Abstract
Non-indigenous freshwater bivalves negatively affect invaded ecosystems through different mechanisms, including inter-specific competition for trophic resources. Here, we investigated in Lake Trasimeno (Central Italy) the diet of the invasive Dreissena polymorpha and the native Anodonta anatina. δ15N and δ13C stable isotopes were measured in winter and summer in bivalves, phytoplankton, and sedimentary organic matter (SOM); the relative dietary contributions of the two resources were determined using Bayesian mixing models. To elucidate the different carbon and nitrogen pools characterizing the study site, isotopic analyses were extended to zooplankton and to representatives of the benthic flora and macroinvertebrate fauna. Independently from the season, the two bivalves showed a limited trophic overlap, as mixing models indicated for D. polymorpha a diet based primarily on phytoplankton, while A. anatina relied mainly on SOM. Dietary differences were less marked in summer, when comparable isotopic values characterized phytoplankton and SOM. In winter, conversely, the trophic differentiation between the two species was more evident, and corresponded with a significant enrichment in SOM δ13C values, likely due to a substantial contribution of carbon deriving from decaying macrophytes. Whether differences in ecological and behavioral traits alone can explain the observed trophic segregation between the two species, or if they have actively shifted their diet to reduce competition for food is discussed. We conclude emphasizing the need of an advanced resolution of the influence of non-indigenous species on the flux of energy and matter in invaded lentic systems, including Lake Trasimeno.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the exception of Antarctica, freshwater bivalves (including the orders Unionida, Venerida, Mytilida, and Myida) are distributed throughout all continents where they are acknowledged to exert a critical influence on the structure and function of lotic and lentic ecosystems (Lopes-Lima et al., 2018). Indeed, besides providing a dominant contribution to the benthic biomass, bivalves can affect the abundance of bacteria, particulates, and primary producers in the water column through top-down effects, ultimately influencing nutrient cycling (Taylo et al., 2015; Vaughn, 2018; McDowell & Sousa, 2019 and literature cited). In addition, they can act as ecosystem engineers, altering the physical structure of the benthic environment via bioturbation or creating colonizable biogenic substrates (Vaughn & Hakenkamp, 2001; Boeker et al., 2016; Ilarri et al., 2019).
Among freshwater bivalves, Unionida have declined dramatically in recent decades and are currently considered one of the most threatened taxonomic groups worldwide (Dudgeon et al., 2006; Lopes-Lima et al., 2014b; Ferreira-Rodríguez et al., 2019). The remarkable declines in spatial distribution, abundance, and species diversity have been related to a suite of anthropogenic disturbances, including habitat loss and fragmentation, water quality degradation, overexploitation, and climate change; the introduction of invasive species has been emphasized as a further, important threat to the conservation of native Unionida (Lopes-Lima et al., 2017, 2018; Böhm et al., 2021). Invasion impacts on native bivalves have been generally related with a number of factors, including differential ability to respond to abiotic (e.g., environmental stress) and biotic pressures (e.g., predators including parasites), modification of the physical or biogeochemical conditions of the substrate and water column, as well as competition for space (Sousa et al., 2014; Bielen et al., 2016; Ożgo et al., 2020; Taskinen et al., 2021). In addition, a number of reviews and long term studies have suggested that invasive bivalves—dreissenid mussels in particular—may be superior competitors in food acquisition due to, e.g., more flexible diets, or higher ability and efficiency to exploit trophic resources (Higgins & Vander Zanden, 2010; Sousa et al., 2014; Strayer and Malcom, 2018). Evidence supporting this scenario is still relatively limited (Makhutova et al., 2013; Novais et al., 2016; Douda & Čadková, 2018; Modesto et al., 2021), and further experimental investigations are needed to verify under field conditions the potential occurrence of exploitative food competition for invasive and native bivalves.
Here, we compared the diets of the invasive zebra mussel Dreissena polymorpha (Pallas, 1771) (Myida) and of the native duck mussel Anodonta anatina (Linnaeus, 1758) (Unionida) occurring in sympatry in Lake Trasimeno (Central Italy). Complete information on the biology, ecology, and invasion history of D. polymorpha is provided in Karatayev et al. (2007) and in Nalepa and Schloesser (2019). In brief, the species is characterized by a high fecundity (2.7–10 × 105 eggs produced per female per reproductive season), with planktonic free-swimming larvae and benthic sessile adult stages, reaching a typical shell length of 20–30 mm in 3–4 years. In the last two centuries, D. polymorpha has spread from the native Ponto-Caspian Basin toward North American and European freshwaters at alarming rates (Karatayev et al., 2015). The invasion has caused considerable concern worldwide due to the impacts observed in both lotic and lentic environments, related to, e.g., negative effects on the structure of native communities as well as on energy and element fluxes across trophic levels (Ricciardi et al., 1996; Burlakova et al., 2000; Caraco et al., 2006; Sousa et al., 2011). In Italy, the dreissenid was first reported from Lake Garda in the early ‘70s (Giusti & Oppi, 1972); it was subsequently observed in Lake Trasimeno in 1999 (Spilinga et al., 2000), probably introduced by transfer of recreational boats, fish restocking, and aquaculture practices (Charavgis and Cingolani, 2004). The species is currently found almost ubiquitously in the basin on rocks and artificial substrates as well as on soft bottoms at high densities (up to 200.000 ind. m-2: Lancioni and Gaino, 2006; Goretti et al., 2020b). Anodonta anatina, as other unionids, has a complex life cycle involving internal fertilization, larval stages (glochidia) brooded by females (or hermaphrodites) in gill chambers, and subsequent release (3–4 × 105 glochidia per individual) as fish ectoparasites completing their metamorphosis to young mussels in the host (Kat, 1984; Niemeyer, 1993). Adults are characterized by an indeterminate growth and can live up to 13 years reaching a shell length of more than 100 mm (Zieritz and Aldridge, 2011; Müller et al., 2021). The species has a pan-European distribution (Graf, 2007) and occurs in lakes and slow-flowing lotic environments throughout northern and central Italy, including Lake Trasimeno (Froufe et al., 2017).
Dreissena polymorpha and Anodonta spp. are suspension feeders with diets primarily based on phytoplankton (Bastviken et al., 1998; Bontés et al., 2007; Naddafi et al., 2007; Lopes-Lima et al., 2014a) that can be supplemented with a diverse spectrum of alternative resources, including organic particulate re-suspended from bottom sediments (Raikow & Hamilton, 2001; Strayer et al., 2004; Vaughn et al., 2008; Cole and Solomon, 2012). Exploitative competition for food has been repeatedly suggested to explain the negative impacts exerted by the zebra mussel on native unionid bivalves including species of the genus Anodonta (Strayer and Smith, 1996; Baker and Levinton, 2003; Strayer & Malcom, 2018). In the past decade, the analysis of bulk carbon and nitrogen stable isotopes (SIA hereafter) has provided valuable insights into the origin and nature of bivalve trophic ecology (among others, Atkinson et al., 2010; Brauns et al., 2021). Accordingly, in the present study, we measured δ15N and δ13C stable isotope values of soft tissues of D. polymorpha and A. anatina from a littoral site in Lake Trasimeno and of their potential pelagic and benthic trophic resources, i.e., phytoplankton and organic matter of sedimentary origin (SOM hereafter). Bayesian mixing models were further used to quantify the relative contribution of the resources to the diets the two bivalve species and, ultimately, verify their degree of dietary overlap and potential trophic competition.
Given the shallowness of Lake Trasimeno and its susceptibility to sediment resuspension events (see further in the next section), we posited that SOM may contribute to the diet of the bivalves in particular in winter months, when phytoplankton abundance is low and wind-induced resuspension of bottom sediments increases remarkably (Ludovisi & Gaino, 2010; Bresciani et al., 2020). To this end, isotopic analyses were repeated in August and February to verify the effect of seasonal variations in the availability of pelagic and benthic organic matter sources on the dietary habits of the two species. In addition, to contextualize the results and provide a more comprehensive understanding of the contribution of different sources to carbon and nitrogen dynamics in the lake littoral zones, stable isotope analysis was extended to zooplankton together with other representative plant and invertebrate taxa occurring at the study location.
Material and methods
Site description
The study was performed in Lake Trasimeno (43.133283°N, 12.100064°E, Central Italy; Fig. 1). Details on the lake's morphometric and hydrological characteristics can be found in Ludovisi & Gaino (2010) and in Bresciani et al. (2020). In brief, the basin is located 257 m above sea level and is the largest laminar lake in Italy (124 Km2). It is shallow (average depth: 4.7 m, maximum depth: 6.3 m), has a single artificial outlet, and is fed by several ephemeral creeks. Given the relatively small extent of the watershed (396 Km2), its hydrological regime is driven by precipitation, and strong seasonal and inter-annual oscillations in water level and quality are observed (Ludovisi & Poletti, 2003; Ludovisi & Gaino, 2010). The lake is mesoeutrophic and phosphorous-limited, and late-summer phytoplankton blooms are typically observed. In addition, it is polymictic with no significant thermal stratification during the year and frequent wind-driven sediment resuspension events in winter months (Ludovisi & Gaino, 2010; Gaino et al., 2012; Bresciani et al., 2020).
