo group (P < 0.04). Concomitantly, some improvement in the clinical symptoms including cough, dyspnea, myalgia, headache, and olfactory and gustatory dysfunction were detected in the BAs group. Hematologic findings showed a significant decrease in the percentage of neutrophils (P < 0.006) and neutrophil-to-lymphocyte ratio (NLR) levels (P < 0.003), associated with a significant increase in the percentage of lymphocytes in the BAs group compared with the placebo (P < 0.002). Additionally, a significant decrease in CRP, LDH, IL − 6 and TNF − α levels was detected in the BAs group. Following the intervention, fewer patients in the BAs group were PCR-positive for COVID-19 compared to placebo, though not statistically significant.
Conclusion
Overall, the treatment with Inflawell® resulted in shorter hospital stay, alleviation of COVID-19 clinical symptoms and decline in the level of pro-inflammatory cytokines.
Trial registration
The trial has been registered in https://www.irct.ir with unique identifier: IRCT20170315033086N10 (https://en.irct.ir/trial/51631). IRCT is a primary registry in the WHO registry network (https://www.who.int/clinical-trials-registry-platform/network/primary-registries).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global COVID-19 pandemic has resulted in a large number of hospitalization and death due to a lack of effective treatments to manage COVID-19 symptoms. SARS-CoV2 infection, triggers a variable immune response and clinical symptoms ranging from benign and asymptomatic to rapidly progressing symptoms leading to acute respiratory distress syndrome (ARDS) (Hoehl et al. 2020; Grasselli et al. 2020). The primary immune response against SARS-CoV2 involves activation of the innate immune system including recruitment of monocytes-macrophages, neutrophils, natural killer cells and the release of interferons (IFNs), pro-inflammatory cytokines, and chemokines to coordinate an antiviral and cytotoxic response against the viral invasion (Boechat et al. 2021). Hyper-induction of inflammatory cytokines mainly IL-1β, IL-6 and TNF-α produced by innate and adaptive immune cells is one key aspect of COVID-19 that can lead to cytokine release syndrome (Olbei et al. 2021; Costela-Ruiz et al. 2020). Concomitantly, SARS-CoV2 induces the release of chemokines that recruit neutrophils to the affected organs. An aberrant neutrophil activation in severe COVID-19 patients results in excessive neutrophil tissue infiltration and formation of neutrophil extracellular traps (NETs) in a process called NETosis that may lead to lung injury in these patients (Zuo et al. 2020; Wang et al. 2020). A high neutrophil-to-lymphocyte ratio (NLR) as well as T cell lymphopenia, especially decreased CD4 + T cells have been reported in the majority of COVID-19 patients and particularly among the severe cases (Peng et al. 2020). In concert with these findings, NLR has been indicated as an independent risk factor for severe COVID-19 (Alkhatip et al. 2021; Cai et al. 2021; Zeng et al. 2021; Liu et al. 2020), Considering the important role of pro-inflammatory pathways in COVID-19 pathology, several natural compounds with anti-inflammatory and immunomodulatory effects have been proposed as potential adjunctive therapy in conjunction with anti-viral drugs used in the current standard of care treatment (Ho et al. 2021). There is a large body of evidence suggesting that Boswellic acids (BAs), the main pharmacologically active triterpenoids derived from the resin of Boswellia spp., confer medicinal effect(s) due to their anti-inflammatory, immunomodulatory and antioxidant properties (Du et al. 2015; Gomaa et al. 2021). The anti-inflammatory properties of BAs are exerted through inhibition of cyclooxygenase-2, 5-lipoxygenase, inducible NO synthase and modulation of nuclear factor kappa B family of transcription factors (Baram 2019; Roy et al. 2019). In-silico studies have demonstrated binding of BAs to the spike protein and RNA polymerase of SARS-CoV2 suggesting that BAs may inhibit the viral cell entry and replication (Caliebe et al. 2021; Kadhim et al. 2021). Therefore, BAs not only provide anti-inflammatory benefits but may also render anti-viral activity, further supporting their use for the prevention or treatment of COVID-19.