The littoral zones are generally muddy, with dense beds of aquatic macrophytes belonging to the genera Stuckenia, Myriophyllum, and Vallisneria extending seasonally in particular along the southern coasts of the lake (Marchegiano et al., 2017). The macroinvertebrate community is composed of a diverse assemblage of annelid, mollusc, insect, and crustacean taxa including the invasive decapod Procambarus clarkii (Girard, 1852) and amphipod Dikerogammarus villosus (Sowinsky, 1894) (Goretti et al., 2014; VV.AA., 2015; Catasti et al., 2017; Goretti et al., 2020b; Mancini et al., 2021). Native bivalves comprise the sphaeriid Sphaerium corneum (Linnaeus, 1758) and the unionids Unio elongatulus C.Pfeiffer, 1825, Anodonta anatina, A. cygnea (Linnaeus, 1758), and A. exulcerata Porro, 1838 (VV.AA., 2015; Froufe et al., 2017).
Sample collection
Sampling operations were carried out on August 20th, 2015, and on February 4th, 2016, in the southern sector of the basin in the locality of Sant’Arcangelo (43.089788° N, 12.156246° E; Fig. 1). To collect phytoplankton and zooplankton under conditions of high-water clarity and minimum sediment resuspension, sampling dates corresponded with days always preceded by at least 72 h of good weather and low/negligible wind speed. The sampled area, representative of the general benthic conditions characterizing the littoral environments of the lake (Marchegiano et al., 2017; Goretti et al., 2020a), was located in a shallow embayment (approximate mean depth = 1 m) with muddy bottoms and artificial rocky shores. The riparian vegetation was mainly represented by stands of the common reed Phragmites australis (Cav.) Trin. ex Steud. 1841 while Myriophillum spicatum L., Stuckenia pectinata (L.) Böerner, and Vallisneria spiralis L. dominate among submerged macrophytes. In winter, large natural accumulations of leaf litter and organic detritus originating from the aforementioned floral species are generally found along the shores and bottoms (Mancinelli et al., 2018).
At each sampling occasion, plankton samples were taken at approximately 50 m from the coast from a pier extending in the embayment. Five 6-L samples of surface water (0–50 cm) were collected by a hand-held Ruttner bottle and transported to laboratory for phytoplankton analyses in refrigerated containers. In addition, five 200-L samples of surface water were collected using a diaphragm suction pump and filtered in situ on a 50 μm mesh size screen for zooplankton analyses.
Thirty individuals of D. polymorpha were randomly scraped from submerged rocks and artificial structures, while A. anatina specimens were hand-collected by wading. Since sampling operations imposed a significant disturbance of the embayment soft bottoms, ultimately making increasingly difficult the detection of specimens, five Anodonta individuals were collected per sampling occasion. A pond net (mesh size = 1 mm) was swept five times through submerged macrophytes and leaf litter accumulations to collect samples of the dominant plant and macroinvertebrate species. After collection, all floral and faunal samples were placed, depending on individual size, in Falcon tubes or in other plastic buckets in filtered lake water and sealed. In addition, samples of the superficial sediment layer (6 replicates per season) were collected using a methacrylate core (400 mm length, 114 mm ϕ) driven into the sediment to a depth of approximately 10 cm. The core was extracted after sealing its upper end and the overlying water was removed by aspiration to avoid resuspension. Subsequently, the superficial layer (0-1 cm) of each core was gently scooped with a plastic spoon and collected in Falcon tubes, while the remaining sediment was transferred in plastic bags. All collected samples were stored in refrigerated containers (4 °C) until transfer to the laboratory.
Laboratory procedures
Water samples collected for phytoplankton analysis were filtered through GF/C fiber glass filters (1.2 μm mesh size). The samples collected for zooplankton analysis were centrifuged in order to remove floating particles and filamentous algae, prior to be filtered through GF/C fiber glass filters (1.2 μm mesh size). For both phytoplankton and zooplankton, the material collected on the GF/C filters was examined using an inverted microscope (×200 magnification for phytoplankton and ×40 for zooplankton) to qualitatively confirm the dominance of phyto- or zooplanktonic organisms over other sestonic material. Once examined, the material retained by the filters was gently scraped with a scalpel and dried in Eppendorf tubes at 60 °C for at least one week. Identical drying conditions were applied to all the remaining plant and animal samples. Plants were identified to species level and rinsed in distilled water to remove extraneous materials. All sediment samples were sieved on a 1-mm screen to collect invertebrates for later identification and analysis. Superficial sediment layer samples were sieved and homogenized, while leaf litter and other macroscopic detrital particles (coarse particulate organic matter, CPOM hereafter) retained in the sieve were collected. All plant and sediment samples were eventually dried (60 °C, > 1 week).
In general, invertebrates were identified to species level using conventional taxonomic keys and enumerated; they were subsequently kept in distilled water for 12 h to clear gut contents and euthanized by thermal shock (− 80 °C for 10 min). Subsequently amphipods, decapods, chironomids, and oligochaetes (see Results) were individually dried (60 °C, > 1 week). A calliper was used to measure to the nearest mm the shell length of euthanized D. polymorpha specimens as the distance between posterior to anterior tips along the median axis; bivalves were then dissected and had their foot excised and dried (60 °C, > 1 week). Noticeably, for A. anatina, classical taxonomic keys were inadequate in providing an unquestionable identification of the sampled individuals, given the high intraspecific variability in morphometric traits generally observed in species belonging to the genera Anodonta (Guarneri et al., 2014; Riccardi et al., 2019). Accordingly, after shell length measurement, the excised foot of each individual was divided into two subsamples. The first subsample was dried as previously described; the second was preserved in ethanol 70% at − 20 °C and subsequently subjected to molecular species attribution performed through PCR–RFLP (PCR-restriction fragment length polymorphism) on the nuclear locus ITS1(internal transcribed spacer) specifically chosen to avoid DUI (doubly uniparental inheritance) problems. Total DNA was isolated individually in duplicate from the foot subsample using the Wizard® Genomic DNA Purification Kit (Promega) by means of a modified protocol (Lucentini et al., 2010) and the quantity and quality of DNA were assessed on 1% agarose gel and by spectrophotometric analysis, as already used for other bivalve species (Lucentini et al., 2010). Amplification and digestion of ITS1 region were carried out following Zieritz et al. (2012) to unequivocally assign each individual to a species by means of a validated PCR–RFLP protocol. Restriction products were checked by electrophoresis in 2.2% TBE buffer-agarose gel containing SafeView Nucleic Acid Stain (NBS Biologicals) and visualized under UV light. The totality of the examined Anodonta specimens was identified as A. anatina.
Stable isotope analysis
All oven-dried samples were ground to a fine powder with a mortar and pestle. Each sediment sample was preventively split into two aliquots, one of which was acidified (HCl, 2 N) using the drop-by-drop procedure to remove carbonates (Jacob et al., 2005). Consequently, subsamples of different sizes were taken from each taxon/matrix [i.e., macrophytes: 3.1 ± 0.1 mg; sediment: 29.1 ± 0.1 mg (non-acidified) and 9.9 ± 0.1 mg (acidified); phytoplankton and zooplankton: 2.8 ± 0.4 mg; bivalves, amphipods, decapods, oligochaetes, and chironomids: 2.1 ± 0.1 mg] and pressed into ultra-pure tin capsules (Costech Analytical Technologies). Multiple specimens were pooled when single individuals did not provide a sufficient mass for the subsample (see Table 1 in Results). Carbon and nitrogen stable isotope values were determined using an Elemental Analyser (Thermo Scientific Flash EA 1112) connected with an Isotope Ratio Mass Spectrometer (Thermo Scientific Delta Plus XP). Concentrations of total carbon (C) and nitrogen (N) were expressed as g Kg−1 tissue dry weight; isotopic values were expressed in conventional per mil δ notation in relation to international standards (PeeDee Belemnite for carbon and atmospheric N2 for nitrogen). Analytical precision based on the standard deviation of replicates of internal standards (International Atomic Energy Agency IAEA-NO-3 for δ15N and IAEA-CH-6 for δ13C) was 0.2‰ for both δ13C and δ15N. Acidified samples were used to measure sediment δ13C values, while untreated samples were used for δ15 N values.
Some animal taxa showed a C:N ratio higher than 3.5–4 (Table S1, online information), thus indicating a considerable contribution of lipids to tissues carbon pool (Post et al., 2007). Lipids are depleted in 13C compared to proteins and carbohydrates and may significantly bias δ13C estimations (Logan et al., 2008). Accordingly, in samples with C:N > 3.5, δ13C values were corrected for lipid content using the correction algorithms based on tissue C:N ratios proposed by Syväranta & Rautio (2010) for zooplankton and by Post et al. (2007) for the remaining samples.
Data analysis
In general, values in the text are expressed as means ± 1SD if not otherwise specified. All statistical procedures were implemented in the R statistical environment development (ver. 4.1.1; R Development Core Team, 2021). Two-tailed Student's t-tests (α = 0.05) were used to check for seasonal or inter-specific differences in δ13C and δ15 N values in phytoplankton, SOM, and in the two bivalves; data were tested for conformity to assumptions of variance homogeneity (Cochran’s C test) and normality (Shapiro–Wilks test) and transformed when required. Given the relatively low sample sizes (Table 1), the R software pwr (Champely et al., 2020) was used to perform a power analysis for each test showing a significant outcome (see Results). Without exceptions, the analyses indicated that the power to detect the observed effects at the 0.05 level was always > 93%.
A two-member Bayesian mixing model was implemented to determine the proportional contribution of phytoplankton and SOM to the diet of each bivalve species using the R package SIMMR (Parnell, 2020). SIMMR includes an updated Bayesian mixing algorithm based on the SIAR package (Parnell et al., 2010) to produce a probability distribution that represents the likelihood a given food source contributes to the consumer's biomass. In addition, similar to SIAR, it produces a range of feasible solutions to the mixing problem to which are assigned credibility intervals (CIs) (in this study, 95% CI; Parnell et al., 2010). Within the SIMMR framework, δ13C and δ15N values were adjusted for one trophic level. The identification of appropriate trophic enrichment factors (hereafter TEF) is a key issue in isotopic ecology, as they significantly affect the output of mixing model procedures (Phillips et al., 2014). Taxonomy and the nature of the diet are among the sources of variability that may influence TEFs (Vanderklift and Ponsard, 2003; Caut et al., 2009; Brauns et al., 2018), imposing, when possible, the use of specifically determined values (see, e.g., Annabi et al., 2018; Twining et al., 2020). The carbon and nitrogen TEF values for one trophic level used here (i.e., Δδ13C = 0.8 ± 1‰, Δδ15N = 3.6 ± 0.9‰, n = 8) were calculated as the average of the values collated by Brauns et al. (2018) from laboratory studies on marine bivalves feeding on phytoplankton, implemented with data from Kasai et al. (2016) on the freshwater venerid Corbicula sandai Morelet, 1886 (Table S2, online information). 10,000 simulations per model were run; Gelman diagnostics were carried out on models output to determine whether confidence intervals were comprised between 1 and 1.1, and a higher number of simulations were performed if necessary.
To express the results of the simulations only in terms of mean contributions of each source with standard deviations may hide multimodality or the extent of variations in dietary preference within consumer populations (Semmens et al., 2013). Accordingly, for each potential resource, the results were presented as mean contributions as well as probability densities of proportional dietary contributions using the density plot function in SIMMR. Posterior probabilities of similarity (POS), defined as the Bayesian probability that two proportional dietary contributions are identical (Blasco & Blasco, 2017), were estimated to verify for the two bivalve species whether in each sampling occasion phytoplankton and SOM provided significantly different contributions to the respective diets. In addition, since the sample size of bivalve consumers employed here for isotopic analysis (5–8; Table1) represents the lower boundary considered appropriate for the implementation of a two-member Bayesian mixing model procedure (Ward et al., 2011; Phillips et al., 2014; see also Kadoya et al., 2012), the R package samplesim (Casajus et al., 2021) was used to investigate the effect of sample size on estimates and precision of stable isotope mixing solutions. In brief, the package allows to modify samples sizes assuming a normal distribution of a given mean and standard deviation. Samples of different sizes are created from the distribution, and mixing proportions are estimated using the SIAR algorithm for multiple replicates of each sample size. Here, we used 999 replicates with sample sizes = 5, 10, 20, 40, and 80.
Concentration dependencies were not incorporated in the models because elemental concentration values in SOM were expected to be extremely diluted given the low percentage of organic matter (max 7% in dry weight; estimated as weight loss on ignition at 450 °C for 6 h; Mancinelli unpublished data); therefore, using concentration dependencies would have unrealistically and abnormally increased the contribution of this source (Raoult et al., 2018).
Results
Dreissena polymorpha, Anodonta anatina, phytoplankton, zooplankton, and SOM samples were collected on both August and February, together with the dipteran Chironomus plumosus (Linnaeus, 1758), the amphipod Echinogammarus veneris (Heller, 1865), and the non-indigenous oligochaete Branchiura sowerbyi (Beddard, 1892) (Table 1). Five species of aquatic macrophytes and six juvenile specimens of the non-indigenous Louisiana crayfish Procambarus clarkii (ranging between 24 and 32 mm in total length measured from the tip of the rostrum to the rear edge of telson; Mancinelli, personal observation) were collected only in August, while CPOM was collected in significant amounts only in February (Table 1). On average, D. polymorpha individuals collected in February were significantly larger than in August (17.6 ± 5.9 vs. 13.2 ± 3.6 mm shell length; t-test, t = 4.3, P < 0.0001, 58 d.f.), while for A. anatina specimens, differences in size between the two sampling occasions were negligible (94.7 ± 13.9 vs. 88.4 ± 11.8 mm in February and August, respectively; t = 1.1, P = 0.34, 8 d.f.).
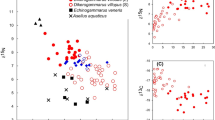
In August, phytoplankton and SOM showed comparable δ15 N values (5.5 ± 0.4‰ vs. 5.4 ± 0.3‰; t-test, t = 0.5, P = 0.62, 9 d.f.), but differed in δ13C (t = 4.5, P = 0.003, 9 d.f.), with more depleted values observed for SOM than for phytoplankton (− 27.2 ± 0.5‰ vs. − 25.7 ± 0.4‰; Fig. 2A). Macrophytes, in contrast, showed highly enriched δ13C values (Fig. 2A), ranging between − 20.7 ± 0.6‰ [Cladophora glomerata (L.) Kütz.] and -11.9 ± 1.7‰ (Chara globularis Thuill.), and variable δ15 N values ranging between 1.9‰ (Myriophyllum spicatum) and 5.8‰ (Cladophora glomerata). Depleted δ13C values consistent with those of SOM and phytoplankton were observed for zooplankton, as well as for the deposit-feeders Chironomus plumosus and Branchiura sowerbyi; in contrast, crustaceans (i.e., Echinogammarus veneris and Procambarus clarkii) showed enriched δ13C and δ15 N values consistent with those generally determined for macrophytes (Fig. 2A).
δ13C and δ15N isotopic bi-plot illustrating the carbon and nitrogen values (means ± 1SD) of Dreissena polymorpha and Anodonta anatina as compared with those of phytoplankton, zooplankton, and SOM in summer (A) and in winter (B). For the sake of comparison, δ13C and δ15N values of macrophytes (summer), CPOM (winter), the amphipod Echinogammarus veneris (both seasons), juveniles of the decapod Procambarus clarkii (summer), the chironomid Chironomus plumosus (both seasons), and the oligochaete Branchiura sowerbyi (both seasons) are included. Taxa are indicated using the acronyms listed in Table 1; for those taxa characterized by C/N values > 3.5, δ13C values have been lipid-corrected (see text for details and Table S1 in online information)
In February, phytoplankton showed negligible variations in δ15 N compared to August (t = 1.27, P = 0.12, 8 d.f.), while a limited (1.2‰ on average), yet significant depletion was observed for δ13C (t = 2.39, P = 0.02, 8 d.f, Fig. 2B). Conversely, SOM isotopic values varied significantly between seasons, showing more depleted δ15 N values (t = -9.53, P < 0.0001, 10 d.f.) and enriched δ13C values (t = 21.4, P < 0001, 10 d.f.) in February than in August. Carbon isotopic values, in particular, were enriched by approximately 5.8‰, and were consistent with those observed for living macrophytes in August (Fig. 2A) and for CPOM collected in February (Fig. 2B).
δ13C and δ15 N in Dreissena polymorpha showed a temporal pattern of variation generally mirroring that of phytoplankton (Fig. 2A and 2B), even though the species showed more depleted δ13C and δ15 N values in February than in August (t-tests, P always < 0.05). Similarly, δ13C and δ15 N values in Anodonta anatina varied remarkably between the two sampling occasions (Fig. 2A and 2B; t-tests, P always < 0.05). In particular, a significant enrichment in 13C was observed in February, consistent with that observed for SOM (Fig. 2B); noticeably, similar patterns of variation characterized the zooplankton as well as C. plumosus and B. sowerbyi (Fig. 2B). The results of SIMMR indicated that in August phytoplankton was included in the diet of both D. polymorpha and A. anatina (Table 2; Fig. 3A); however, its contribution varied considerably, being dominant for D. polymorpha (70% and 77%, mean and median proportional contribution), while being approximately 30% (23% median contribution) for A. anatina, for which SOM appeared to prevail in the diet (Table 2). Noticeably, for D. polymorpha the probability densities of the proportional dietary contributions of phytoplankton and SOM overlapped considerably (Fig. 3A); furthermore, the posterior probabilities of similarity test indicated a non-significant difference (P = 0.21). In contrast, A. anatina showed more segregated, even though marginally non-significant (P = 0.09) proportional dietary contributions (Fig. 3A). Noticeably, a substantial effect of sample size was observed on mixing solutions, as simulations indicated that depending on the number of analyzed individuals phytoplankton might have reached a median proportional contribution of up to 88% and less than 13% in the diet of D. polymorpha and A. anatina, respectively (Fig. S1, online information).