Here we studied the effect of BAs, formulated as Inflawell® syrup, for adjunctive therapy to support body’s immune response against SARS-CoV2 infection. We conducted a phase II randomized, double-blind, placebo-controlled trial to study the beneficial effect of BAs on the immune system of patients with moderate COVID-19.
Methods
Study design and randomization
A phase II randomized, double-blind, placebo-controlled parallel trial was conducted at a single site from January 25 to March 5, 2021. Hospitalized COVID-19 patients ageing ≥ 18 years and determined to be PCR positive were enrolled in this study. They showed at least one of these clinical manifestations: (1) respiratory distress (breathing at ≥ 25 times per minute), (2) percentage of oxygen saturation level at rest being ≤ 92%, and (3) having fever. Subsequent to the initial screening, patients were evaluated for the following exclusion criteria: (1) having a history of liver, kidney, and/or heart failure, (2) inability to take the oral medication, (3) pregnancy and lactation or having a positive pregnancy test, (4) participation in two or more clinical studies simultaneously, (5) admission to intensive care unit (ICU), and (6) cancer diagnosis at the time of COVID-19 infection. Randomization was performed based on the four-sized block approach (Suresh 2011). To allocate patients into the two intervention groups, a non-ordering computer sequence code list was generated. Random allocation codes were concealed in envelopes with medication jars and referral sequences. Patients received allocation codes according to the patient referral sequences, and entered into the study. All study participants and researchers were blinded from treatment assignments until the end of the study and all processes were monitored by the study coordinator to ensure the integrity of the process.
Test material
A Boswellia extract formulation, Inflawell® syrup developed at Kondor Pharma Inc. (Canada) was used in this study. Each serving (10 ml) of Inflawell® syrup consists of 333 mg of Boswellia serrata resin extract standardized to contain 40% boswellic acids (K-Vie™ formulation) dissolved in coconut oil and water. The placebo was prepared by dissolving fructose in coconut oil and water and color was matched by the addition of food coloring.
Intervention
Following patient recruitment, eligible subjects were randomly allocated to two groups: Inflawell® intervention group (BAs group) that received 10 ml of Inflawell® syrup thrice daily (30 ml/day), and the control group (placebo group) that received the placebo syrup. Bottles were the same in shape and size. Inflawell® and placebo syrups were administered along with the standard COVID-19 medications in both groups and patients’ symptoms were followed for 14 days. The volume of remaining syrup in the bottles was recorded weekly to confirm the patient’s compliance.
Clinical data, specimen collection, and evaluation of outcome
Patients were examined at the onset of the study and on day 14. Demographic data, physical status including height and body weight, body mass index (BMI), body temperature, were recorder. In addition, the heart and respiratory rates and peripheral venous blood oxygen saturation value, SpO2, as well as the medical history and medications were recorded. Blood samples were obtained at day 0 and 14 and serum was immediately collected by centrifugation at 3000 rpm, at 4 °C for 10 min and stored at − 80 °C for further evaluation of the secondary outcomes. The following blood markers were measured: complete blood count (CBC), C-reactive protein (CRP), creatinine, lactate dehydrogenase (LDH), alanine amino transferase (ALT), creatine phosphokinase (CPK), erythrocyte sedimentation rate (ESR) (mm/h), prothrombin time (PT), partial thromboplastin time (PTT) and international normalized ratio (INR). Serum levels of IL-1β, IL-6, TNF-α and IL-10 were also measured twice at each time point using sandwich-type enzyme-linked immunosorbent assay (ELISA). Human assay kits were purchased from Abcam (Cambridge, UK) and International GmbH, IBL (Hamburg, Germany). SARS-CoV-2 RT-PCR test was performed on days 0 and 14 on the nasopharyngeal samples. In this study, the primary endpoint was the duration of hospitalization and the secondary endpoints were levels of CRP, LDH, IL-1β, IL-6, TNF-α, IL-10 and SARS-CoV-2 PCR result at day 14 post treatment.