Posterior probability distributions of the contributions of phytoplankton and sedimentary organic matter (SOM) to the diet of Dreissena polymorpha and Anodonta anatina in summer (A) and in winter (B). Bar plots represent posterior model estimates of contributions in terms of median, interquartile range, and max/min values; for further details on the results of Bayesian stable isotope mixing models, see Table 2
Compared with August, in February the trophic segregation between the two species was more evident, with D. polymorpha relying mainly on phytoplankton, and A. anatina primarily exploiting SOM (Table 2). Additionally, the probability densities of phytoplankton and SOM proportional dietary contributions showed no overlap for both bivalves, the similarity probability was negligible (D. polymorpha: P = 0.002; A. anatina: P < 0.0001), and no considerable sample size-related effects were observed, as the estimated proportional contributions of the resources remained virtually unchanged with an increase in the sample size of the consumers (Fig. S1).
Discussion
Our isotopic investigation indicated that in Lake Trasimeno, independently from the season, the invasive Dreissena polymorpha and the native Anodonta anatina showed different trophic habits, with phytoplankton and SOM prevailing in the diet of the dreissenid and the anodontid, respectively. Dietary differences were particularly apparent in February (Table 2; Fig. 3B; see also Fig. 2B). In August, the respective proportional contributions of the two resources were remarkably dissimilar in terms of mean and median values (Table 2), yet both the mixing models procedure and the similarity tests on posterior probabilities highlighted a notable uncertainty in the results, in particular for Dreissena (Fig. 3A).
A comprehensive understanding of these findings requires to cast them within the background provided by the isotopic data on additional animal and plant taxa collected during the study. In summer, it is apparent that two main carbon pools characterized the benthic system of the study location. The first was represented by 13C-enriched living macrophytes (Fig. 2A; see also Fig. 4 for a graphical synthesis), likely to support second-level crustacean consumers, such as the herbivorous/detritivorus amphipod Echinogammarus veneris and juveniles of the omnivore Procambarus clarkii (Mancinelli et al., 2002; 2007; Alcorlo & Baltanás, 2013). The second pool included 13C-depleted phytoplankton and SOM, with overlapping δ15 N values and differing significantly in δ13C by approximately 1.4‰ (Fig. 2A). Indeed, the similar isotopic values of the two sources can explain the uncertain results of the mixing model output (Fig. 3A). The use of mixing models to quantify animal diets requires the sources to have isotopically distinct values; small differences (e.g., 1‰ or lower), even when statistically significant, may have no practical ecological implications, ultimately hampering the robustness of the results (Phillips et al., 2014). In these respects, the dual isotopic approach adopted here should include in future investigations additional biochemical methodologies based on, e.g., fatty acids (Makhutova et al., 2013; Fujibayashi et al., 2016) or a wider spectrum of isotopic markers (Weber et al., 2017) to allow for a more robust resolution of the summer diet of D. polymorpha as well as of A. anatina. In addition, future studies should also analyze a larger number of individuals. Together with resources showing limited isotopic differences, relatively low sample sizes in consumers can determine outputs showing highly diffuse source contributions (Brett, 2014; Phillips et al., 2014), as observed here in August. The simulation test on the effect of the sample size of the two bivalves actually indicated that a larger number of analyzed individuals might have increased the robustness of the mixing model results obtained in August for both D. polymorpha and A. anatina (Fig. S1). In contrast, in February, the simulation test confirmed the robustness of the results independently from the sample size (Fig. S1). It is apparent, however, that future analyses carried out on larger sample sizes will allow researchers to investigate, e.g., size-related dietary changes or seasonal variations in the isotopic niche of the two bivalves, not addressed here and generally overlooked in freshwater bivalves [but see Suh & Shin, 2013 for the estuarine Ruditapes philippinarum (Adams & Reeve, 1850)].
Graphical summary of seasonal variations in trophic relationships (black arrows) and trasfers of material (gray arrow) linking basal resources (i.e., phytoplankton, sedimentary organic matter, and macrophytes) with Dreissena polymorpha, Anodonta anatina, and other invertebrates in the benthic environment of Lake Trasimeno littoral zones
Noticeably, the observed isotopic similarity between phytoplankton and SOM may suggest a summer pelagic–benthic coupling (sensu Soetaert et al., 2000), which D. polymorpha and other suspension feeders possibly contribute to through biodeposition of feces and pseudo-feces (Gergs et al., 2009; Ozersky et al. 2012; but see further in the text). The accumulation of fresh organic matter in superficial sediments after phytoplanktonic blooms (common in August in Lake Trasimeno: Ludovisi and Gaino, 2010; Bresciani et al., 2020; see also Xu et al., 2019 and literature cited for examples from other shallow lentic systems) can be considered as the main causative mechanism of the coupling. This hypothesis is partially supported by the non-significant differences in C:N ratios (SOM = 13 ± 4.6; phytoplankton = 8.8 ± 1.2; t-test with separate variance estimates: t = − 2.1, P = 0.09, 5.6 d.f.) and by the limited depletion in δ13C values between SOM and phytoplankton, consistent with what is generally observed during early diagenetic processes of pelagic organic matter in freshwater systems (e.g., Lehmann et al., 2002).
The general picture that emerges in summer is that phytoplankton supports directly D. polymorpha and indirectly, through pelagic–benthic coupling, A. anatina, together with other depositivores such as Branchiura sowerbyi and Chironomus plumosus (Fig. 4). It is worth mentioning that the δ13C and δ15 N values of C. plumosus were significantly more depleted than those of SOM (Fig. 2A; t-tests, P < 0.05 for both elements), suggesting that the trophic link may be mediated by 13C and 15 N-depleted methanotrophic bacteria (Jones et al., 2008; Tsuchiya et al., 2020).
In February, conversely, while phytoplankton showed depleted δ13C values comparable with those observed in August, δ13C in SOM was significantly higher, an enrichment likely to be determined by macrophyte-derived decaying CPOM (Fig. 2B; Fig. 4). Under these conditions, the trophic segregation between the two bivalves was more apparent (Table 3; Fig. 3B) and further corroborated by post hoc similarity tests.
The habitat preferences of the two species may explain their dietary differences. Dreissena polymorpha can form clumps (“druses”) and colonize unconsolidated sediments, but, as observed here and in previous studies performed in Lake Trasimeno (Lancioni & Gaino, 2006), they are generally attached to natural and artificial hard substrata, thus filter-feeding in the water column (Mellina & Rasmussen, 1994). In contrast, A. anatina lives buried in sediment superficial layers or at the water–sediment interface, where it can show a relatively high mobility (Schwalb & Pusch, 2007). Additionally, anodontids can forage by pedal feeding, i.e., by using the foot to sweep detrital and colloidal particles, microphytobenthos, and bacteria from the sediment surface directly into the shell (Nichols et al., 2005; Brendelberger & Klauke, 2009).
Nevertheless, SOM provided an average contribution between 20 and 30% to the diet of D. polymorpha depending on the season (Fig. 3; Table 3). A relatively high trophic plasticity has already been indicated in D. polymorpha, and a dual reliance on both phytoplankton and SOM has been shown in both lacustrine and riverine populations (Garton et al., 2005; Cole & Solomon, 2012). Wind-induced resuspension events in Lake Trasimeno occur year-round yet are more frequent and intense in winter (Havens et al., 2009; Ludovisi and Gaino, 2010; Gaino et al., 2012); hence, D. polymorpha may adopt in this season a strategy of selective feeding, as already highlighted for the species on phytoplankton (e.g., Baker et al., 1998; Vanderploeg et al., 2001; Naddafi et al., 2007). A relatively high trophic plasticity has been also indicated for A. anatina, and laboratory investigations have shown that phytoplankton, if available, can be efficiently consumed by the duck mussel (Bontés et al., 2007; Dionisio Pires et al., 2007). In spite of this, the species showed in this study independently from the season a net prevalence of SOM in its diet. Even though the already mentioned behavioral and ecological dissimilarities can explain the dietary differences observed between the two species, these may also be interpreted as the result of a trophic segregation adopted to minimize competition for food. In particular, it can be hypothesized that the high efficiency of D. polymorpha in feeding on phytoplankton (Roditi et al., 1996; Dionisio Pires et al., 2004) gives rise to a competitive advantage to D. polymorpha with respect to A. anatina for this food source, imposing to the latter a SOM-based diet. Further controlled feeding experiments may help in clarifying the actual nature of the interaction between the two bivalves, as well as between D. polymorpha and zooplankton: the bivalve’s clearance on phytoplankton may negatively affect pelagic consumers (Jack & Thorp, 2000; Kissman et al., 2010), a potential interaction suggested by the consistency in their δ13C and δ15 N values observed here in summer (Fig. 2A).
A further aspect to be considered is that the 13C-enrichment in SOM reflected in those of B. sowerbyi and C. plumosus, as well as in zooplankton (Fig. 3B), a result that can be explained considering the feeding habits of dominant zooplanktonic species in Lake Trasimeno. Spring–summer zooplankton is typically characterized by strictly herbivorous species [mainly Bosmina longirostris (O.F.Müller, 1776), Daphnia galeata (Sars, 1864) and Diaphanosoma brachyurum (Liévin, 1848)] and to a minor extent by copepods of the genus Cyclops, having a herbivorous–detritivorous generalist diet; conversely, Cyclops spp. generally prevail in winter zooplankton (Hamza et al., 1995; Ludovisi et al., 2005). Such a seasonal taxonomic differentiation in zooplankton was confirmed by the qualitative observations made to verify the nature of the seston (see Material and Methods), with summer samples showing a greater abundance of cladocerans than winter samples, dominated by Cyclops.
It is worth noting that the general scenario discussed here is based on the assumption that the isotopic composition of an organism is linked only to that of its diet. In principle, non-trophic factors may have contributed to the δ13C and δ15 N values of D. polymorpha and A. anatina in summer and winter. For example, δ13C in both freshwater and marine bivalves has been indicated to be influenced by seasonal shifts in energy allocation strategies (Geist et al., 2005; Paulet et al., 2006), and reproduction-related δ13C enrichments have been often observed in summer (Malet et al., 2007). In addition, both D. polymorpha and A. anatina reduce remarkably their metabolic activity and filtration rates under low temperature conditions (Schneider, 1992; Lurman et al., 2014). This implies that in winter both species may experience periods of starvation, and fasting in bivalves may cause a considerable δ15 N enrichment in soft tissues (Yokoyama et al., 2005). In our study, the occurrence of both the aforementioned phenomena cannot be excluded; however, it is likely that they did exert a secondary influence on the isotopic values of D. polymorpha and A. anatina, as they both showed depleted δ13C and enriched δ15 N values in summer as compared to winter (Fig. 2).
In conclusion, the present study provided novel information on the trophic habits of the invasive D. polymorpha and of the native A. anatina in Lake Trasimeno, and a number of issues were advanced on carbon dynamics between the benthic and pelagic compartments in the lacustrine littoral zone (Fig. 4). The results of our investigation may provide a useful framework to investigate in future the impact of D. polymorpha as well as of other invasive species on the flux of energy and elements in the basin. However, our results considered as preliminary, and additional, year-round investigations are required to verify their generality. Specifically, the dietary patterns observed in the present study in winter and summer need to be verified on a seasonal or even shorter temporal scale, given the irregularity of wind-induced resuspension events and the high monthly variability in abundance and composition of the phytoplanktonic assemblage characterizing Lake Trasimeno (Taticchi, 1992; Havens et al., 2009; Bresciani et al., 2020).
Noticeably, the limited sampling effort applied in the present context allowed the collection, together with D. polymorpha, of two other non-indigenous invertebrates, i.e., B. sowerbyi and P. clarkii. Branchiura sowerbyi impact on invaded systems has received to date scant attention, while considerable information is available on the ecological impacts of P. clarkii on native benthic invertebrates, including anodontid bivalves (Meira et al., 2019 and literature cited). However, the actual effects of P. clarkii on A. anatina in Lake Trasimeno are to date unexplored. In addition, the Chinese pond mussel Sinanodonta woodiana (I.Lea, 1834), invasive in Italy (Cilenti et al., 2019), has been repeatedly recorded in Lake Trasimeno after 2017 (Froufe et al., 2017; Goretti et al., 2020b). Also for this introduced anodontid, no information is available on its impacts on native bivalve species occurring in the lake. Thus, beside providing reference information on trophically mediated interactions between D. polymorpha and A. anatina, this study may also provide an incentive toward a more advanced resolution of the role played by native bivalve species in the flux of energy and matter in Lake Trasimeno, and how it is directly or indirectly influenced by introduced invertebrate and fish species.
Data Availability
Enquiries about data availability should be directed to the authors.
Change history
24 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Alcorlo, P. & A. Baltanás, 2013. The trophic ecology of the red swamp crayfish (Procambarus clarkii) in Mediterranean aquatic ecosystems: a stable isotope study. Limnetica 32: 121–138.
Annabi, A., R. Bardelli, S. Vizzini & G. Mancinelli, 2018. Baseline assessment of heavy metals content and trophic position of the invasive blue swimming crab Portunus segnis (Forskål, 1775) in the Gulf of Gabès (Tunisia). Marine Pollution Bulletin 136: 454–463.
Atkinson, C. L., S. P. Opsahl, A. P. Covich, S. W. Golladay & L. M. Conner, 2010. Stable isotopic signatures, tissue stoichiometry, and nutrient cycling (C and N) of native and invasive freshwater bivalves. Journal of the North American Benthological Society 29: 496–505.
Baker, S. M. & J. S. Levinton, 2003. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 505: 97–105.
Baker, S. M., J. S. Levinton, J. P. Kurdziel & S. E. Shumway, 1998. Selective feeding and biodeposition by zebra mussels and their relation to changes in phytoplankton composition and seston load. Journal of Shellfish Research 17: 1207–1214.
Bastviken, D. T. E., N. F. Caraco & J. J. Cole, 1998. Experimental measurements of zebra mussel (Dreissena polymorpha) impacts on phytoplankton community composition. Freshwater Biology 39: 375–386.
Bielen, A., I. Bošnjak, K. Sepčić, M. Jaklič, M. Cvitanić, J. Lušić, J. Lajtner, T. Simčič & S. Hudina, 2016. Differences in tolerance to anthropogenic stress between invasive and native bivalves. Science of the Total Environment 543: 449–459.
Blasco, A. & P. D. A. Blasco, 2017. Bayesian Data Analysis for Animal Scientists, Vol. 265. Springer, New York:
Boeker, C., T. Lueders, M. Mueller, J. Pander & J. Geist, 2016. Alteration of physico-chemical and microbial properties in freshwater substrates by burrowing invertebrates. Limnologica 59: 131–139.
Böhm, M., N. I. Dewhurst-Richman, M. Seddon, S. E. H. Ledger, C. Albrecht, D. Allen, A. E. Bogan, J. Cordeiro, K. S. Cummings, A. Cuttelod, G. Darrigran, W. Darwall, Z. Fehér, C. Gibson, D. L. Graf, F. Köhler, M. Lopes-Lima, G. Pastorino, K. E. Perez, K. Smith, D. van Damme, M. V. Vinarski, T. von Proschwitz, T. von Rintelen, D. C. Aldridge, N. A. Aravind, P. B. Budha, C. Clavijo, D. Van Tu, O. Gargominy, M. Ghamizi, M. Haase, C. Hilton-Taylor, P. D. Johnson, Ü. Kebapçı, J. Lajtner, C. N. Lange, D. A. W. Lepitzki, A. Martínez-Ortí, E. A. Moorkens, E. Neubert, C. M. Pollock, V. Prié, C. Radea, R. Ramirez, M. A. Ramos, S. B. Santos, R. Slapnik, M. O. Son, A.-S. Stensgaard & B. Collen, 2021. The conservation status of the world’s freshwater molluscs. Hydrobiologia 848: 3231–3254.
Bontés, B. M., A. M. Verschoor, L. M. Dionisio Pires, E. van Donk & B. W. Ibelings, Functional response of Anodonta anatina feeding on a green alga and four strains of cyanobacteria, differing in shape, size and toxicity. In: Gulati, R. D., E. Lammens, N. De Pauw & E. Van Donk (eds) Shallow Lakes in a Changing World, Dordrecht, 2007. Springer Netherlands, p 191–204.
Brauns, M., I. G. Boëchat, A. P. C. de Carvalho, D. Graeber, B. Gücker, T. Mehner & D. von Schiller, 2018. Consumer-resource stoichiometry as a predictor of trophic discrimination (Δ13C, Δ15N) in aquatic invertebrates. Freshwater Biology 63: 1240–1249.
Brauns, M., T. Berendonk, S. Berg, F. Grunicke, D. Kneis, S. Krenek, T. Schiller, J. Schneider, A. Wagner & M. Weitere, 2021. Stable isotopes reveal the importance of terrestrially derived resources for the diet of the freshwater pearl mussel (Margaritifera margaritifera). Aquatic Conservation: Marine and Freshwater Ecosystems 31: 2496–2505.
Brendelberger, H. & C. Klauke, 2009. Pedal feeding in freshwater unionid mussels: particle-size selectivity. Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 30: 1082–1084.
Bresciani, M., M. Pinardi, G. Free, G. Luciani, S. Ghebrehiwot, M. Laanen, S. Peters, V. Della Bella, R. Padula & C. Giardino, 2020. The use of multisource optical sensors to study phytoplankton spatio-temporal variation in a Shallow Turbid Lake. Water 12: 284.
Brett, M. T., 2014. Resource polygon geometry predicts Bayesian stable isotope mixing model bias. Marine Ecology Progress Series 514: 1–12.
Burlakova, L., A. Karatayev & D. K. Padilla, 2000. The impact of Dreissena polymorpha (Pallas) invasion on unionid bivalves. International Review of Hydrobiology 85: 529–541.