Statistical analysis
Data were analyzed using IBM SPSS and GraphPad Prism software. Normal distribution was assessed using Kolmogorov–Smirnov test. Quantitative variables were shown as mean ± standard error of mean (SEM). Unpaired t test or Mann–Whitney U test was used for baseline quantitative variables according to parametric or nonparametric analysis, respectively. Comparative variation of dependent variables was measured by analysis of covariance (ANCOVA). For imbalance adjustment of pre-test data, the baseline of each parameter was used as a covariate and P value ≤ 0.05 was considered to be statistically significant. Qualitative variables were analyzed using the Chi-Square test.
Ethics statement
The study protocol was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (SBMU), Reference number: IR.SBMU.MSP.REC.1399.311, dated 2020-10-13. The trial has been registered in https://www.irct.ir with unique identifier: IRCT20170315033086N10 (https://en.irct.ir/trial/51631). IRCT is a primary registry in the WHO registry network (https://www.who.int/clinical-trials-registry-platform/network/primary-registries). Written and signed informed consent was obtained from all patients.
Results
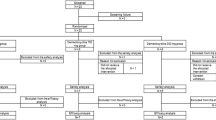
Out of 123 patients initially screened for the study at the Tehran University of Medical Sciences Hospital, 32 patients (26%) did not meet the eligibility criteria due to having negative RT-PCR test (16 patients), showing no typical COVID-19 symptoms (10 patients), previous COVID-19 infection (4 patients), and not fitting the age demographic range (2 patients). The rest (74%) were potentially eligible for enrollment. Among eligible subjects, 41 patients were excluded due to: refusing to participate in the study (18 patients), active cancer (1 patient), and simultaneous participation in another clinical study (5 patients), being transferred to ICU (4 patients), suffering from liver and heart failures (2 and 11 patients, respectively). The remaining 50 patients met all enrollment criteria and were assigned to Inflawell® syrup/BAs (n = 25) and placebo (n = 25) groups. During the trial, 3 additional patients were removed from the study due to aggravation of symptoms and needing to be moved to ICU. Hence, the final number of patients in each group was 24 patients in the BAs group, and 23 patients in the placebo group. All patients continued receiving the standard treatments during the 14-day period (Fig. 1).
Demographical characteristics and comorbidities
The mean age of participants in BAs and placebo groups was 51.92 yrs (31–76 yrs) and 55.84 yrs (35–78 yrs), respectively (Table 1). The mean BMI was 27.58 ± 0.71 for the BAs group and 28.05 ± 0.57 for the placebo group. While 22 patients (44%) had no clinical history, 28 patients (56%) had at least one comorbidity. The most common comorbidities were diabetes mellitus (28%) and hypertension (22%). In addition, 6% had hypothyroidism, 2% had the chronic obstructive pulmonary disease (COPD), 2% had asthma and 2% had ischemic heart disease (Table 1). Among enrolled patients, one patient treated with BAs and two placebo-administrated subjects were removed from the study due to admittance to ICU (Fig. 2).
Clinical outcomes
The most common symptoms reported at the onset of the study were body pain (43 patients, 91.5%) and headache (34 patients, 72.3%), followed by shortness of breath (47 patients, 100%), cough (46 patients, 97.9%), and olfactory/gustatory sense dysfunction (8 patients, 17.02% and 5 patients, 10.63%, respectively). After intervention, the clinical manifestations were improved in both groups but as shown in Tables 2 and 3, improvement in patients in the BAs group was markedly greater than placebo. Furthermore, the increase in oxygen saturation was significantly greater in the BAs group compared with the placebo group on day14 (96 vs 93%, with a P < 0.0001, Table 2). As shown in Table 3, post intervention, the olfactory and gustatory disorders improved in both BAs group and placebo group. While three patients in the placebo group reported to have a loss of olfactory sense with two having gustatory problems that did not improve at day 14, all patients in the BAs group expressed improvement in both senses at the completion of the study. The average days of hospitalization for BAs group were shorter compared to the placebo group (4.20 ± 0.68 vs. 5.15 ± 0.45 days, P < 0.04) (Table 4, Fig. 3).