Caraco, N. F., J. J. Cole & D. L. Strayer, 2006. Top down control from the bottom: regulation of eutrophication in a large river by benthic grazing. Limnology and Oceanography 51: 664–670.
Casajus, N., C. Cameron, D. Ehrich & N. Lecomte, 2021. samplesim: an R package to investigate sample size effects in stable isotope mixing solutions. R package version 1.0. URL: https://github.com/ahasverus/samplesim.
Catasti, M., V. Della Bella, A. M. Di Giulio, E. Goretti, M. Morpurgo, M. Pallottini & E. Tricarico, 2017. Un nuovo alieno in Umbria: il “gamberetto killer” del lago Trasimeno. Micron 37: 16–19.
Caut, S., E. Angulo & F. Courchamp, 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Champely, S., C. Ekstrom, P. Dalgaard, J. Gill, S. Weibelzahl, A. Anandkumar, C. Ford , R. Volcic & H. De Rosario, 2020. pwr: Power analysis functions along the lines of Cohen (1988). R package version 1.3–0. http://cran.r-project.org/web/packages/pwr.
Cilenti, L., G. Mancinelli, T. Scirocco & A. Specchiulli, 2019. First record of Sinanodonta woodiana (Lea, 1834) in an artificial reservoir in the Molise region, Southeast Italy. BioInvasions Records 8: 320–328.
Cole, J. J. & C. T. Solomon, 2012. Terrestrial support of zebra mussels and the Hudson River food web: a multi-isotope, Bayesian analysis. Limnology and Oceanography 57: 1802–1815.
Dionisio Pires, L. M., R. R. Jonker, E. Van Donk & H. J. Laanbroek, 2004. Selective grazing by adults and larvae of the zebra mussel (Dreissena polymorpha): application of flow cytometry to natural seston. Freshwater Biology 49: 116–126.
Dionisio Pires, L. M., B. M. Bontes, L. Samchyshyna, J. Jong, E. Van Donk & B. W. Ibelings, 2007. Grazing on microcystin-producing and microcystin-free phytoplankters by different filter-feeders: implications for lake restoration. Aquatic Sciences 69: 534–543.
Douda, K. & Z. Čadková, 2018. Water clearance efficiency indicates potential filter-feeding interactions between invasive Sinanodonta woodiana and native freshwater mussels. Biological Invasions 20: 1093–1098.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z.-I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A.-H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Ferreira-Rodríguez, N., Y. B. Akiyama, O. V. Aksenova, R. Araujo, M. Christopher Barnhart, Y. V. Bespalaya, A. E. Bogan, I. N. Bolotov, P. B. Budha, C. Clavijo, S. J. Clearwater, G. Darrigran, V. T. Do, K. Douda, E. Froufe, C. Gumpinger, L. Henrikson, C. L. Humphrey, N. A. Johnson, O. Klishko, M. W. Klunzinger, S. Kovitvadhi, U. Kovitvadhi, J. Lajtner, M. Lopes-Lima, E. A. Moorkens, S. Nagayama, K.-O. Nagel, M. Nakano, J. N. Negishi, P. Ondina, P. Oulasvirta, V. Prié, N. Riccardi, M. Rudzīte, F. Sheldon, R. Sousa, D. L. Strayer, M. Takeuchi, J. Taskinen, A. Teixeira, J. S. Tiemann, M. Urbańska, S. Varandas, M. V. Vinarski, B. J. Wicklow, T. Zając & C. C. Vaughn, 2019. Research priorities for freshwater mussel conservation assessment. Biological Conservation 231: 77–87.
Froufe, E., M. Lopes-Lima, N. Riccardi, S. Zaccara, I. Vanetti, J. Lajtner, A. Teixeira, S. Varandas, V. Prié, A. Zieritz, R. Sousa & A. E. Bogan, 2017. Lifting the curtain on the freshwater mussel diversity of the Italian Peninsula and Croatian Adriatic coast. Biodiversity and Conservation 26: 3255–3274.
Fujibayashi, M., O. Nishimura & H. Tanaka, 2016. Evaluation of food sources assimilated by unionid mussels using fatty acid trophic markers in Japanese freshwater ecosystems. Journal of Shellfish Research 35: 231–235.
Gaino, E., F. Scoccia, S. Piersanti, M. Rebora, L. G. Bellucci & A. Ludovisi, 2012. Spicule records of Ephydatia fluviatilis as a proxy for hydrological and environmental changes in the shallow Lake Trasimeno (Umbria, Italy). Hydrobiologia 679: 139–153.
Garton, D. W., C. D. Payne & J. P. Montoya, 2005. Flexible diet and trophic position of dreissenid mussels as inferred from stable isotopes of carbon and nitrogen. Canadian Journal of Fisheries and Aquatic Sciences 62: 1119–1129.
Geist, J., K. Auerswald & A. Boom, 2005. Stable carbon isotopes in freshwater mussel shells: environmental record or marker for metabolic activity? Geochimica Et Cosmochimica Acta 69: 3545–3554.
Gergs, R., K. Rinke & K.-O. Rothhaupt, 2009. Zebra mussels mediate benthic–pelagic coupling by biodeposition and changing detrital stoichiometry. Freshwater Biology 54: 1379–1391.
Giusti, F. & E. Oppi, 1972. Dreissena polymorpha (Pallas) nuovamente in Italia. Memorie Del Museo Civico Di Storia Naturale Di Verona 10: 45–49.
Goretti, E., C. Marcucci, A. Di Veroli, A. Fabrizi & E. Gaino, 2014. The tubificids (Annelida, Oligochaeta) of Lake Trasimeno and Lake Piediluco in Central Italy, with a study of SEM morphology of some species. Turkish Journal of Zoology 38: 334–341.
Goretti, E., M. Pallottini, S. Pagliarini, M. Catasti, G. La Porta, R. Selvaggi, E. Gaino, A. M. Di Giulio & A. Ali, 2020a. Use of larval morphological deformities in Chironomus plumosus (chironomidae: Diptera) as an indicator of freshwater environmental contamination (Lake Trasimeno, Italy). Water 12: 1–17.
Goretti, G., M. Pallottini, G. La Porta & V. Della Bella, 2020b. Invertebrati acquatici alloctoni dell’Umbria In Della Bella, V. (ed) Caratterizzazione e Diffusione delle Specie Aliene Acquatiche e di Ambienti Umidi in Umbria. ARPA Umbria, Perugia, 123–136.
Graf, D. L., 2007. Palearctic freshwater mussel (Mollusca: Bivalvia: Unionoida) diversity and the Comparatory Method as a species concept. Proceedings of the Academy of Natural Sciences of Philadelphia 156: 71–88.
Guarneri, I., O. P. Popa, L. Gola, L. Kamburska, R. Lauceri, M. Lopes-Lima, L. O. Popa & N. Riccardi, 2014. A morphometric and genetic comparison of Sinanodonta woodiana (Lea, 1834) populations: does shape really matter? Aquatic Invasions 9: 183–194.
Hamza, W., P. Pandolfi & M. Taticchi, 1995. Planktonic interactions and their role in describing the trophic status of a shallow lake in Central Italy (Lago Trasimeno). Memorie Dell’istituto Italiano Di Idrobiologia Dott Marco De Marchi 53: 125–140.
Havens, K. E., A. C. Elia, M. I. Taticchi & R. S. Fulton, 2009. Zooplankton–phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 628: 165–175.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta–analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Ilarri, M. I., A. T. Souza, L. Amorim & R. Sousa, 2019. Decay and persistence of empty bivalve shells in a temperate riverine system. Science of the Total Environment 683: 185–192.
Jack, J. D. & J. H. Thorp, 2000. Effects of the benthic suspension feeder Dreissena polymorpha on zooplankton in a large river. Freshwater Biology 44: 569–579.
Jones, R. I., C. E. Carter, A. Kelly, S. Ward, D. J. Kelly & J. Grey, 2008. Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 89: 857–864.
Kadoya, T., Y. Osada & G. Takimoto, 2012. IsoWeb: a bayesian isotope mixing model for diet analysis of the whole food web. PLoS ONE 7: e41057.
Karatayev, A. Y., D. Boltovkoy, D. K. Padilla & L. E. Burlakova, 2007. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26: 205–213.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2015. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746: 97–112.
Kasai, A., D. Ishizaki & T. Isoda, 2016. Isotopic trophic-step fractionation of the freshwater clam Corbicula sandai. Fisheries Science 82: 491–498.
Kat, P. W., 1984. Parasitism and the Unionacea (Bivalvia). Biological Reviews 59: 189–207.
Kissman, C. E. H., L. B. Knoll & O. Sarnelleb, 2010. Dreissenid mussels (Dreissena polymorpha and Dreissena bugensis) reduce microzooplankton and macrozooplankton biomass in thermally stratified lakes. Limnology and Oceanography 55: 1851–1859.
Lancioni, T. & E. Gaino, 2006. The invasive zebra mussel Dreissena polymorpha in Lake Trasimeno (Central Italy): distribution and reproduction. Italian Journal of Zoology 73: 335–346.