Laboratory findings
All COVID-19 patients in the study showed an elevated level of neutrophils, NLR, CRP, LDH, and ESR and a decrease in lymphocytes at baseline. Following the 14 days of treatment, hematological results showed that NLR was significantly reduced in the BAs group (2.74 ± 0.37) compared to the placebo group (4.61 ± 0.60) (P < 0.003). Conversely, the lymphocyte count in the BAs group was significantly increased in comparison with the placebo group (P < 0.002) (Table 5 and Fig. 4). The levels of CRP and LDH significantly declined at day 14 in the BAs group compared to the placebo received patients (P < 0.03 and P < 0.04, respectively). Despite a decreasing trend in ESR and ALT in the BAs group, the difference was not statistically significant in comparison to the placebo group (P = 0.164 and P = 128, respectively). No significant changes were seen in other parameters.
Effect of BAs treatment on pro/anti-inflammatory mediators
The serum concentrations of IL-1β, IL-6 and TNF-α were measured at days 0 and 14. The mean level of IL-6 in the BAs group (3.26 ± 0.41) was significantly lower than the placebo group (5.63 ± 0.51) (P < 0.001). A significant difference was also detected in the serum concentration of TNF-α in the BAs group (69.9 ± 3.51) compared to the placebo group (93.05 ± 6.05) (P < 0.0001). No significant intergroup change was observed in IL-1β levels (P = 0.124). IL-10 level showed an increase in the BAs group over the course of the treatment (14.48 ± 5.221) while it did not change significantly in the placebo group (7.605 ± 1.957) after 14 days (P = 0.18) (Table 6).
Effect of BAs treatment on SARS-CoV-2 RT-PCR test
All enrolled individuals had positive RT-PCR test for SARS-CoV-2 at day 0. After the intervention (day 14), 70.83% of the patients in the BAs group had a negative test result whereas in the placebo group only 47.8% were tested negative, however, the difference between the two groups was not statistically significant (P = 0.108) (Fig. 5; Table 7).
Discussion
Despite the current widespread vaccination and use of anti-viral drugs, the management of COVID-19 symptoms remains challenging. Using herbal medicines to improve immune response to SARS-CoV2 infection alongside the current standard of care might provide a viable approach for adjunctive therapy. In addition, the application of safe and effective herbal medicines standardized for active compounds could hinder the spread of SARS-CoV2 among a percentage of the population that oppose vaccination. Several natural and herbal compounds with anti-inflammatory and anti-viral activities have been suggested for COVID-19 treatment (Singh et al. 2021; Li et al. 2021), but limited clinical trial data exists to support their broad applications. In the present study, we used Inflawell® syrup, a formulation comprising Boswellia extract enriched for Boswellic acids (BAs), on moderate COVID-19 patients. The average length of hospitalization was measured as the primary endpoint while viral count (PCR positivity at day 14), NLR, as well as several infections and inflammation markers were evaluated as secondary outcomes. Our study revealed a significantly shorter length of hospital stay in patients treated with BAs compared with placebo. This is a significant finding as such intervention could reduce the current burden on hospitals and the cluttering of the health care system due to the high volume of COVID-19 hospitalizations. In agreement with a shorter length of hospitalization, the patients receiving Inflawell® demonstrated a slightly larger drop in the number of PCR positive cases after 14 days of treatment. The difference, however, was not statistically significant when compared to the placebo group. Due to the rather small sample size in our study, the observed results cannot be accurately attributed to reported antiviral properties of BAs. Nevertheless, negative PCR results compared with the control, could be correlated to immunoregulatory properties.
Moreover, the patients receiving the BAs (Inflawell® group) showed improvement in the clinical symptoms including olfactory and gustatory dysfunction, shortness of breath, cough, body pain and headache. Recent studies have indicated a correlation between the loss of olfactory and gustatory senses with an increase in the feeling of loneliness, depression and anxiety in COVID-19 patients (Kooper 2021; Dudine et al. 2021). Therefore, a faster relief from these symptoms could positively impact patients’ quality of life. Studies have shown that neuroinflammation and the subsequent microglial activation in olfactory pathways along with necrosis in the olfactory bulb is a key player in COVID-19 anosmia (Laurendon et al. 2020; D'Ascanio et al. 2021). In addition, gustatory dysfunction has been shown to be associated with the secretion of inflammatory cytokines from the taste bud cells leading to impairment in the gustatory sense (Cazzolla et al. 2020). The anti-inflammatory activity of the Inflawell® syrup containing BAs might explain the alleviation of the olfactory and gustatory symptoms in our study. A recent clinical trial on the effect of two other anti-inflammatory compounds, palmitoylethanolamide (PEA) and luteolin in COVID-19 patients has demonstrated a similar improvement in the olfactory and gustatory senses of these patients further confirming the benefits of anti-inflammatory compounds for managing these symptoms (D'Ascanio et al. 2021).
The immune response to SARS-CoV2 infection is rather complex and not completely elucidated. In this study, we measured the level of several markers of immune response and inflammation. Assessment of the percentages of neutrophil, lymphocyte and NLR in the BAs group receiving Inflawell® syrup showed an increase in the number of lymphocytes while neutrophil counts and NLR values were reduced compared with placebo. NLR is an independent risk factor of disease severity in COVID-19 patients (Reusch et al. 2021; Hazeldine and Lord 2021). Over-activation of neutrophils and their infiltration into the lung can be detrimental for COVID-19 patients and further result in lung injury and ARDS (Yang et al. 2021; Hazeldine and Lord 2021). In addition, a low lymphocyte count (lymphopenia) has been associated with COVID-19 symptoms (Kong et al. 2020; Ghizlane et al. 2021). The improvement of NLR in patients that were treated with BAs suggests the immunomodulatory effect of these compounds. Variation in NLR can be monitored to effectively assess the efficacy of treatment. A recent study using Shenhuang Granule (SHG), on COVID-19 patients reported similar improvements in NLR and reduction in the clinical symptoms (Zhou et al. 2021). In another clinical trial, Viranorm, a formulation composed of six herbal ingredients, was used every six hours as an add-on treatment on asymptomatic mild COVID-19 patients. Their results also demonstrated a decrease of NLR following administration for 14 days concomitant with improvement in clinical symptoms (Padmanaban 2021). Altogether, these findings support the use of Inflawell® to improve NLR and manage symptoms of COVID-19.
In our study, the levels of CRP and LDH drastically reduced after administration of Inflawell® syrup for 14 days. The serum levels of CRP and LDH have been reported to increase in COVID-19 (Dogan et al. 2021; Poggiali et al. 2020; Wang et al. 2004). CRP is a commonly used marker for infection and inflammation (Sproston and Ashworth 2018) and an elevated LDH has been associated with increased odds of severe COVID-19 disease (Henry et al. 2020). The observed decline in CRP and LDH levels in our study could be attributed to anti-inflammatory effects of BAs in Inflawell® syrup that regulate and balance the inflammatory processes.
Uncontrolled overproduction of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α in COVID-19 could lead to cytokine release syndrome and subsequently to acute pulmonary damage, multiple organ failure, and ultimately death (Pum et al. 2021; Catanzaro et al. 2020; Mahat et al. 2021). In our study, the levels of IL-6 and TNF-α showed a statistically significant decline following the two-week treatment with Inflawell® syrup that may explain the faster patient recovery in the treatment group. IL-6 is one of the key pro-inflammatory cytokines associated with the exacerbation of COVID-19 symptoms and has been a target for anti-IL-6 antibody therapy (Matthay and Luetkemeyer 2021). Our study suggests that Inflawell® can effectively lower the level of IL-6 thereby providing a supplement or alternative to antibody therapy for the management of COVID-19 symptoms.
Conclusion
In summary, our study supports the benefit of BAs formulated as Inflawell® syrup, as a supportive treatment leading to shorter hospitalization and alleviation of some of the clinical symptoms and inflammatory biomarkers associated with COVID-19. A larger multi-center clinical trial, however, would be needed to confirm and expand on the findings.
Data availability
The enquiries about data availability should be directed to the corresponding author.