Lehmann, M. F., S. M. Bernasconi, A. Barbieri & J. A. McKenzie, 2002. Preservation of organic matter and alteration of its carbon and nitrogen isotope composition during simulated and in situ early sedimentary diagenesis. Geochimica Et Cosmochimica Acta 66: 3573–3584.
Logan, J. M., T. D. Jardine, T. J. Miller, S. E. Bunn, R. A. Cunjak & M. E. Lutcavage, 2008. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. Journal of Animal Ecology 77: 838–846.
Lopes-Lima, M., P. Lima, M. Hinzmann, A. Rocha & J. Machado, 2014a. Selective feeding by Anodonta cygnea (Linnaeus, 1771): the effects of seasonal changes and nutritional demands. Limnologica 44: 18–22.
Lopes-Lima, M., A. Teixeira, E. Froufe, A. Lopes, S. Varandas & R. Sousa, 2014b. Biology and conservation of freshwater bivalves: past, present and future perspectives. Hydrobiologia 735: 1–13.
Lopes-Lima, M., R. Sousa, J. Geist, D. C. Aldridge, R. Araujo, J. Bergengren, Y. Bespalaya, E. Bódis, L. Burlakova, D. Van Damme, K. Douda, E. Froufe, D. Georgiev, C. Gumpinger, A. Karatayev, Ü. Kebapçi, I. Killeen, J. Lajtner, B. M. Larsen, R. Lauceri, A. Legakis, S. Lois, S. Lundberg, E. Moorkens, G. Motte, K.-O. Nagel, P. Ondina, A. Outeiro, M. Paunovic, V. Prié, T. von Proschwitz, N. Riccardi, M. Rudzīte, M. Rudzītis, C. Scheder, M. Seddon, H. Şereflişan, V. Simić, S. Sokolova, K. Stoeckl, J. Taskinen, A. Teixeira, F. Thielen, T. Trichkova, S. Varandas, H. Vicentini, K. Zajac, T. Zajac & S. Zogaris, 2017. Conservation status of freshwater mussels in Europe: state of the art and future challenges. Biological Reviews 92: 572–607.
Lopes-Lima, M., L. E. Burlakova, A. Y. Karatayev, K. Mehler, M. Seddon & R. Sousa, 2018. Conservation of freshwater bivalves at the global scale: diversity, threats and research needs. Hydrobiologia 810: 1–14.
Lucentini, L., L. Gigliarelli, M. E. Puletti, L. Volpi & F. Panara, 2010. Comparison of conservative DNA extraction methods for two Galliformes: grey partridge (Perdix perdix italica, Hartert 1917) and red-legged partridge (Alectoris rufa, Linnaeus 1758). Conservation Genetics Resources 2: 381–384.
Ludovisi, A. & E. Gaino, 2010. Meteorological and water quality changes in Lake Trasimeno (Umbria, Italy) during the last fifty years. Journal of Limnology 69: 174–188.
Ludovisi, A. & A. Poletti, 2003. Use of thermodynamic indices as ecological indicators of the development state of lake ecosystems. 1. Entropy Production Indices. Ecological Modelling 159: 203–222.
Ludovisi, A., M. Minozzo, P. Pandolfi & M. I. Taticchi, 2005. Modelling the horizontal spatial structure of planktonic community in Lake Trasimeno (Umbria, Italy) using multivariate geostatistical methods. Ecological Modelling 181: 247–262.
Lurman, G., J. Walter & H. H. Hoppeler, 2014. Seasonal changes in the behaviour and respiration physiology of the freshwater duck mussel, Anodonta anatina. Journal of Experimental Biology 217: 235–243.
Makhutova, O. N., A. A. Protasov, M. I. Gladyshev, A. A. Sylaieva, N. N. Sushchik, I. A. Morozovskaya & G. S. Kalachova, 2013. Feeding spectra of bivalve mollusks Unio and Dreissena from Kanevskoe Reservoir, Ukraine: are they food competitors or not? Zoological Studies 52: 56.
Malet, N., P. G. Sauriau, N. Faury, P. Soletchnik & G. Guillou, 2007. Effect of seasonal variation in trophic conditions and the gametogenic cycle on δ13C and δ15N levels of diploid and triploid Pacific oysters Crassostrea gigas. Marine Ecology Progress Series 346: 203–217.
Mancinelli, G., M. L. Costantini & L. Rossi, 2002. Cascading effects of predatory fish exclusion on the detritus-based food web of a lake littoral zone (Lake Vico, central Italy). Oecologia 133: 402–411.
Mancinelli, G., M. L. Costantini & L. Rossi, 2007. Top-down control of reed detritus processing in a lake littoral zone: experimental evidence of a seasonal compensation between fish and invertebrate predation. International Review of Hydrobiology 92: 117–134.
Mancinelli, G., P. Papadia, A. Ludovisi, D. Migoni, R. Bardelli, F. P. Fanizzi & S. Vizzini, 2018. Beyond the mean: a comparison of trace- and macroelement correlation profiles of two lacustrine populations of the crayfish Procambarus clarkii. Science of the Total Environment 624: 1455–1466.
Mancini, F., R. De Giorgi, A. Ludovisi, S. Vizzini & G. Mancinelli, 2021. Ontogenetic shift in the trophic role of the invasive killer shrimp Dikerogammarus villosus: a stable isotope study. Biological Invasions 23: 1–15.
Marchegiano, M., E. Gliozzi, S. Ceschin, I. Mazzini, T. Adatte, R. Mazza & D. Ariztegui, 2017. Ecology and distribution of living ostracod assemblages in a shallow endorheic lake: the example of the Lake Trasimeno (Umbria, central Italy). Journal of Limnology 76: 469–487.
McDowell, W. G. & R. Sousa, 2019. Mass mortality events of invasive freshwater bivalves: current understanding and potential directions for future research. Frontiers in Ecology and Evolution 7: 1–12.
Meira, A., M. Lopes-Lima, S. Varandas, A. Teixeira, F. Arenas & R. Sousa, 2019. Invasive crayfishes as a threat to freshwater bivalves: Interspecific differences and conservation implications. Science of the Total Environment 649: 938–948.
Mellina, E. & J. B. Rasmussen, 1994. Patterns in the distribution and abundance of zebra mussel (Dreissena polymorpha) in rivers and lakes in relation to substrate and other physicochemical factors. Canadian Journal of Fisheries and Aquatic Sciences 51: 1024–1036.
Modesto, V., E. Dias, M. Ilarri, M. Lopes-Lima, A. Teixeira, S. Varandas, P. Castro, C. Antunes & R. Sousa, 2021. Trophic niche overlap between native freshwater mussels (Order: Unionida) and the invasive Corbicula fluminea. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 2058–2071.
Müller, T., A. M. Labecka, K. Zając & M. Czarnoleski, 2021. Growth patterns of the pan-European freshwater mussel, Anodonta anatina (Linnaeus, 1758) (Bivalvia: Unionidae), vary with sex and mortality in populations. Ecology and Evolution 11: 2907–2918.
Naddafi, R., K. Pettersson & P. Eklöv, 2007. The effect of seasonal variation in selective feeding by zebra mussels (Dreissena polymorpha) on phytoplankton community composition. Freshw Biol 52.
Nalepa, T. F. & D. W. Schloesser, 2019. Quagga and Zebra Mussels: Biology, Impacts, and Control, CRC Press, Boca Raton:
Nichols, S. J., H. Silverman, T. H. Dietz, J. W. Lynn & D. L. Garling, 2005. Pathways of food uptake in native (Unionidae) and introduced (Corbiculidae and Dreissenidae) freshwater bivalves. Journal of Great Lakes Research 31: 87–96.
Niemeyer, B., 1993. Vergleichende Untersuchungen zur bionomischen Strategie der Teichmuschelarten Anodonta cygnea L. und Anodonta anatina L., Universität Hannover.
Novais, A., E. Dias & R. Sousa, 2016. Inter- and intraspecific variation of carbon and nitrogen stable isotope ratios in freshwater bivalves. Hydrobiologia 765: 149–158.
Ozersky, T., D. O. Evans & D. R. Barton, 2012. Invasive mussels alter the littoral food web of a large lake: stable isotopes reveal drastic shifts in sources and flow of energy. PLoS ONE 7: e51249.
Ożgo, M., M. Urbańska, P. Hoos, H. K. Imhof, M. Kirschenstein, J. Mayr, F. Michl, R. Tobiasz, M. von Wesendonk, S. Zimmermann & J. Geist, 2020. Invasive zebra mussel (Dreissena polymorpha) threatens an exceptionally large population of the depressed river mussel (Pseudanodonta complanata) in a postglacial lake. Ecology and Evolution 10: 4918–4927.
Parnell, A. C., 2020. SIMMR: A Stable Isotope Mixing Model. R package version 0.4.2. https://CRAN.R-project.org/package=simmr.
Parnell, A. C., R. Inger, S. Bearhop & A. L. Jackson, 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5: e9672.
Paulet, Y.-M., A. Lorrain, J. Richard & S. Pouvreau, 2006. Experimental shift in diet δ13C: a potential tool for ecophysiological studies in marine bivalves. Organic Geochemistry 37: 1359–1370.