References
Alkhatip AAAMM, Kamel MG, Hamza MK, Farag EM, Yassin HM, Elayashy M, Naguib AA, Wagih M, Abd-Elhay FA-E, Algameel HZ, Yousef MA, Purcell A, Helmy M (2021) The diagnostic and prognostic role of neutrophil-to-lymphocyte ratio in COVID-19: a systematic review and meta-analysis. Expert Rev Mol Diagn 21(5):505–514. https://doi.org/10.1080/14737159.2021.1915773
Baram SM, Karima S et al (2019) Functional improvement and immune-inflammatory cytokines profile of ischaemic stroke patients after treatment with boswellic acids: a randomized, double-blind, placebo-controlled, pilot trial. Inflammopharmacology 27(6):1101–1112. https://doi.org/10.1007/s10787-019-00627-z
Boechat JL, Chora I, Morais A, Delgado L (2021) The immune response to SARS-CoV-2 and COVID-19 immunopathology - current perspectives. Pulmonology 27(5):423–437. https://doi.org/10.1016/j.pulmoe.2021.03.008
Cai J, Li H, Zhang C, Chen Z, Liu H, Lei F, Qin J-J, Liu Y-M, Zhou F, Song X, Zhou J, Zhao Y-C, Wu B, He M, Yang H, Zhu L, Zhang P, Ji Y-X, Zhao G-N, Lu Z, Liu L, Mao W, Liao X, Lu H, Wang D, Xia X, Huang X, Wei X, Xia J, Zhang B-H, Yuan Y, She Z-G, Xu Q, Ma X, Wang Y, Yang J, Zhang X, Zhang X-J, Li H (2021) The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab 33(2):258-269.e253. https://doi.org/10.1016/j.cmet.2021.01.002
Caliebe RH, Scior T, Ammon HPT (2021) Binding of boswellic acids to functional proteins of the SARS-CoV-2 virus: bioinformatic studies. Arch Pharm 354(11):2100160
Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C (2020) Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther 5(1):84. https://doi.org/10.1038/s41392-020-0191-1
Cazzolla AP, Lovero R, Lo Muzio L, Testa NF, Schirinzi A, Palmieri G, Pozzessere P, Procacci V, Di Comite M, Ciavarella D, Pepe M, De Ruvo C, Crincoli V, Di Serio F, Santacroce L (2020) Taste and smell disorders in COVID-19 patients: role of interleukin-6. ACS Chem Neurosci 11(17):2774–2781. https://doi.org/10.1021/acschemneuro.0c00447
Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L (2020) SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev 54:62–75. https://doi.org/10.1016/j.cytogfr.2020.06.001
D’Ascanio L, Vitelli F, Cingolani C, Maranzano M, Brenner MJ, Di Stadio A (2021) Randomized clinical trial olfactory dysfunction after COVID-19: olfactory rehabilitation therapy vs intervention treatment with Palmitoylethanolamide and Luteolin: preliminary results. Eur Rev Med Pharmacol Sci 25(11):4156–4162
Dogan A, Mustafa G, Dervis T, Orhan A (2021) Diagnostic and early prognostic value of serum CRP and LDH levels in patients with possible COVID-19 at the first admission. J Infect Dev Countries. https://doi.org/10.3855/jidc.14072
Du Z, Liu Z, Ning Z, Liu Y, Song Z, Wang C, Lu A (2015) Prospects of boswellic acids as potential pharmaceutics. Planta Med 81(4):259–271. https://doi.org/10.1055/s-0034-1396313
Dudine L, Canaletti C, Giudici F, Lunardelli A, Abram G, Santini I, Baroni V, Paris M, Pesavento V, Manganotti P, Ronchese F, Gregoretti B, Negro C (2021) Investigation on the loss of taste and smell and consequent psychological effects: a cross-sectional study on healthcare workers who contracted the COVID-19 infection. Front Public Health 9:511
Ghizlane EA, Manal M, Abderrahim EK, Abdelilah E, Mohammed M, Rajae A, Amine BM, Houssam B, Naima A, Brahim H (2021) Lymphopenia in Covid-19: a single center retrospective study of 589 cases. Ann Med Surg 69:102816. https://doi.org/10.1016/j.amsu.2021.102816
Gomaa AA, Mohamed HS, Abd-Ellatief RB, Gomaa MA (2021) Boswellic acids/Boswellia serrata extract as a potential COVID-19 therapeutic agent in the elderly. Inflammopharmacology. https://doi.org/10.1007/s10787-021-00841-8
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A (2020) Baseline characteristics and outcomes of 1591 patients infected With SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 323(16):1574–1581. https://doi.org/10.1001/jama.2020.5394
Hazeldine J, Lord JM (2021) Neutrophils and COVID-19: active participants and rational therapeutic targets. Front Immunol 12:680134–680134. https://doi.org/10.3389/fimmu.2021.680134
Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G (2020) Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med 38(9):1722–1726. https://doi.org/10.1016/j.ajem.2020.05.073
Ho P, Zheng J-Q, Wu C-C, Hou Y-C, Liu W-C, Lu C-L, Zheng C-M, Lu K-C, Chao Y-C (2021) Perspective adjunctive therapies for COVID-19: beyond antiviral therapy. Int J Med Sci 18(2):314–324. https://doi.org/10.7150/ijms.51935
Hoehl S, Rabenau H, Berger A, Kortenbusch M, Cinatl J, Bojkova D, Behrens P, Böddinghaus B, Götsch U, Naujoks F, Neumann P, Schork J, Tiarks-Jungk P, Walczok A, Eickmann M, Vehreschild M, Kann G, Wolf T, Gottschalk R, Ciesek S (2020) Evidence of SARS-CoV-2 Infection in Returning Travelers from Wuhan, China. N Engl J Med 382(13):1278–1280. https://doi.org/10.1056/NEJMc2001899
Kadhim MM, Washeel Salman A, Mrebee Zarzoor A, Kadhum WR (2021) Inhibition of SARS-CoV-2 reproduction using Boswellia carterii: a theoretical study. J Mol Liq 337:116440. https://doi.org/10.1016/j.molliq.2021.116440
Kong M, Zhang H, Cao X, Mao X, Lu Z (2020) Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect 148:e139–e139. https://doi.org/10.1017/S0950268820001557
Kooper DCH, Mkadmi H (2021) Self-reported olfactory and gustatory dysfunction in patients with COVID-19 infection*. Rhinology Online 4:140–146
Laurendon T, Radulesco T, Mugnier J, Gérault M, Chagnaud C, El Ahmadi AA, Varoquaux A (2020) Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 95(5):224–225. https://doi.org/10.1212/wnl.0000000000009850
Li S, Cheng C-S, Zhang C, Tang G-Y, Tan H-Y, Chen H-Y, Wang N, Lai AY-K, Feng Y (2021) Edible and herbal plants for the prevention and management of COVID-19. Front Pharmacol 12:900
Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y (2020) Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81(1):e6–e12. https://doi.org/10.1016/j.jinf.2020.04.002
Mahat RK, Panda S, Rathore V, Swain S, Yadav L, Sah SP (2021) The dynamics of inflammatory markers in coronavirus disease-2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Epidemiol Glob Health 11:100727–100727. https://doi.org/10.1016/j.cegh.2021.100727
Matthay MA, Luetkemeyer AF (2021) IL-6 receptor antagonist therapy for patients hospitalized for COVID-19: who, when, and how? JAMA 326(6):483–485. https://doi.org/10.1001/jama.2021.11121
Olbei M, Hautefort I, Modos D, Treveil A, Poletti M, Gul L, Shannon-Lowe CD, Korcsmaros T (2021) SARS-CoV-2 causes a different cytokine response compared to other cytokine storm-causing respiratory viruses in severely Ill patients. Front Immunol 12:381
Padmanaban KG, Radheshyam N (2021) Role of herbal immunomodulator (Viranorm) as add-on treatment in asymptomatic or mildly symptomatic COVID-19 confirmed cases. Indian J Clin Pract 32(3):187–193
Peng X, Ouyang J, Isnard S, Lin J, Fombuena B, Zhu B, Routy J-P (2020) Sharing CD4+ T cell loss: when COVID-19 and HIV collide on immune system. Front Immunol 11:596631–596631. https://doi.org/10.3389/fimmu.2020.596631
Poggiali E, Zaino D, Immovilli P, Rovero L, Losi G, Dacrema A, Nuccetelli M, Vadacca GB, Guidetti D, Vercelli A, Magnacavallo A, Bernardini S, Terracciano C (2020) Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta 509:135–138. https://doi.org/10.1016/j.cca.2020.06.012