Phillips, D. L., R. Inger, S. Bearhop, A. L. Jackson, J. W. Moore, A. C. Parnell, B. X. Semmens & E. J. Ward, 2014. Best practices for use of stable isotope mixing models in food web studies. Canadian Journal of Zoology 92: 823–835.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montana, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
R Development Core Team, 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Raikow, D. F. & S. K. Hamilton, 2001. Bivalve diets in a midwestern U.S. stream: a stable isotope enrichment study. Limnology and Oceanography 46: 514–522.
Raoult, V., T. F. Gaston & M. D. Taylor, 2018. Habitat–fishery linkages in two major south-eastern Australian estuaries show that the C4 saltmarsh plant Sporobolus virginicus is a significant contributor to fisheries productivity. Hydrobiologia 811: 221–238.
Ricciardi, A., F. G. Whoriskey & J. B. Rasmussen, 1996. Impact of the Dreissena invasion on native unionid bivalves in the upper St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 53: 1434–1444.
Riccardi, N., E. Froufe, A. E. Bogan, A. Zieritz, A. Teixeira, I. Vanetti, S. Varandas, S. Zaccara, K. O. Nagel & M. Lopes-Lima, 2019. Phylogeny of European Anodontini (Bivalvia: Unionidae) with a redescription of Anodonta exulcerata. Zoological Journal of the Linnean Society 189: 745–761.
Roditi, H. A., N. F. Caraco, J. J. Cole & D. L. Strayer, 1996. Filtration of Hudson River water by the zebra mussel (Dreissena polymorpha). Estuaries 19: 824–832.
Schneider, D. W., 1992. A bioenergetics model of zebra mussel, Dreissena polymorpha, growth in the Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 49: 1406–1416.
Schwalb, A. N. & M. T. Pusch, 2007. Horizontal and vertical movements of unionid mussels in a lowland river. Journal of the North American Benthological Society 26: 261–272.
Semmens, B. X., E. J. Ward, A. C. Parnell & P. D. L., S. Bearhop, R. Inger, A. Jackson & J. W. Moore, 2013. Statistical basis and outputs of stable isotope mixing models: comment on Fry. Marine Ecology Progress Series 490: 285–289.
Soetaert, K., J. J. Middelburg, P. M. J. Herman & K. Buis, 2000. On the coupling of benthic and pelagic biogeochemical models. Earth-Science Reviews 51: 173–201.
Sousa, R., F. Pilotto & D. C. Aldridge, 2011. Fouling of European freshwater bivalves (Unionidae) by the invasive zebra mussel (Dreissena polymorpha). Freshw Biol 56.
Sousa, R., A. Novais, R. Costa & D. L. Strayer, 2014. Invasive bivalves in fresh waters: impacts from individuals to ecosystems and possible control strategies. Hydrobiologia 735: 233–251.
Spilinga, C., U. Chiappafreddo & Q. Pirisinu, 2000. Dreissena polymorpha (Pallas) al lago Trasimeno. Rivista Di Idrobiologia 39: 145–152.
Strayer, D. L. & H. M. Malcom, 2018. Long-term responses of native bivalves (Unionidae and Sphaeriidae) to a Dreissena invasion. Freshwater Science 37: 697–711.
Strayer, D. L. & L. C. Smith, 1996. Relationships between zebra mussels (Dreissena polymorpha) and unionid clams during the early stages of the zebra mussel invasion of the Hudson River. Freshwater Biology 36: 771–780.
Strayer, D. L., J. A. Downing, W. R. Haag, T. L. King, J. B. Layzer, T. J. Newton & J. S. Nichols, 2004. Changing perspectives on pearly mussels, North America’s most imperiled animals. Bioscience 54: 429–439.
Suh, Y. J. & K.-H. Shin, 2013. Size-related and seasonal diet of the manila clam (Ruditapes philippinarum), as determined using dual stable isotopes. Estuarine, Coastal and Shelf Science 135: 94–105.
Syväranta, J. & M. Rautio, 2010. Zooplankton, lipids and stable isotopes: importance of seasonal, latitudinal, and taxonomic differences. Canadian Journal of Fisheries and Aquatic Sciences 67: 1721–1729.
Taskinen, J., M. Urbańska, F. Ercoli, W. Andrzejewski, M. Ożgo, B. Deng, J. M. Choo & N. Riccardi, 2021. Parasites in sympatric populations of native and invasive freshwater bivalves. Hydrobiologia 848: 3167–3178.
Taticchi, M. I., 1992. Studies on Lake Trasimeno and other water bodies in Umbria Region (Central Italy). Memorie Istituto Italiano Idrobiologia 50: 259–317.
Taylo, J. M., M. J. Vanni & A. S. Flecker, 2015. Top-down and bottom-up interactions in freshwater ecosystems: emerging complexities. In La Pierre, K. J. & T. C. Hanley (eds), Trophic Ecology: Bottom-up and Top-down Interactions across Aquatic and Terrestrial Systems Cambridge University Press, Cambridge, Ecological Reviews: 55–85.
Tsuchiya, K., K. Komatsu, R. Shinohara, A. Imai, S.-I.S. Matsuzaki, R. Ueno, V. S. Kuwahara & A. Kohzu, 2020. Variability of benthic methane-derived carbon along seasonal, biological, and sedimentary gradients in a polymictic lake. Limnology and Oceanography 65: 3017–3031.
Twining, C. W., S. J. Taipale, L. Ruess, A. Bec, D. Martin-Creuzburg & M. J. Kainz, 2020. Stable isotopes of fatty acids: current and future perspectives for advancing trophic ecology. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 375: 20190641.
Vanderklift, M. & S. Ponsard, 2003. Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136: 169–182.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 58: 1208–1221.
Vaughn, C. C., 2018. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C. C. & C. C. Hakenkamp, 2001. The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46: 1431–1446.
Vaughn, C. C., S. J. Nichols & D. E. Spooner, 2008. Community and foodweb ecology of freshwater mussels. Journal of the North American Benthological Society 27: 409–423.
VV.AA., 2015. Parco Regionale del Lago Trasimeno. Aspetti faunistici – Anfibi Rettili Pesci e Invertebrati. Regione Umbria - Servizio sistemi naturalistici e zootecnia. https://www.regione.umbria.it/documents/18/2512711/Trasimeno_anfibi_rettili_pesci_inv_ott_15.pdf/359f3c6e-6c97-45b3-9cb9-5b6a57443f7e. Accessed 19 Nov 2020.
Ward, E. J., B. X. Semmens, D. L. Phillips, J. W. Moore & N. Bouwes, 2011. A quantitative approach to combine sources in stable isotope mixing models. Ecosphere 2: art19.
Weber, A. M., J. E. Bauer & G. Thomas Watters, 2017. Assessment of nutritional subsidies to freshwater mussels using a multiple natural abundance isotope approach. Freshwater Biology 62: 615–629.
Xu, J., H. Lyu, X. Xu, Y. Li, Z. Li, S. Lei, S. Bi, M. Mu, C. Du & S. Zeng, 2019. Dual stable isotope tracing the source and composition of POM during algae blooms in a large and shallow eutrophic lake: all contributions from algae? Ecological Indicators 102: 599–607.
Yokoyama, H., A. Tamaki, K. Harada, K. Shimoda, K. Koyama & Y. Ishihi, 2005. Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Marine Ecology Progress Series 296: 115–128.
Zieritz, A. & D. C. Aldridge, 2011. Sexual, habitat-constrained and parasite-induced dimorphism in the shell of a freshwater mussel (Anodonta anatina, Unionidae). Journal of Morphology 272: 1365–1375.
Zieritz, A., B. Gum, R. Kuehn & J. Geist, 2012. Identifying freshwater mussels (Unionoida) and parasitic glochidia larvae from host fish gills: a molecular key to the North and Central European species. Ecology and Evolution 2: 740–750.
Acknowledgements
Funding from the project ‘‘Tratti bio-ecologici chiave in una specie invasiva: Procambarus clarkii’’ (Fundamental Research Funding), Department of Chemistry, Biology, and Biotechnology, University of Perugia to A.L., and from the Italian Ministry of Instruction, University, and Research (Law n. 232 December 11th, 2016) to G.M. is acknowledged. The constructive comments by two anonymous reviewers that helped improving the manuscript are gratefully acknowledged. All the authors provided their consent to participate to the study and to the publication of its results and declared no competing interests. No ethical issues related with the use of animals in the performed analyses were involved. Original data are available upon request to the corresponding author. This paper has been conceived during a sabbatical year of one the authors (G.M.) in the Department of Chemistry, Biology, and Biotechnology, University of Perugia (Italy).
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. Funding was provided by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca, and by Università degli Studi di Perugia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Handling Editor: Eric R. Larson
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ludovisi, A., Goretti, E., Pallottini, M. et al. Stable isotope analysis reveals trophic segregation between the invasive zebra mussel Dreissena polymorpha and the native duck mussel Anodonta anatina in Lake Trasimeno (Italy). Hydrobiologia 849, 2091–2108 (2022). https://doi.org/10.1007/s10750-022-04846-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04846-4