Pum A, Ennemoser M, Adage T, Kungl AJ (2021) Cytokines and chemokines in SARS-CoV-2 infections-therapeutic strategies targeting cytokine storm. Biomolecules 11(1):91. https://doi.org/10.3390/biom11010091
Reusch N, De Domenico E, Bonaguro L, Schulte-Schrepping J, Baßler K, Schultze JL, Aschenbrenner AC (2021) Neutrophils in COVID-19. Front Immunol 12:952
Roy NK, Parama D, Banik K, Bordoloi D, Devi AK, Thakur KK, Padmavathi G, Shakibaei M, Fan L, Sethi G, Kunnumakkara AB (2019) An update on pharmacological potential of boswellic acids against chronic diseases. Int J Mol Sci 20(17):4101. https://doi.org/10.3390/ijms20174101
Singh NA, Kumar P, Jyoti KN (2021) Spices and herbs: Potential antiviral preventives and immunity boosters during COVID-19. Phytother Res. https://doi.org/10.1002/ptr.7019.10.1002/ptr.7019
Sproston NR, Ashworth JJ (2018) Role of C-Reactive protein at sites of inflammation and infection. Front Immunol 9:754–754. https://doi.org/10.3389/fimmu.2018.00754
Suresh K (2011) An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci 4(1):8–11. https://doi.org/10.4103/0974-1208.82352
Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L (2020) Clinical outcomes in 55 patients with severe acute respiratory syndrome Coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis 221(11):1770–1774. https://doi.org/10.1093/infdis/jiaa119
Wang J-T, Sheng W-H, Fang C-T, Chen Y-C, Wang J-L, Yu C-J, Chang S-C, Yang P-C (2004) Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis 10(5):818–824. https://doi.org/10.3201/eid1005.030640
Yang S-C, Tsai Y-F, Pan Y-L, Hwang T-L (2021) Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed J 44(4):439–446. https://doi.org/10.1016/j.bj.2020.09.001
Zeng Z-Y, Feng S-D, Chen G-P, Wu J-N (2021) Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: a prospective cohort study. BMC Infect Dis 21(1):80. https://doi.org/10.1186/s12879-021-05796-3
Zhou S, Feng J, Xie Q, Huang T, Xu X, Zhou D, Zhang W, Sun S, Liu X, Wu X, Che J, Fan T, Zou D, Wang J, Zhan D, Peng D, Feng Y, Yu G, Yuan Z, Fang B (2021) Traditional Chinese medicine shenhuang granule in patients with severe/critical COVID-19: a randomized controlled multicenter trial. Phytomedicine 89:153612–153612. https://doi.org/10.1016/j.phymed.2021.153612
Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS (2020) Neutrophil extracellular traps in COVID-19. JCI Insight. https://doi.org/10.1172/jci.insight.138999
Acknowledgements
Undoubtedly, execution of this research under COVID-19 pandemic conditions and the limitations imposed by it, was only possible due to cooperation and empathy of a dedicated and compassionate team. Herein, I do cordially extend my profuse gratitude and appreciation to Ms. A. Daraei, the Head of Clinical Chemistry Laboratory, Ms. Sarira Shahnavaz in the Immunology Department, the staff of Tehran University of Medical Sciences Hospital Complex, especially the nurse of the Infectious Diseases Department, Ms. Fatemeh Hosseini Shad, the Pars Teb Laboratory staff, Dr. Meysam Mahdavi and Dr. Ali Kharazian, for the conductance of all routine clinical tests, all COVID-19 patients and other friends that helped us in carrying out this research.
Funding
This project is supported by Shahid Beheshti University of Medical Sciences (Grant number, 3086N10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barzin Tond, S., Balenci, L., Khajavirad, N. et al. Inflawell® improves neutrophil-to-lymphocyte ratio and shortens hospitalization in patients with moderate COVID-19, in a randomized double-blind placebo-controlled clinical trial. Inflammopharmacol 30, 465–475 (2022). https://doi.org/10.1007/s10787-022-00928-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-00928-w